Abstract
Background: Obesity, particularly visceral and ectopic adiposity, increases the risk of type 2 diabetes.
Objective: The aim of this study was to determine if restriction of dietary carbohydrate is beneficial for body composition and metabolic health.
Methods: Two studies were conducted. In the first, 69 overweight/obese men and women, 53% of whom were European American (EA) and 47% of whom were African American (AA), were provided with 1 of 2 diets (lower-fat diet: 55%, 18%, and 27% of energy from carbohydrate, protein, and fat, respectively; lower-carbohydrate diet: 43%, 18%, and 39%, respectively) for 8 wk at a eucaloric level and 8 wk at a hypocaloric level. In the second study, 30 women with polycystic ovary syndrome (PCOS) were provided with 2 diets (lower-fat diet: 55%, 18%, and 27% of energy from carbohydrate, protein, and fat, respectively; lower-carbohydrate diet: 41%, 19%, and 40%, respectively) at a eucaloric level for 8 wk in a random-order crossover design.
Results: As previously reported, among overweight/obese adults, after the eucaloric phase, participants who consumed the lower-carbohydrate vs. the lower-fat diet lost more intra-abdominal adipose tissue (IAAT) (11 ± 3% vs. 1 ± 3%; P < 0.05). After weight loss, participants who consumed the lower-carbohydrate diet had 4.4% less total fat mass. Original to this report, across the entire 16-wk study, AAs lost more fat mass with a lower-carbohydrate diet (6.2 vs. 2.9 kg; P < 0.01), whereas EAs showed no difference between diets. As previously reported, among women with PCOS, the lower-carbohydrate arm showed decreased fasting insulin (−2.8 μIU/mL; P < 0.001) and fasting glucose (−4.7 mg/dL; P < 0.01) and increased insulin sensitivity (1.06 arbitrary units; P < 0.05) and “dynamic” β-cell response (96.1 · 109; P < 0.001). In the lower-carbohydrate arm, women lost both IAAT (−4.8 cm2; P < 0.01) and intermuscular fat (−1.2 cm2; P < 0.01). In the lower-fat arm, women lost lean mass (−0.6 kg; P < 0.05). Original to this report, after the lower-carbohydrate arm, the change in IAAT was positively associated with the change in tumor necrosis factor α (P < 0.05).
Conclusion: A modest reduction in dietary carbohydrate has beneficial effects on body composition, fat distribution, and glucose metabolism. This trial was registered at clinicaltrials.gov as NCT00726908 and NCT01028989.
Keywords: insulin secretion, insulin sensitivity, PCOS, glycemic load, visceral fat, body composition
Introduction
Obesity, particularly intra-abdominal and ectopic adiposity, is associated with insulin resistance. Insulin resistance, in combination with β cell dysfunction, contributes to the development of type 2 diabetes (T2D)4 (1). To address the current worldwide increase in T2D prevalence, feasible nonpharmacologic approaches are needed for preventing and reversing obesity and obesity-related metabolic dysfunction.
The consumption of large amounts of processed carbohydrate-containing foods may be one of the major factors leading to both obesity and metabolic dysfunction. The consumption of processed carbohydrates leads to elevated insulin secretion, which, in turn, promotes glucose oxidation, impairs fat oxidation, facilitates de novo lipogenesis, and promotes storage of fat (2), while at the same time leading to insulin resistance, inflammation (3), and oxidative stress (4). The combination of insulin resistance and hyperinsulinemia also disrupts lipid metabolism and increases the risk of cardiovascular disease, the most common source of mortality among individuals with T2D (5).
In contrast, when dietary carbohydrate is restricted and insulin declines, metabolic processes shift to favor fat oxidation over lipid storage. As a result, the lipid profile improves, and lipotoxic processes that impair β cell function and insulin action resolve. A number of studies documented the benefits of lower-carbohydrate diets on measures of metabolic health and medication use in patients with T2D (6–11) and for weight loss and improved metabolic health in healthy individuals (12). Despite these well-documented benefits, carbohydrate restriction or reduction is not currently recommended for prevention or treatment of diabetes by the major organizations and institutions to which physicians turn for guidance (13, 14).
The impact of dietary carbohydrate on metabolism may differ with individual phenotype. We and others have reported that the degree of insulin sensitivity and the amount of insulin secreted after a glucose challenge affect the extent to which dietary carbohydrate quantity and quality affect the change in body weight and body composition over 12–18 mo (15–18). Individuals who are more sensitive to insulin, or who secrete a greater amount of insulin in response to a glucose challenge, appear to be more sensitive to the stimulatory effects of dietary carbohydrate on fat deposition. These observations may be particularly relevant to ethnic minorities. African Americans (AAs), Mexican Americans, and Native Americans secrete a greater amount of insulin than do European Americans (EAs) for a given amount of exposure to oral or intravenous glucose (19–21). All of these minority groups are at greater risk of both obesity and T2D compared with EAs. Thus, although it is possible that diet modification could play a particularly relevant role in improving metabolic health in ethnic minorities, this hypothesis has not been tested.
This article discusses the results of 2 recent studies comparing carbohydrate-restricted diets with lower-fat diets in 2 populations at elevated risk of T2D. In both studies, the 2 experimental diets were matched for protein content to avoid potential confounding effects of altered protein intake on body composition or metabolic outcomes. The first study, conducted in nondiabetic but overweight or obese AA and EA adults, examined body composition and body fat distribution during conditions of both weight maintenance and weight loss. The second study, conducted in women with polycystic ovary syndrome (PCOS), examined these outcomes as well as outcomes related to metabolic health (insulin sensitivity and β cell function) during weight maintenance. Women with PCOS are characterized by insulin resistance, hyperinsulinism, and perhaps visceral adiposity (22–24) and are therefore at increased risk of T2D.
Methods
Overweight/obese adults.
Sixty-nine men and women aged 21–50 y were enrolled in the study. Details of the study were published previously (25–27). Briefly, inclusion criteria were BMI (in kg/m2) of 25–45, weight <136 kg, age 21–50 y, nondiabetic, and no weight change of >2.3 kg over the past 6 mo. Approximately 50% of participants were AAs and ∼50% were EAs, by design, to examine potential differences in the response to diet on the basis of ethnicity. Glucose tolerance was evaluated at screening by using a 2-h oral-glucose-tolerance test, and only those who had 2-h glucose in the normal or mildly impaired range (≤155 mg/dL) were eligible for the study. Twenty-seven participants had impaired fasting glucose (≥100 mg/dL). Participants were informed of the experimental design, and oral and written consent was obtained. The study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham.
Baseline testing included body composition by DXA, body fat distribution by computed tomography (CT) scan, and the acute insulin response to glucose (AIRg). AIRg is the incremental AUC for insulin during the first 10 min after intravenous glucose injection at a dose of 300 mg/kg. After completing baseline testing, participants were assigned to 1 of 2 diets that differed in percentage of energy from carbohydrate (55% or 43%) and fat (27% or 39%), with both having 18% protein, and were provided with all food at a eucaloric level for an 8-wk period. At week 4, participants were administered a breakfast meal test (28). For this test, blood was collected before and for 4 h during and after consumption of a breakfast meal that was part of their assigned diet. Serum concentrations of insulin and glucose were assessed at several time points. After completion of the 8-wk eucaloric period, participants entered a second 8-wk intervention period in which energy intake was decreased by 1000 kcal/d. Body composition and fat distribution were assessed at the end of both eucaloric and hypocaloric phases. Data were analyzed within all participants combined, and within each ethnic group.
Original to this report, circulating markers of inflammation were assessed by immunoassay in fasted morning sera before and after the intervention. High-sensitivity C-reactive protein (CRP) was assessed by turbidometric methods by using a SIRRUS analyzer (Stanbio Laboratory), with reagents obtained from Pointe Scientific, and TNF-α and IL-6 by using electrochemiluminscence (Meso Scale Discovery). Minimum detectable concentrations for each assay were 0.05 mg/L, 0.507 pg/mL, and 0.25 pg/mL, respectively. Mean intra-assay CVs were 7.49%, 7.61%, and 6.68%, respectively. Mean interassay CVs were 2.13%, 5.47%, and 9.72%, respectively. Data were analyzed by paired t test and by ANCOVA for the main outcome of 8-wk concentration, with baseline concentration as a covariate. In addition, to determine if changes in intra-abdominal adipose tissue (IAAT) were associated with changes in markers of inflammation, multiple linear regression analysis was conducted within each diet group to adjust for the change in total body fat mass. All analyses were conducted with the use of SAS software (version 9.3; SAS Institute); the α level was set at 0.05.
Original to this report, data were analyzed within each ethnic group. Because AAs relative to EAs have a greater insulin response to glucose, it was of interest to determine if the response to dietary carbohydrate content differed in AAs vs. EAs. For these analyses, data from the entire 16-wk intervention were combined. Preliminary analyses indicated that because some participants lost weight and fat during the eucaloric phase, the body composition response to the entire 16-wk intervention gave the clearest picture of how the change in diet macronutrient composition affected body composition. Thus, the change in body fat mass (kg) was assessed by using ANCOVA, with fat mass at 16 wk as the dependent variable, ethnicity as the class variable, and baseline fat mass and insulin sensitivity as covariates. Insulin sensitivity was selected as a covariate because it interacts with diet composition in determining changes in body composition over time (15, 16). The least squares means procedure was used to generate adjusted means and SEMs for graphical presentation. All analyses were conducted by using SAS software (version 9.3); the α level was set at 0.05. Descriptive statistics are presented as means ± SDs; main outcome data are presented as means ± SEMs.
Women with PCOS.
Details of the study were published previously (29). Briefly, 30 women with PCOS were enrolled in the study. Participants were informed of the experimental design, and oral and written consent was obtained. The study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham.
The study was conducted by using a crossover design. Comprehensive metabolic testing was conducted before and after each 8-wk arm, with a 4-wk washout period between arms. After completing baseline testing, participants were assigned, by using a randomization scheme, to 1 of 2 diets: a lower-fat diet with a macronutrient composition of 55% carbohydrate, 18% protein, and 27% fat (% of energy from each) or a lower-carbohydrate diet with a macronutrient composition of 41% carbohydrate, 19% protein, and 40% fat. All food was provided for the duration of each arm.
Dynamic β cell response to glucose [dynamic phase serum insulin response to glucose (PhiD)] was determined before and after each diet arm by using glucose and C-peptide data obtained during a liquid meal tolerance test (30). Insulin sensitivity was calculated by using a formula based on insulin and glucose values throughout the meal test (31). Original to this report, circulating markers of inflammation were assessed by immunoassay as described in the previous section, Overweight/obese adults. Body composition by DXA and body fat distribution by CT scan were determined before and after each arm. Cross-sectional areas of IAAT and intermuscular adipose tissue (IMAT) were quantified from CT scans by using SliceOmatic software (TomoVision).
Main outcomes were fasting glucose, fasting insulin, insulin sensitivity, β cell responsiveness, body composition, and body fat distribution. Secondary outcomes were circulating markers of inflammation. Data were analyzed by paired t test for changes within each treatment arm and by ANCOVA for differences between diet arms (α = 0.05). Descriptive statistics are presented are means ± SDs; main and secondary outcome data are presented as means ± SEMs. To determine if changes in IAAT were independently associated with changes in markers of inflammation, multiple linear regression analysis was conducted within each diet group for the dependent variable “change in CRP/TNF-α/IL-6,” with changes in IAAT and total body fat mass as independent variables.
Results
Overweight/obese adults.
Descriptive characteristics of the study population, by ethnicity, are shown in Table 1. AA and EA participants did not differ with respect to age, BMI, or fat mass. However, AAs had 40% greater AIRg (P < 0.01).
TABLE 1.
Participant characteristics by ethnicity among overweight/obese adults1
| EA | AA | |
| Sex (M/F), n | 18/18 | 13/20 |
| BMI, kg/m2 | 31.8 ± 3.7 | 33.2 ± 4.7 |
| Age, y | 36.1 ± 8.0 | 34.1 ± 8.6 |
| Weight, kg | 97.2 ± 18.5 | 102.0 ± 19.0 |
| Fat mass, kg | 38.9 ± 9.2 | 40.6 ± 8.8 |
| AIRg, μIU/mL × 10 min | 824 ± 628 | 1420 ± 917** |
Values are means ± SDs; n = 36 EAs, n = 33 AAs. **Different from EAs, P < 0.01. AA, African American; AIRg, acute insulin response to glucose; EA, European American.
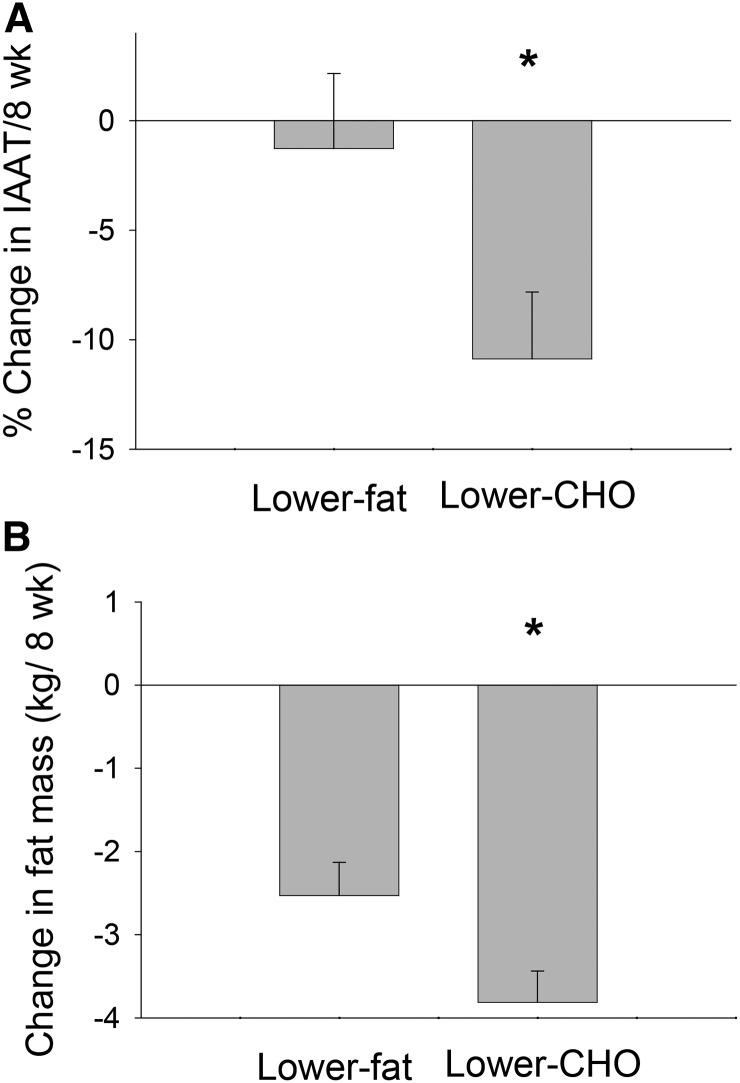
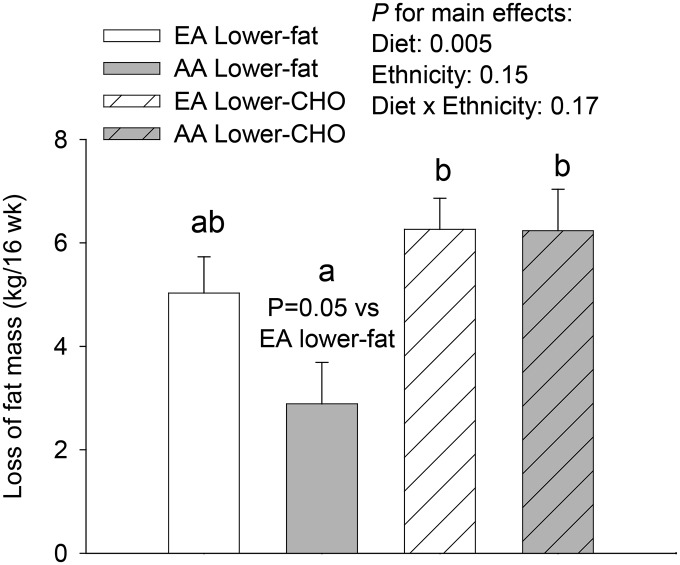
As previously reported (27), in the eucaloric phase, loss of IAAT was significantly greater (P < 0.05; Figure 1A) in participants who consumed the lower-carbohydrate diet (11%) than in those who consumed the lower-fat diet. Furthermore, when comparing the 2 diet groups at the end of the eucaloric phase, participants who consumed the lower-carbohydrate diet had 11% less intra-abdominal fat (IAAT) than did those who consumed the lower-fat diet (P < 0.05, adjusted for total fat mass and baseline IAAT). In the hypocaloric phase, total fat mass loss was greater in participants who consumed the lower-carbohydrate (4.4%) diet vs. those who consumed the lower-fat diet (P < 0.05; Figure 1B). Original to this report, markers of inflammation did not change in response to either of the diets (P > 0.05) when data were analyzed by paired t test or ANCOVA (Table 2). Furthermore, changes in markers of inflammation were not associated with the change in IAAT. Original to this report, over the course of the entire study, loss of total body fat was greater in AAs, but not EAs, in individuals receiving the lower-carbohydrate diet (Figure 2).
FIGURE 1.
Change in IAAT over 8 wk during the eucaloric phase (A) and in total fat mass over 8 wk during the hypocaloric phase (B) in overweight/obese adults consuming a lower-CHO or lower-fat diet. Values are means ± SEMs. A: Lower-fat diet, n = 29; lower-carbohydrate diet, n = 34; B: lower-fat diet, n = 28; lower-CHO diet, n = 31. *Different from lower-fat arm, P < 0.05. Both panels were adapted from reference 27. CHO, carbohydrate; IAAT, intra-abdominal adipose tissue.
TABLE 2.
Serum concentrations of markers of inflammation at baseline and at 8 wk in overweight/obese adults and women with PCOS consuming lower-fat and lower-CHO diets1
| Lower-fat diet |
Lower-CHO diet |
|||
| Week 0 | Week 8 | Week 0 | Week 8 | |
| Overweight/obese adults | ||||
| CRP, mg/L | 0.46 ± 0.06 | 0.45 ± 0.07 | 0.66 ± 0.05 | 0.58 ± 0.06 |
| TNF-α, pg/mL | 0.95 ± 0.01 | 0.94 ± 0.01 | 0.96 ± 0.02 | 0.94 ± 0.02 |
| IL-6, pg/mL | 0.40 ± 0.02 | 0.38 ± 0.02 | 0.45 ± 0.02 | 0.42 ± 0.02 |
| Women with PCOS | ||||
| CRP, mg/L | 0.68 ± 0.06 | 0.67 ± 0.08 | 0.65 ± 0.06 | 0.66 ± 0.06 |
| TNF-α, pg/mL | 0.80 ± 0.03 | 0.78 ± 0.02 | 0.74 ± 0.03 | 0.75 ± 0.02 |
| IL-6, pg/mL | 0.46 ± 0.03 | 0.41 ± 0.04 | 0.39 ± 0.03 | 0.39 ± 0.03 |
Values are means ± SEMs. For overweight/obese adults (parallel-arm design): lower-fat diet, n = 26; lower-CHO diet, n = 36; for women with PCOS (crossover design): lower-fat diet, n = 23; lower-CHO diet, n = 27. CHO, carbohydrate; CRP, C-reactive protein; PCOS, polycystic ovary syndrome.
FIGURE 2.
Loss of total body fat over 16 wk (8 wk eucaloric followed by 8 wk 1000-kcal/d energy deficit) in overweight/obese AA and EA adults consuming a lower-CHO or lower-fat diet. Values are means ± SEMs. EA lower-fat diet arm, n = 14; AA lower-fat arm, n = 14; EA lower-CHO arm, n = 18, AA lower-CHO arm, n = 13. Different lowercase letters indicate significant differences between groups. Within AAs, those who consumed the lower-CHO diet lost more fat than did those consuming the lower-fat diet (P < 0.05). Within the lower-fat diet groups, the difference between ethnic groups in fat loss was significant at P = 0.05. AA, African-American; CHO, carbohydrate; EA, European-American.
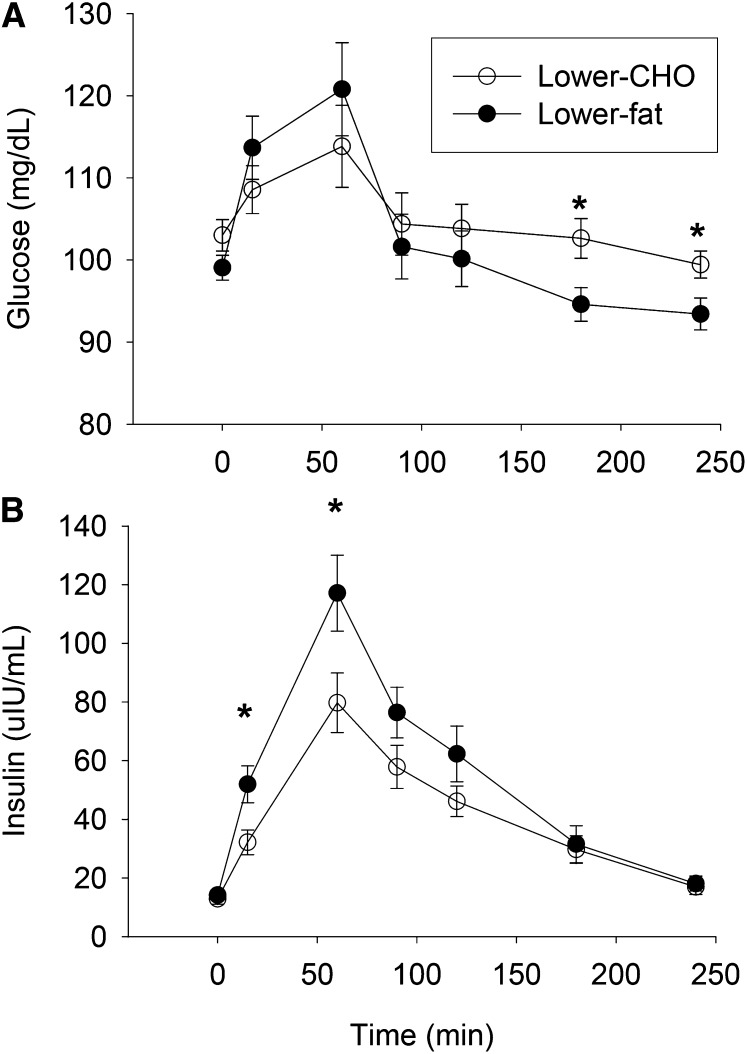
Results from the breakfast meal test at 4 wk indicated that the insulin response to the 2 diets differed, with peak insulin concentration (P < 0.05) and incremental insulin AUC (P < 0.01) being significantly lower after the lower-carbohydrate breakfast meal (Figure 3) (28). AUC glucose did not differ between the 2 meals; however, serum glucose concentration was lower at 3 and 4 h during the lower-fat meal (P < 0.05).
FIGURE 3.
Serum glucose (A) and insulin (B) concentrations in overweight/obese adults after consumption of lower-CHO or lower-fat breakfast meals. Values are means ± SEMs. *Groups differ at that time, P < 0.05. Lower-fat diet, n = 29; lower-CHO diet, n = 35. Adapted from reference 28 with permission. CHO, carbohydrate.
Women with PCOS.
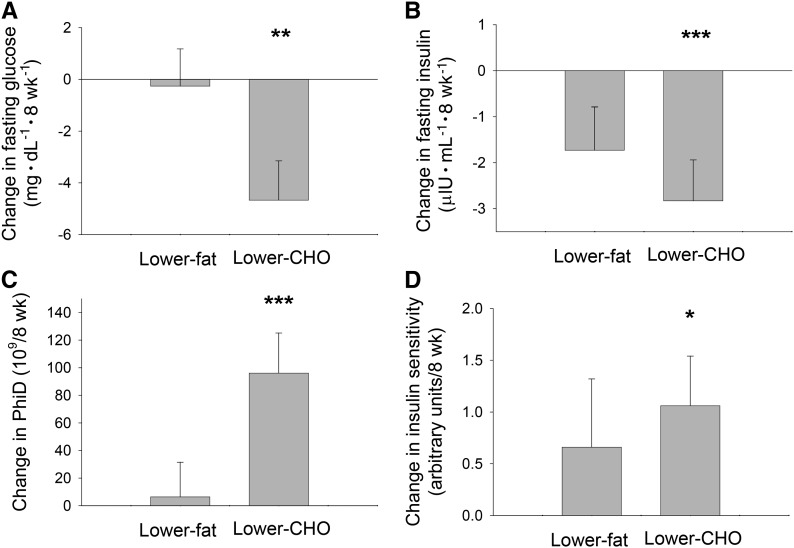
Participant characteristics at baseline are shown in Table 3. As reported previously (29), a paired t test indicated that the lower-carbohydrate diet resulted in significant decreases in fasting insulin (P < 0.001) and fasting glucose (P < 0.01) and significant increases in insulin sensitivity (P < 0.05) and PhiD (P < 0.001) (Figure 4). No changes in these outcomes were observed while consuming the lower-fat diet. Original to this report, markers of inflammation did not change in response to either of the diets (Table 2). However, after the lower-carbohydrate arm, the change in IAAT was associated with the change in TNF-α (standardized coefficient = 0.45, P = 0.04) independent of the change in total body fat mass.
TABLE 3.
Characteristics of participants in the PCOS study at baseline1
| Characteristic | Value |
| BMI, kg/m2 | 31.8 ± 5.7 |
| Age, y | 31.2 ± 5.8 |
| Serum analytes | |
| Fasting glucose, mg/dL | 96.0 ± 9.0 |
| Fasting insulin, μIU/mL | 8.6 ± 6.6 |
| Testosterone, ng/dL | 53.7 ± 28.3 |
| SHBG, nmol/L | 49.3 ± 21.0 |
| FAI | 4.3 ± 2.8 |
Values are means ± SDs; n = 30. Adapted from reference 29 with permission. FAI, Free Androgen Index [(total testosterone in nmol/L/SHBG in nmol/L) × 100]; PCOS, polycystic ovary syndrome; SHBG, sex hormone binding globulin.
FIGURE 4.
Changes in fasting serum glucose (A), fasting serum insulin (B), PhiD (C), and insulin sensitivity (D) from baseline to week 8 in women with polycystic ovary syndrome who consumed lower-CHO and lower-fat diets. Values are means ± SEMs. Lower-fat diet, n = 23; lower-CHO diet, n = 27. Different from baseline: *P < 0.05, **P < 0.01, ***P < 0.001. Adapted from reference 29 with permission. CHO, carbohydrate; PhiD, dynamic phase serum insulin response to glucose.
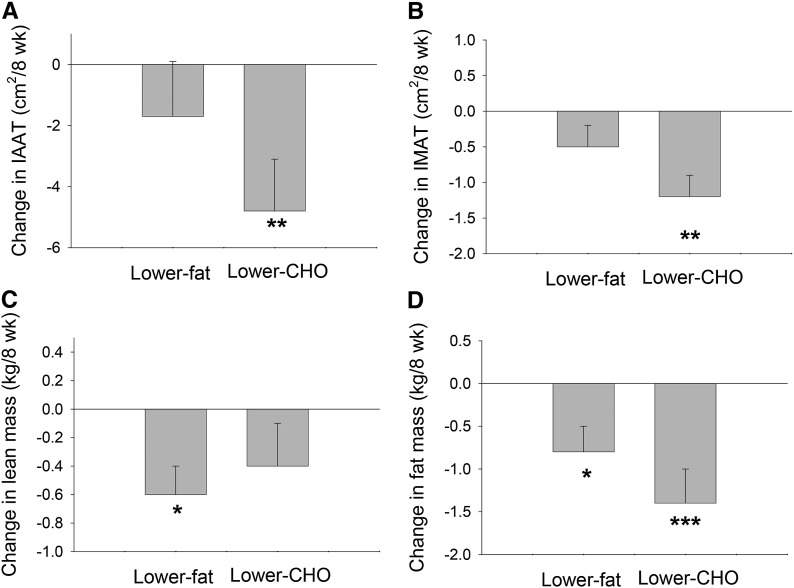
As previously reported (32), while in the lower-carbohydrate arm, women lost both IAAT (P < 0.01) and IMAT (P < 0.01). In contrast, while in the lower-fat arm, women lost lean mass (P < 0.05) (Figure 5). No changes in IAAT or IMAT were observed during the lower-fat arm, and no change in lean mass was observed over the course of the lower-carbohydrate arm. Although loss of total fat mass was significant with both diets, it was greater with the lower-carbohydrate diet (P < 0.05; adjusted for baseline total fat mass and change in lean mass).
FIGURE 5.
Changes in IAAT (A), IMAT (B), total body lean mass (C), and total body fat mass (D) from baseline to week 8 in women with polycystic ovary syndrome who consumed lower-CHO and lower-fat diets. Values are means ± SEMs. Lower-fat diet, n = 23; lower-CHO diet, n = 27. Different from baseline: *P < 0.05, **P < 0.01, ***P < 0.001. Adapted from reference 32 with permission. CHO, carbohydrate; IAAT, intra-abdominal adipose tissue; IMAT, intermuscular adipose tissue.
Discussion
Among nondiabetic, overweight/obese adults, we found that the consumption of a lower-carbohydrate vs. a lower-fat diet resulted in selective depletion of IAAT during weight-maintenance conditions and enhanced depletion of total body fat under weight-loss conditions. Insulin response to a breakfast “test” meal was lower with the lower-carbohydrate diet (when compared with the lower-fat diet). These observations suggest that carbohydrate restriction reduces insulin secretion, which may facilitate fat mobilization (33), particularly from the intra-abdominal area, a depot associated with metabolic dysfunction that is enlarged in individuals with T2D (34, 35).
Although IAAT is considered a proinflammatory adipose depot, we did not observe changes in markers of inflammation over the course of the intervention, regardless of diet assignment. Nor did we observe a correlation between changes in IAAT and changes in markers of inflammation. In a previous study that involved weight loss with the use of a hypocaloric prescription, we observed decreases in all markers of inflammation assessed, with the decrease in TNF-α best explained by the decrease in IAAT (36). Furthermore, on a cross-sectional basis, TNF-α was associated with IAAT but not with total fat mass or any other individual fat depot (37). On the basis of the observation that dietary carbohydrates have proinflammatory effects (3, 38), we anticipated that the lower-carbohydrate diet would reduce inflammation either directly or via depletion of IAAT. It is possible that our lower-carbohydrate prescription (43% carbohydrate) was not sufficiently low to reduce inflammation or that the greater amount of saturated fat (12–13%) in this diet negated any potentially beneficial effect of carbohydrate reduction. It will be important in future studies to identify the aspects of diet composition that minimize inflammation.
We also observed that the loss of body fat with carbohydrate (vs. fat) restriction was greater among AA individuals, a group characterized by a strikingly high AIRg. We previously observed that insulin-sensitive AA women who consumed a diet relatively high in glycemic load showed greater gain in body fat under free-living conditions (16). This phenotype-by-diet interaction was not observed in EA women. It is tempting to speculate that the greater insulin responsiveness of AAs contributes to their sensitivity to diet composition. Specifically, that dietary carbohydrate, by altering insulin secretion, affects insulin-stimulated deposition of lipid in adipose tissue. Prospective studies are needed to examine the interactive effects of insulin sensitivity, insulin secretion, and diet composition on change in body composition in AAs and EAs. It is possible that diets individually tailored to specific phenotypes would result in greater success with both weight loss and weight-loss maintenance, particularly for ethnic minorities who are at elevated risk of both obesity and T2D.
In women with PCOS, a lower-carbohydrate diet intervention resulted in decreases in fasting glucose and fasting insulin and a concomitant improvement in insulin sensitivity. We also observed an increase in first-phase β cell response (PhiD). A low or inadequate first-phase β cell response is one of the first signs of impaired β cell function (39) and appears to occur due to glucose toxicity (40–43). In the current study, the reduction in fasting glucose observed with the lower-carbohydrate diet may have permitted the increase in PhiD.
PCOS is one of the most common endocrine disorders in premenopausal women (44). Although its etiology is not entirely clear, it is thought that a genetic predisposition to insulin resistance of skeletal muscle leads to an elevation in insulin secretion that stimulates testosterone production from the ovaries, which remain sensitive to insulin action. Infertility, hirsutism, and obesity are characteristics of the disorder. In addition, women with PCOS are at elevated risk of developing both T2D and cardiovascular disease, presumably due to their insulin resistance and hyperinsulinism. Although treatment with oral contraceptives and other drugs that alter the reproductive-endocrine axis can alleviate symptoms, there is a need for nonpharmacologic treatment options. That diet modification through carbohydrate restriction could alleviate symptoms by lowering insulin secretion is a possibility worth pursuing.
Women with PCOS also showed favorable changes in body composition (lower fat mass and preservation of lean mass) and fat distribution (lower IAAT and IMAT) with the lower-carbohydrate diet. These changes would be expected to improve metabolic health. The role of IMAT in the etiology of chronic metabolic disease has not been widely investigated. However, greater IMAT was observed in men with T2D and has been associated with greater prevalence of hyperglycemia (45). Furthermore, greater IMAT has been associated with elevation in markers of inflammation (46). These observations suggest that fatty infiltration of skeletal muscle is either a contributor to, or a marker of, impaired metabolic health.
Overweight women with PCOS have been characterized by elevated markers of inflammation relative to weight-matched healthy controls (47–49). Markers of inflammation are inversely associated with insulin sensitivity (37), and elevated biomarkers of inflammation are a risk factor for T2D (50). Thus, we predicted that a diet that improved metabolic health would result in a reduction in circulating markers of inflammation. In support of this hypothesis, we observed that after the lower-carbohydrate arm, the change in IAAT was associated with the change in TNF-α, independent of the change in total body fat mass. This observation suggests that depletion of IAAT with carbohydrate restriction may have mediated a decrease in inflammation. In women with or without PCOS, in vitro TNF-α production was correlated with circulating concentrations of testosterone and androstenedione (51). It is possible that the decrease in testosterone observed in this study after the lower-carbohydrate diet (29) may have been related to inflammation. Longer-term studies are needed to better gauge the effectiveness of carbohydrate restriction in reducing inflammation and associated reproductive and metabolic defects in women with PCOS.
A strength of both studies was that the protein content of the diets did not differ. Elevated dietary protein may contribute to maintenance of, or gain in, lean body mass (52). In addition, dietary protein affects insulin secretion (53) and satiety (54). Because we were assessing the influence of carbohydrate reduction on insulin secretion, we wanted to avoid any potential confounding influence of differences in dietary protein. We provided food throughout the study; thus, we did not anticipate that differences in satiety/hunger would affect study results, because food intake was determined by the study protocol. However, one of the goals of the studies was to evaluate the effect of the diets on hunger/satiety (28). For this reason, the protein content of the 2 diets was carefully matched.
In summary, among 2 groups of individuals at elevated risk of T2D (overweight/obese/prediabetic adults, women with PCOS), restriction of dietary carbohydrate (relative to restriction of dietary fat) resulted in favorable changes in body composition, fat distribution, and glucose metabolism that may reduce the risk of T2D.
Acknowledgments
We thank Maryellen Williams and Cindy Zeng of the University of Alabama at Birmingham (UAB) Metabolism/Human Physiology Core Laboratory (Nutrition Obesity Research Center, Diabetes Research Center, Center for Clinical and Translational Science) for help with laboratory analyses and Betty Darnell and Suzanne Choquette of the UAB Center for Clinical and Translational Science for help in the experimental design and diet development. BAG designed the study, wrote the manuscript, and had primary responsibility for the final content; and BAG and AMG conducted the research and analyzed the data. Both of the authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, African American; AIRg, acute insulin response to glucose; CRP, C-reactive protein; CT, computed tomography; EA, European American; IAAT, intra-abdominal adipose tissue; IMAT, intermuscular adipose tissue; PCOS, polycystic ovary syndrome; PhiD, dynamic phase serum insulin response to glucose; T2D, type 2 diabetes.
References
- 1.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab 2004;89:463–78. [DOI] [PubMed] [Google Scholar]
- 2.Magkos F, Wang X, Mittendorfer B. Metabolic actions of insulin in men and women. Nutrition 2010;26:686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buyken AE, Goletzke J, Joslowski G, Felbick A, Cheng G, Herder C, Brand-Miller JC. Association between carbohydrate quality and inflammatory markers: systematic review of observational and interventional studies. Am J Clin Nutr 2014;99:813–33. [DOI] [PubMed] [Google Scholar]
- 4.de Carvalho Vidigal F, Guedes Cocate P, Goncalves Pereira L, de Cassia Goncalves Alfenas R. The role of hyperglycemia in the induction of oxidative stress and inflammatory process. Nutr Hosp 2012;27:1391–8. [DOI] [PubMed] [Google Scholar]
- 5.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001;44(Suppl 2):S14–21. [DOI] [PubMed] [Google Scholar]
- 6.Mayer SB, Jeffreys AS, Olsen MK, McDuffie JR, Feinglos MN, Yancy WS. Two diets with different hemoglobin A and antiglycemic medication effects despite similar weight loss in type 2 diabetes. Diabetes Obes Metab 2014;16(1):90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med 2005;142:403–11. [DOI] [PubMed] [Google Scholar]
- 8.Gannon MC, Nuttall FQ. Control of blood glucose in type 2 diabetes without weight loss by modification of diet composition. Nutr Metab (Lond) 2006;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gannon MC, Nuttall FQ. Effect of a high-protein diet on ghrelin, growth hormone, and insulin-like growth factor-I and binding proteins 1 and 3 in subjects with type 2 diabetes mellitus. Metabolism 2011;60:1300–11. [DOI] [PubMed] [Google Scholar]
- 10.Nuttall FQ, Gannon MC. The metabolic response to a high-protein, low-carbohydrate diet in men with type 2 diabetes mellitus. Metabolism 2006;55:243–51. [DOI] [PubMed] [Google Scholar]
- 11.Hussain TA, Mathew TC, Dashti AA, Asfar S, Al-Zaid N, Dashti HM. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 2012;28:1016–21. [DOI] [PubMed] [Google Scholar]
- 12.Hu T, Bazzano LA. The low-carbohydrate diet and cardiovascular risk factors: evidence from epidemiologic studies. Nutr Metab Cardiovasc Dis 2014;24:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, Neumiller JJ, Nwankwo R, Verdi CL, Urbanski P, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013;36:3821–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Standards of medical care in diabetes–2013. Diabetes Care 2013;36(Suppl 1):S11–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gower BA, Hunter GR, Chandler-Laney PC, Alvarez JA, Bush NC. Glucose metabolism and diet predict changes in adiposity and fat distribution in weight-reduced women. Obesity (Silver Spring) 2010;18:1532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gower BA, Alvarez JA, Bush NC, Hunter GR. Insulin sensitivity affects propensity to obesity in an ethnic-specific manner: results from two controlled weight loss intervention studies. Nutr Metab (Lond) 2013;10(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA 2007;297:2092–102. [DOI] [PubMed] [Google Scholar]
- 18.McClain AD, Otten JJ, Hekler EB, Gardner CD. Adherence to a low-fat vs. low-carbohydrate diet differs by insulin resistance status. Diabetes Obes Metab 2013;15:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in African-American and Caucasian children. J Clin Endocrinol Metab 2002;87:2218–24. [DOI] [PubMed] [Google Scholar]
- 20.Haffner SM, D'Agostino R, Jr, Saad MF, Rewers M, Mykkanen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 1996;45:742–8. [DOI] [PubMed] [Google Scholar]
- 21.Stefan N, Stumvoll M, Weyer C, Bogardus C, Tataranni PA, Pratley RE. Exaggerated insulin secretion in Pima Indians and African-Americans but higher insulin resistance in Pima Indians compared to African-Americans and Caucasians. Diabet Med 2004;21:1090–5. [DOI] [PubMed] [Google Scholar]
- 22.Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab 1983;57:356–9. [DOI] [PubMed] [Google Scholar]
- 23.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774–800. [DOI] [PubMed] [Google Scholar]
- 24.Borruel S, Fernandez-Duran E, Alpanes M, Marti D, Alvarez-Blasco F, Luque-Ramirez M, Escobar-Morreale HF. Global adiposity and thickness of intraperitoneal and mesenteric adipose tissue depots are increased in women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab 2013;98:1254–63. [DOI] [PubMed] [Google Scholar]
- 25.Goree LL, Chandler-Laney P, Ellis AC, Casazza K, Granger WM, Gower BA. Dietary macronutrient composition affects beta cell responsiveness but not insulin sensitivity. Am J Clin Nutr 2011;94:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gower BA, Goree LL, Chandler-Laney PC, Ellis AC, Casazza K, Granger WM. A higher-carbohydrate, lower-fat diet reduces fasting glucose concentration and improves beta-cell function in individuals with impaired fasting glucose. Metabolism 2012;61:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goss AM, Goree LL, Ellis AC, Chandler-Laney PC, Casazza K, Lockhart ME, Gower BA. Effects of diet macronutrient composition on body composition and fat distribution during weight maintenance and weight loss. Obesity (Silver Spring) 2013;21:1139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandler-Laney PC, Morrison SA, Goree LL, Ellis AC, Casazza K, Desmond R, Gower BA. Return of hunger following a relatively high carbohydrate breakfast is associated with earlier recorded glucose peak and nadir. Appetite 2014;80:236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gower BA, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, Granger WM, Goss AM, Bates GW. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin Endocrinol (Oxf) 2013;79:550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001;50:150–8. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda M, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing. Diabetes Care 1999;22:1462–70. [DOI] [PubMed] [Google Scholar]
- 32.Goss AM, Chandler-Laney P, Ovalle F, Goree L, Azziz R, Desmond R, Bates G, Gower B. Effects of a eucaloric reduced carbohydrate diet on body composition and fat distribution in women with PCOS. Metabolism 2014;63(10):1257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 2011;60:2441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol 1997;145:614–9. [DOI] [PubMed] [Google Scholar]
- 35.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994;17:961–9. [DOI] [PubMed] [Google Scholar]
- 36.Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA. Effect of diet with and without exercise training on markers of inflammation and fat distribution in overweight women. Obesity (Silver Spring) 2011;19(6):1131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyatt TC, Phadke R, Hunter GR, Bush N, Munoz AJ, Gower BA. Insulin sensitivity in African-American and Caucasian women: association with inflammation. Obesity (Silver Spring) 2009;17:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Punder K, Pruimboom L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients 2013;5:771–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdul-Ghani MA, Matsuda M, Jani R, Jenkinson CP, Coletta DK, Kaku K, DeFronzo RA. The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab 2008;295:E401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godsland IF, Jeffs JAR, Johnston DG. Loss of beta cell function as fasting glucose increases in the non-diabetic range. Diabetologia 2004;47:1157–66. [DOI] [PubMed] [Google Scholar]
- 41.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo R. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolims (SAM) study. Diabetologia 2004;47:31–9. [DOI] [PubMed] [Google Scholar]
- 42.Meyer J, Sturis J, Katschinski M, Arnold R, Goke B, Byrne MM. Acute hyperglycemia alters the ability of the normal beta-cell to sense and respond to glucose. Am J Physiol Endocrinol Metab 2002;282:E917–22. [DOI] [PubMed] [Google Scholar]
- 43.Rossetti L, Shulman GI, Zawalich W, DeFronzo R. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest 1987;80:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012;33:981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miljkovic-Gacic I, Gordon CL, Goodpaster BH, Bunker CH, Patrick AL, Kuller LH, Wheeler VW, Evans RW, Zmuda JM. Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am J Clin Nutr 2008;87:1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, Kuller LH, Pahor M, Schaap LA, Visser M, et al. Health ABC study. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring) 2009;17(5):1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diamanti-Kandarakis E, Paterakis T, Kandarakis HA. Indices of low-grade inflammation in polycystic ovary syndrome. Ann N Y Acad Sci 2006;1092:175–86. [DOI] [PubMed] [Google Scholar]
- 48.González F, Rote NS, Minium J, Kirwan JP. Evidence of proatherogenic inflammation in polycystic ovary syndrome. Metabolism 2009;58:954–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glintborg D, Andersen M, Richelsen B, Bruun JM. Plasma monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha are increased in patients with polycystic ovary syndrome (PCOS) and associated with adiposity, but unaffected by pioglitazone treatment. Clin Endocrinol (Oxf) 2009;71(5):652–8. [DOI] [PubMed] [Google Scholar]
- 50.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107. [DOI] [PubMed] [Google Scholar]
- 51.González F, Kirwan JP, Rote NS, Minium J. Evidence of mononuclear cell preactivation in the fasting state in polycystic ovary syndrome. Am J Obstet Gynecol 2014 Jun 20 (Epub ahead of print; DOI: 10.1016/j.ajog.2014.06.044). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mettler S, Mitchell N, Tipton KD. Increased protein intake reduces lean body mass loss during weight loss in athletes. Med Sci Sports Exerc 2010;42:326–37. [DOI] [PubMed] [Google Scholar]
- 53.Nuttall FQ, Gannon MC. Plasma glucose and insulin response to macronutrients in nondiabetic and NIDDM subjects. Diabetes Care 1991;14:824–38. [DOI] [PubMed] [Google Scholar]
- 54.Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M. Protein, weight management, and satiety. Am J Clin Nutr 2008;87:1558S–61S. [DOI] [PubMed] [Google Scholar]