Abstract
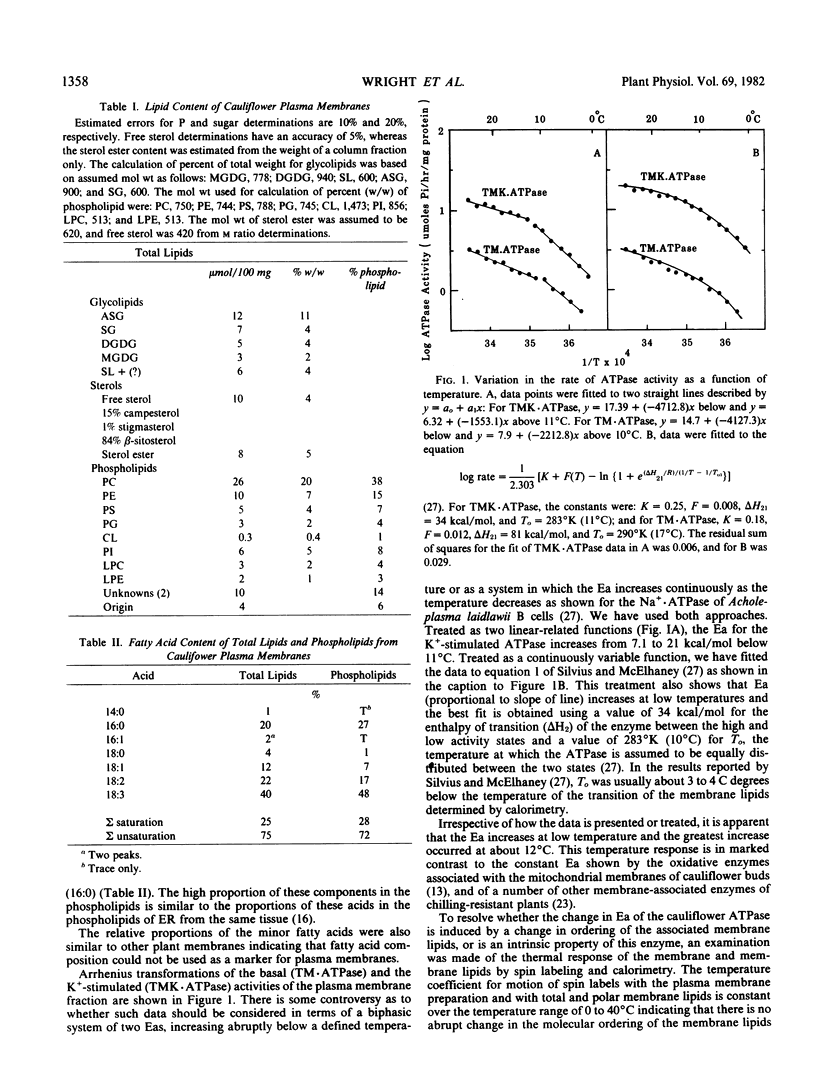
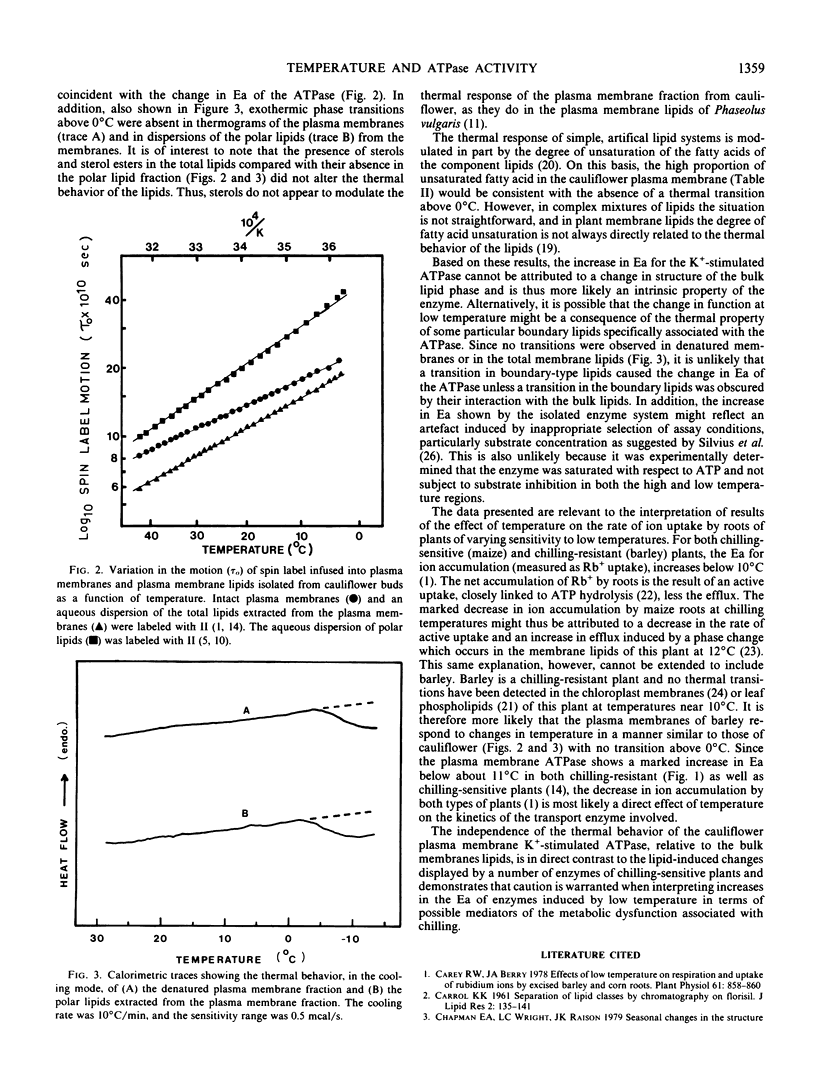
A plasma-membrane fraction rich in ion-stimulated ATPase activity was isolated from cauliflower (Brassica oleracea L.) buds. The activity of the ATPase was dependent on Mg2+ and stimulated 4-fold by K+. The lipids of the membrane fraction contained 57% by weight of phospholipid, 16% glycolipid including sterol glycosides, and 27% neutral lipids. Sterols and sterol esters comprised 9% by weight of the total lipid fraction, and the m ratio of total sterol to phospholipid was 0.5. Fatty acid unsaturation of the membrane lipids was 75%. Arrhenius plots of the Mg2+ and Mg2+ + K+ stimulated ATPase activity were biphasic with an increase in activation energy occurring below about 12°C, a response typical of some membrane-associated enzymes of chilling-sensitive plants. No thermal transitions were detected in the membranes or membrane lipids between 0 and 30°C using differential scanning calorimetry and electron spin resonance spectroscopy. This type of thermal behavior is typical of membranes of chilling-resistant plants. It was concluded that the low temperature increase in activation energy of the ion-stimulated, membrane-associated ATPase is an intrinsic property of the enzyme system and not the result of a transition in the bulk membrane lipid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARROLL K. K. Separation of lipid classes by chromatography on Florisil. J Lipid Res. 1961 Apr;2:135–141. [PubMed] [Google Scholar]
- Carey R. W., Berry J. A. Effects of low temperature on respiration and uptake of rubidium ions by excised barley and corn roots. Plant Physiol. 1978 May;61(5):858–860. doi: 10.1104/pp.61.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper P. J., Livne A., Meyerstein N. Changes in lipid composition and osmotic fragility of erythrocytes of hamster induced by heat exposure. Biochim Biophys Acta. 1971 Nov 5;248(2):300–305. doi: 10.1016/0005-2760(71)90018-x. [DOI] [PubMed] [Google Scholar]
- McMurchie E. J., Pomeroy M. K. Isolation and properties of ion-stimulated ATPase activity associated with cauliflower plasma membranes. Plant Physiol. 1981 Sep;68(3):626–630. doi: 10.1104/pp.68.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan W. G., Smillie R. M. Multi-temperature effects on Hill reaction activity of barley chloroplasts. Biochim Biophys Acta. 1976 Sep 13;440(3):461–475. doi: 10.1016/0005-2728(76)90034-7. [DOI] [PubMed] [Google Scholar]
- Nolan W. G., Smillie R. M. Temperature-induced Changes in Hill Activity of Chloroplasts Isolated from Chilling-sensitive and Chilling-resistant Plants. Plant Physiol. 1977 Jun;59(6):1141–1145. doi: 10.1104/pp.59.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. C., Ladbrooke B. D., Chapman D. Molecular interactions in mixed lecithin systems. Biochim Biophys Acta. 1970 Jan 6;196(1):35–44. doi: 10.1016/0005-2736(70)90163-x. [DOI] [PubMed] [Google Scholar]
- Pike C. S., Berry J. A. Membrane phospholipid phase separations in plants adapted to or acclimated to different thermal regimes. Plant Physiol. 1980 Aug;66(2):238–241. doi: 10.1104/pp.66.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Batt R. D. Quantitative analysis of sulfolipid (sulfoquinovosyl diglyceride) and galactolipids (monogalactosyl and digalactosyl diglycerides) in plant tissues. Anal Biochem. 1968 Jan;22(1):74–88. doi: 10.1016/0003-2697(68)90261-3. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., McElhaney R. N. Membrane lipid physical state and modulation of the Na+,Mg2+-ATPase activity in Acholeplasma laidlawii B. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1255–1259. doi: 10.1073/pnas.77.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. R., Read B. D., McElhaney R. N. Membrane enzymes: artifacts in Arrhenius plots due to temperature dependence of substrate-binding affinity. Science. 1978 Feb 24;199(4331):902–904. doi: 10.1126/science.146257. [DOI] [PubMed] [Google Scholar]
- Travis R. L., Booz M. L. Partial Characterization of a Potassium-stimulated Adenosine Triphosphatase from the Plasma Membrane of Meristematic and Mature Soybean Root Tissue. Plant Physiol. 1979 Mar;63(3):573–577. doi: 10.1104/pp.63.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. P., Merrilees P. A. The removal of water and nonlipid contaminants from lipid extracts. Lipids. 1970 Apr;5(4):367–370. doi: 10.1007/BF02532100. [DOI] [PubMed] [Google Scholar]


