Abstract
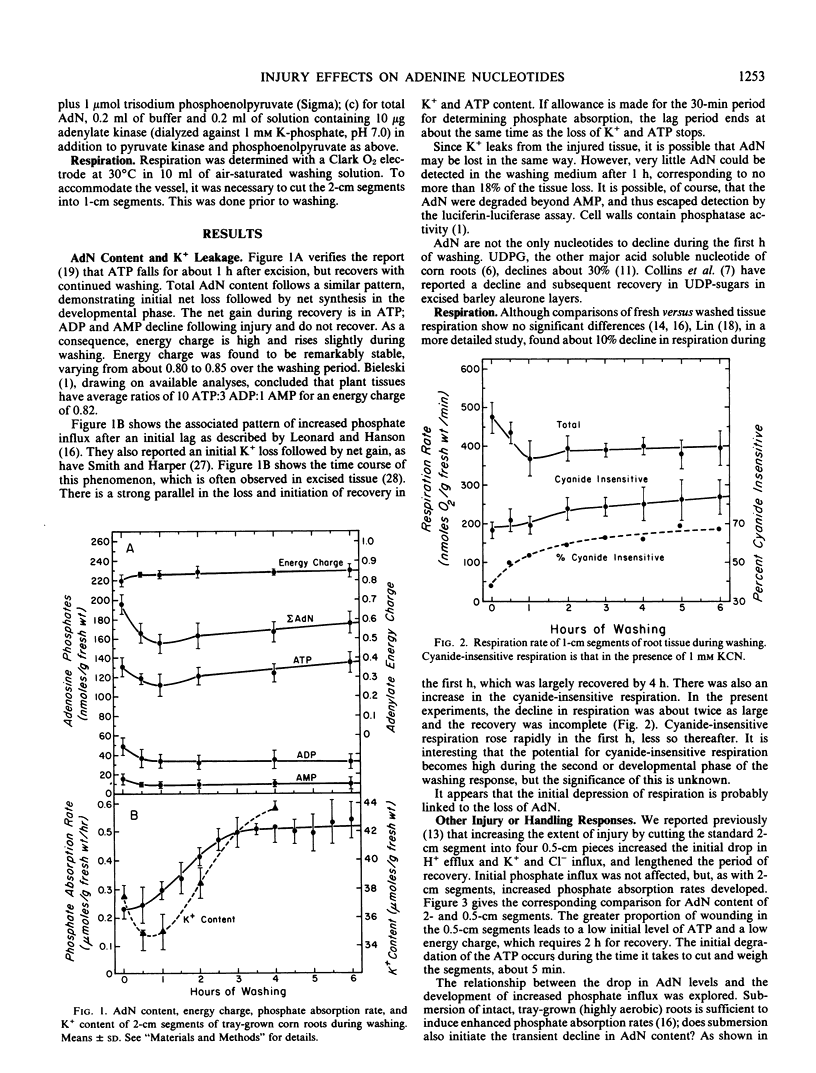
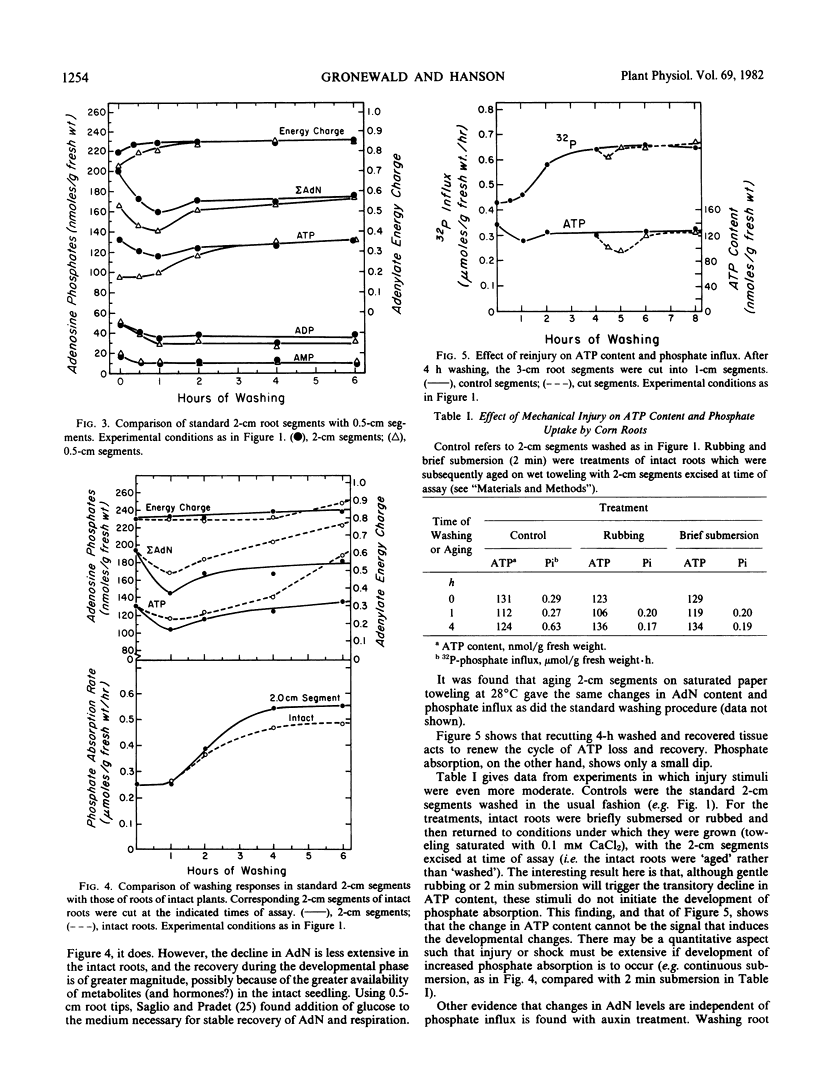
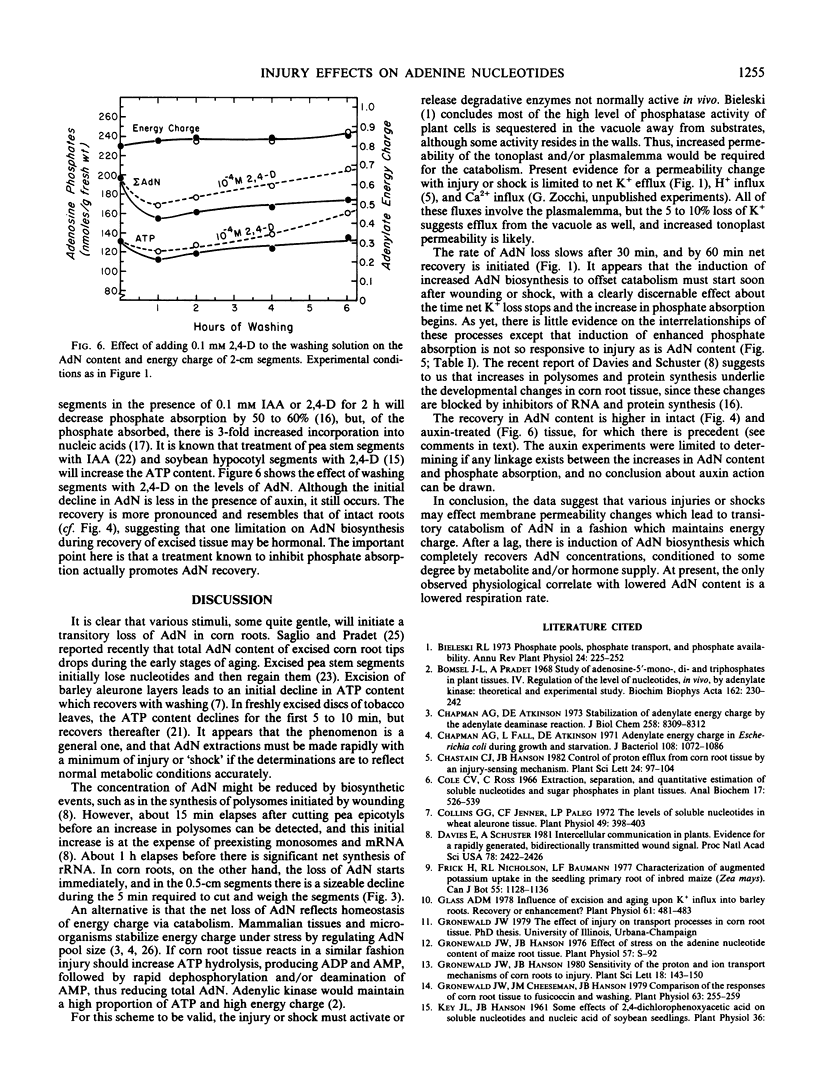
The adenine nucleotide content of the 2-centimeter segments excised from tray-grown corn (Zea mays L., WF9 × Mo17) roots declines for the first hour after excision. Concomitant with the loss of adenine nucleotides is a decline in respiration and a leakage of K+. With continued washing, these parameters partially or completely recover and increased phosphate influx develops. Increasing the wound effect by cutting 0.5-centimeter segments gives a more rapid and pronounced degradation of adenine nucleotides and slower recovery. Conversely, the mild injury caused by submerging intact roots induces less degradation and produces greater net adenine nucleotide synthesis during recovery; adding auxin to the washing medium produces a similar result. With all treatments, there is stabilization of energy charge at about 0.85.
Brief submersion or rubbing of intact roots, as well as recutting washed and recovered root segments, will initiate the transient loss of adenine nucleotides but will not induce increased phosphate influx.
It is suggested that the loss in adenine nucleotides may reflect homeostasis in energy charge via catabolism arising from membrane permeability changes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomsel J. L., Pradet A. Study of adenosine 5'-mono-,di- and triphosphates in plant tissues. IV. Regulation of the level of nucleotides, in vivo, by adenylate kinase: theoretical and experimental study. Biochim Biophys Acta. 1968 Aug 20;162(2):230–242. doi: 10.1016/0005-2728(68)90105-9. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Stabilization of adenylate energy charge by the adenylate deaminase reaction. J Biol Chem. 1973 Dec 10;248(23):8309–8312. [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. V., Ross C. Extraction, separation, and quantitative estimation of soluble nucleotides and sugar phosphates in plant tissues. Anal Biochem. 1966 Dec;17(3):526–539. doi: 10.1016/0003-2697(66)90188-6. [DOI] [PubMed] [Google Scholar]
- Collins G. G., Jenner C. F., Paleg L. G. The levels of soluble nucleotides in wheat aleurone tissue. Plant Physiol. 1972 Mar;49(3):398–403. doi: 10.1104/pp.49.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Schuster A. Intercellular communication in plants: Evidence for a rapidly generated, bidirectionally transmitted wound signal. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2422–2426. doi: 10.1073/pnas.78.4.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. D. Influence of Excision and Aging upon K Influx into Barley Roots: Recovery or Enhancement? Plant Physiol. 1978 Apr;61(4):481–483. doi: 10.1104/pp.61.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronewald J. W., Cheeseman J. M., Hanson J. B. Comparison of the responses of corn root tissue to fusicoccin and washing. Plant Physiol. 1979 Feb;63(2):255–259. doi: 10.1104/pp.63.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Increased Membrane-bound Adenosine Triphosphatase Activity Accompanying Development of Enhanced Solute Uptake in Washed Corn Root Tissue. Plant Physiol. 1972 Mar;49(3):436–440. doi: 10.1104/pp.49.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Induction and development of increased ion absorption in corn root tissue. Plant Physiol. 1972 Mar;49(3):430–435. doi: 10.1104/pp.49.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Hanson J. B. Increase in electrogenic membrane potential with washing of corn root tissue. Plant Physiol. 1974 Nov;54(5):799–801. doi: 10.1104/pp.54.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Hanson J. B. Phosphate absorption rates and adenosine 5'-triphosphate concentrations in corn root tissue. Plant Physiol. 1974 Sep;54(3):250–256. doi: 10.1104/pp.54.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol P. K. Rapid Metabolic Changes in the Wounding Response of Leaf Discs following Excision. Plant Physiol. 1976 Jan;57(1):80–84. doi: 10.1104/pp.57.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P. H., Pradet A. Soluble Sugars, Respiration, and Energy Charge during Aging of Excised Maize Root Tips. Plant Physiol. 1980 Sep;66(3):516–519. doi: 10.1104/pp.66.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. C., Harper J. C. Influence of the root tip and the duration of washing on k retention by excised apical root segments of corn. Plant Physiol. 1977 Feb;59(2):335–336. doi: 10.1104/pp.59.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]


