Abstract
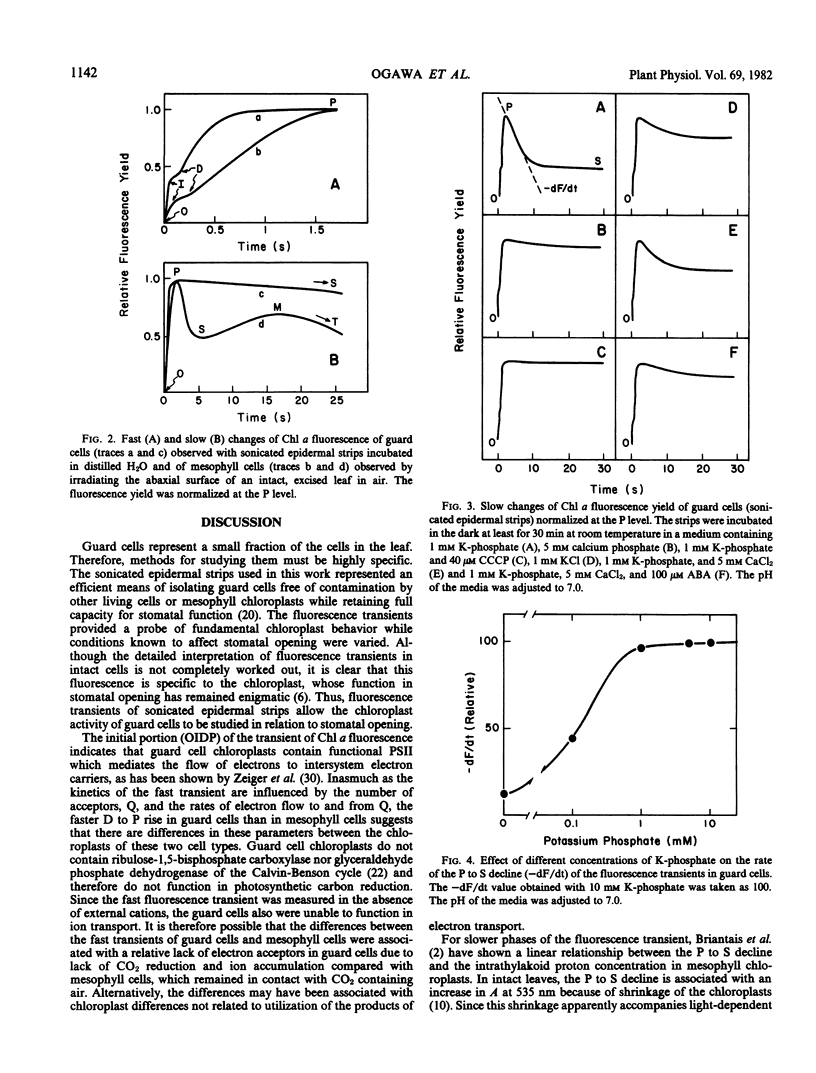
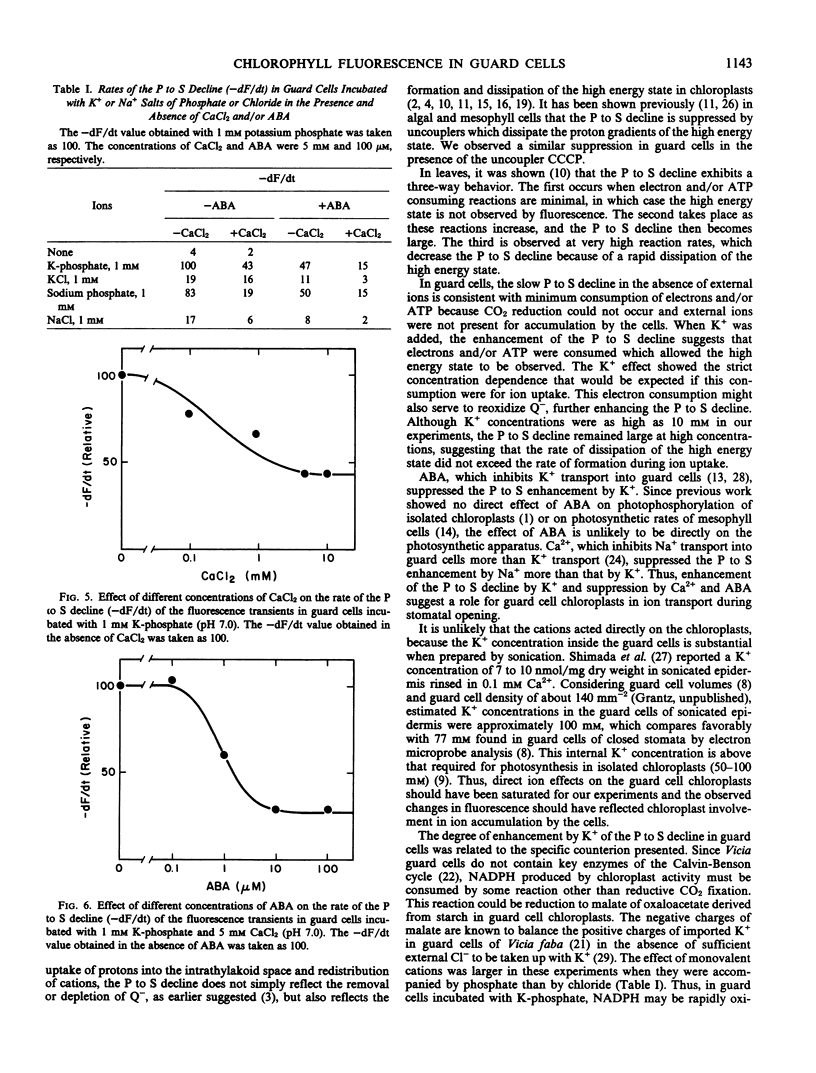
The effects of cations and abscisic acid on chloroplast activity in guard cells of Vicia faba were investigated by analysis of the transient of chlorophyll a fluorescence. When epidermal strips containing guard cells as the only living cells were incubated in water and illuminated with strong light, chlorophyll a fluorescence rose rapidly to a high intensity and then declined slowly to a stationary level. The rate of this decline was enhanced by K+ or Na+, and the effect of these cations was greater when added with phosphate than with chloride as the anion. Ca2+ suppressed the enhancement by Na+ and, to a lesser extent, that by K+. Abscisic acid also suppressed the enhancement by K+ and Na+. Since the fluorescence decline reflects the increase of intrathylakoid H+ concentration necessary for photophosphorylation, the acceleration of the decline by K+ (or Na+ in the absence of Ca2+) implicates chloroplast activity in ion accumulation by guard cells in the light. The differential effects of phosphate and chloride suggest that chloroplast activity may be involved in malate formation in guard cells in the light.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briantais J. M., Vernotte C., Picaud M., Krause G. H. A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim Biophys Acta. 1979 Oct 10;548(1):128–138. doi: 10.1016/0005-2728(79)90193-2. [DOI] [PubMed] [Google Scholar]
- Humble G. D., Hsiao T. C. Light-dependent Influx and Efflux of Potassium of Guard Cells during Stomatal Opening and Closing. Plant Physiol. 1970 Sep;46(3):483–487. doi: 10.1104/pp.46.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humble G. D., Raschke K. Stomatal opening quantitatively related to potassium transport: evidence from electron probe analysis. Plant Physiol. 1971 Oct;48(4):447–453. doi: 10.1104/pp.48.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause G. H. The high-energy state of the thylakoid system as indicated by chlorophyll fluorescence and chloroplast shrinkage. Biochim Biophys Acta. 1973 Apr 5;292(3):715–728. doi: 10.1016/0005-2728(73)90019-4. [DOI] [PubMed] [Google Scholar]
- Mawson B. T., Colman B., Cummins W. R. Abscisic Acid and photosynthesis in isolated leaf mesophyll cell. Plant Physiol. 1981 Feb;67(2):233–236. doi: 10.1104/pp.67.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty P., Govindjee Light-induced changes in the fluorescence yield of chlorophyll a in Anacystis nidulans. I. Relationship of slow fluorescence changes with structural changes. Biochim Biophys Acta. 1973 Apr 27;305(1):95–104. doi: 10.1016/0005-2728(73)90235-1. [DOI] [PubMed] [Google Scholar]
- Munday J. C., Jr, Govindjee Light-induced changes in the fluorescence yield of chlorophyll A in vivo. 3. The dip and the peak in the fluorescence transient of Chlorella pyrenoidosa. Biophys J. 1969 Jan;9(1):1–21. doi: 10.1016/s0006-3495(69)86365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N., Sugahara K. Control of excitation transfer in photosynthesis. 3. Light-induced decrease of chlorophyll a fluorescence related to photophosphorylation system in spinach chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):182–192. doi: 10.1016/0005-2728(69)90046-2. [DOI] [PubMed] [Google Scholar]
- Outlaw W. H., Lowry O. H. Organic acid and potassium accumulation in guard cells during stomatal opening. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4434–4438. doi: 10.1073/pnas.74.10.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Manchester J., Dicamelli C. A., Randall D. D., Rapp B., Veith G. M. Photosynthetic carbon reduction pathway is absent in chloroplasts of Vicia faba guard cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6371–6375. doi: 10.1073/pnas.76.12.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Mayne B. C., Zenger V. E., Manchester J. Presence of Both Photosystems in Guard Cells of Vicia faba L: IMPLICATIONS FOR ENVIRONMENTAL SIGNAL PROCESSING. Plant Physiol. 1981 Jan;67(1):12–16. doi: 10.1104/pp.67.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas J. E. Photophosphorylation Can Provide Sufficient Adenosine 5'-Triphosphate to Drive K Movements during Stomatal Opening. Plant Physiol. 1972 Apr;49(4):649–650. doi: 10.1104/pp.49.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kirk C. A., Raschke K. Presence of Chloride Reduces Malate Production in Epidermis during Stomatal Opening. Plant Physiol. 1978 Mar;61(3):361–364. doi: 10.1104/pp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E., Armond P., Melis A. Fluorescence Properties of Guard Cell Chloroplasts: EVIDENCE FOR LINEAR ELECTRON TRANSPORT AND LIGHT-HARVESTING PIGMENTS OF PHOTOSYSTEMS I AND II. Plant Physiol. 1981 Jan;67(1):17–20. doi: 10.1104/pp.67.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]



