Abstract
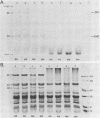
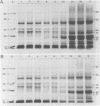
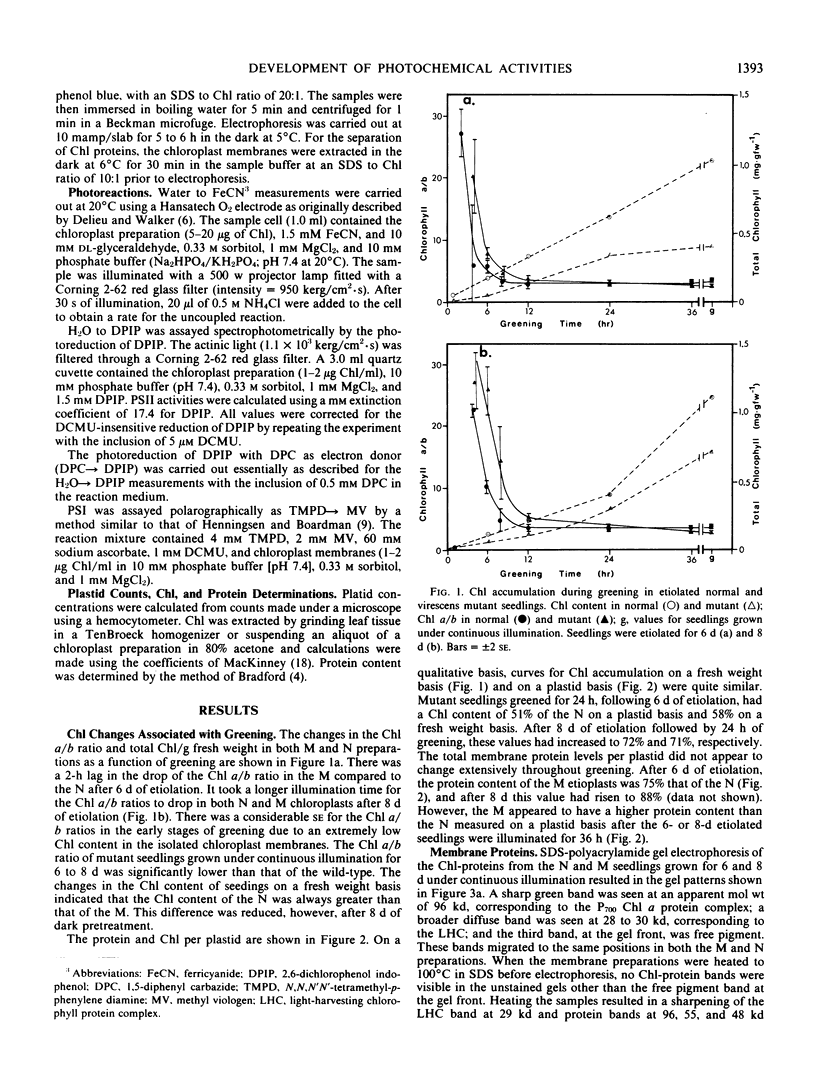
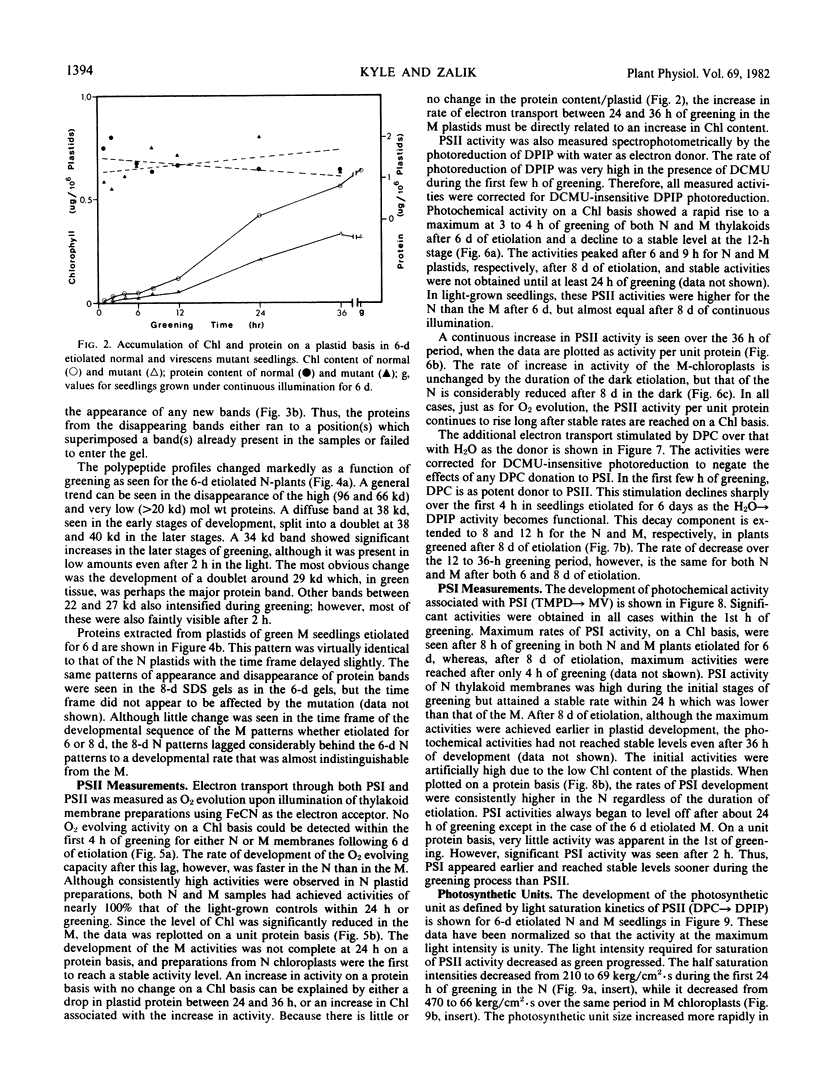
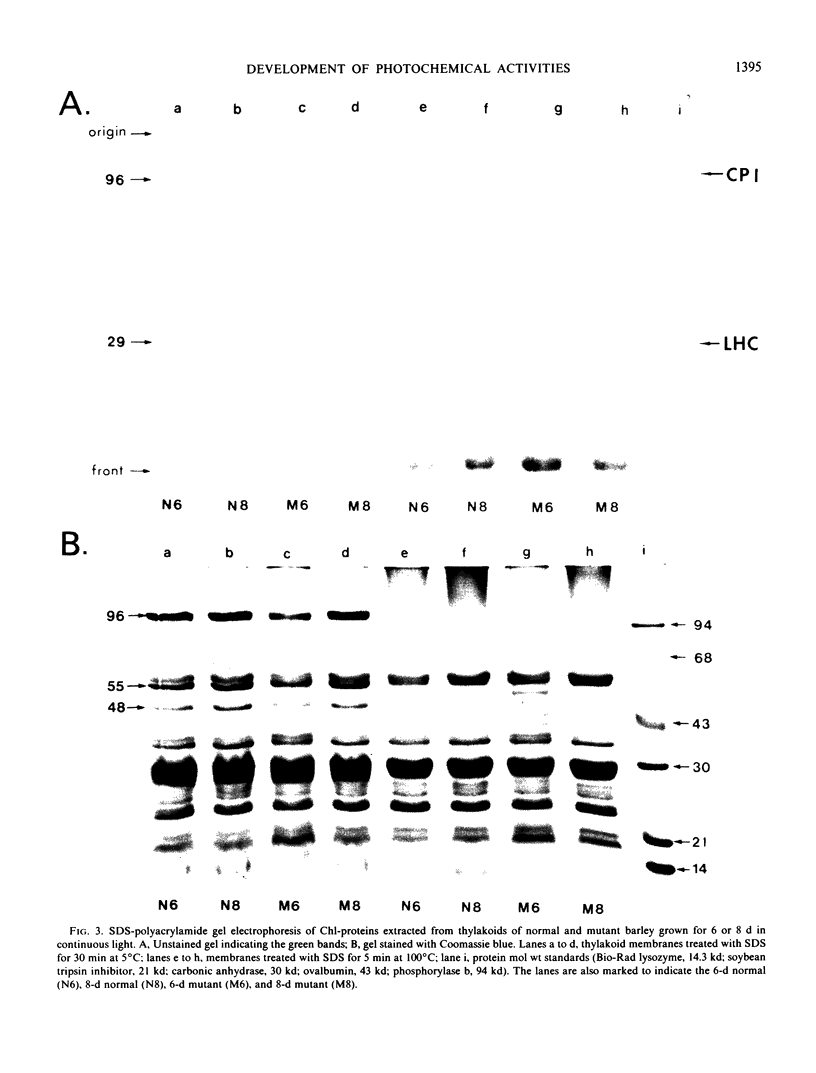
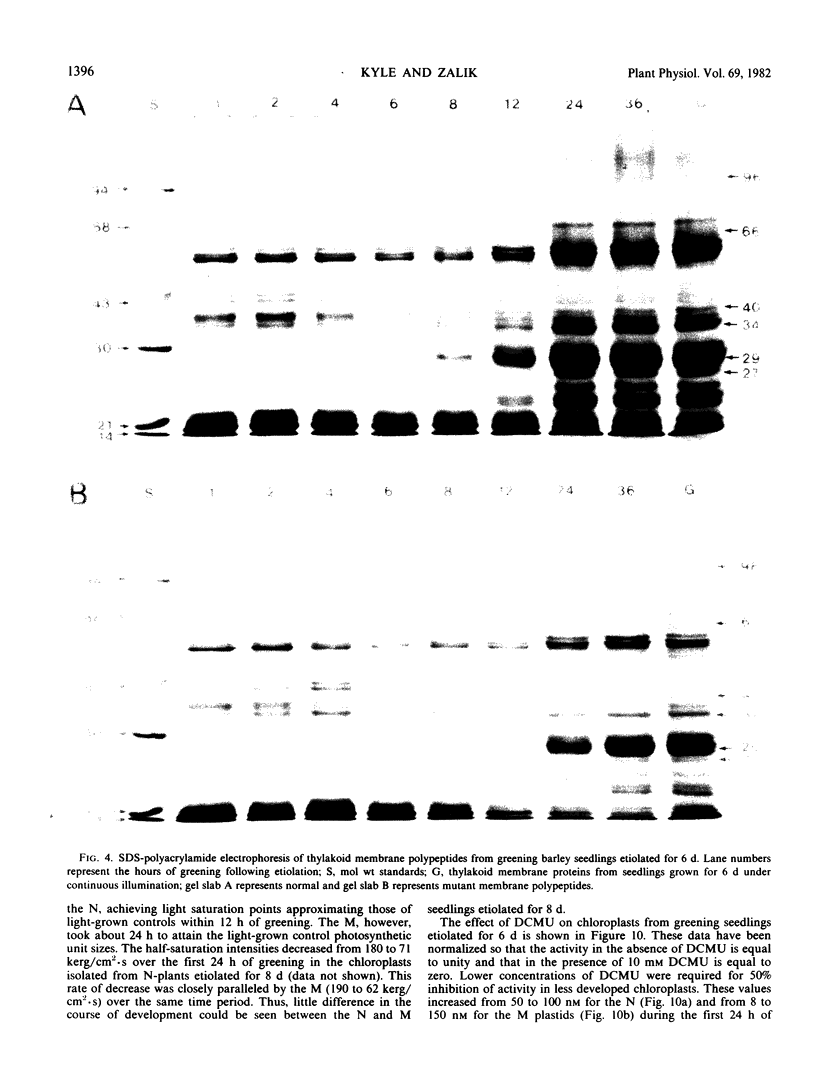
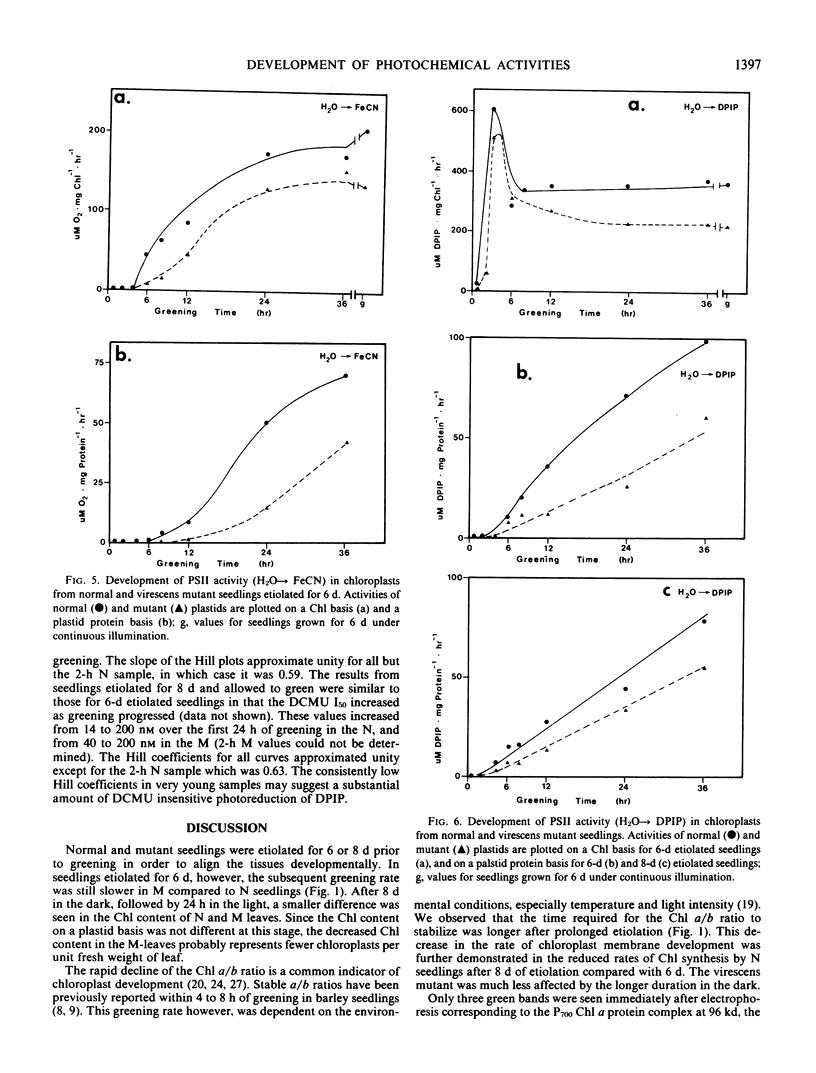
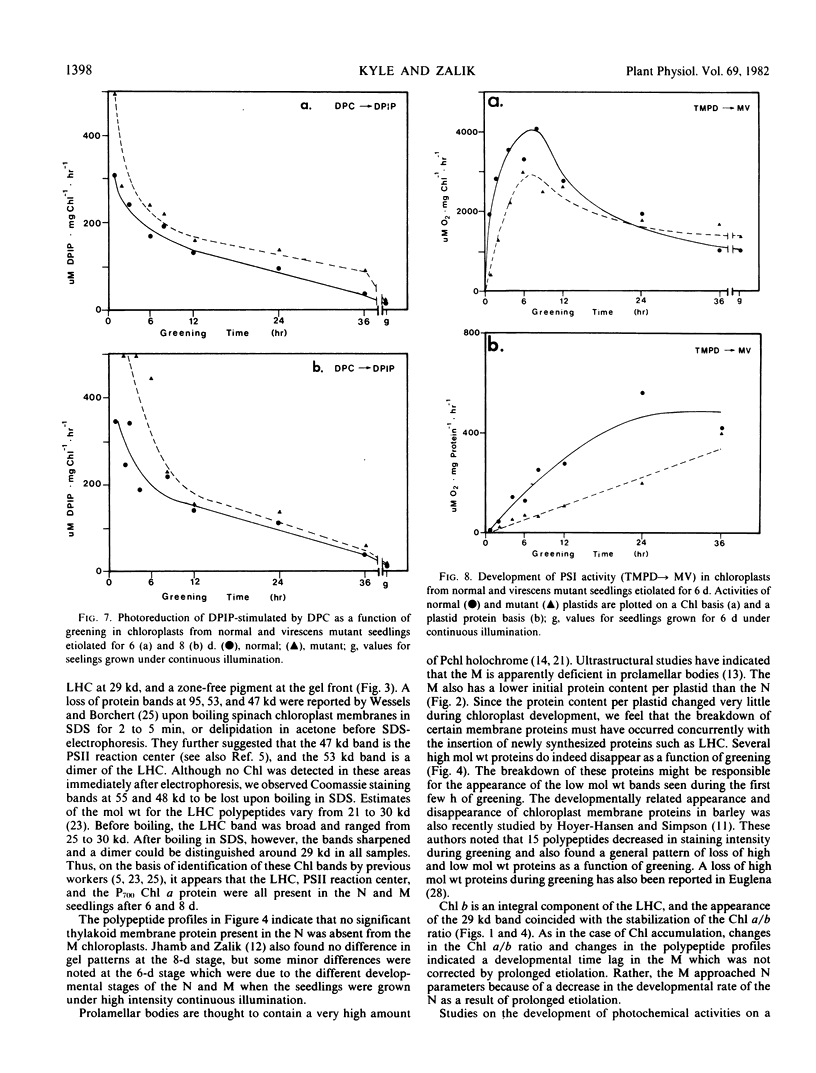
The development of photochemical activity in relation to pigment and membrane protein accumulation in chloroplasts of greening wild-type barley (Hordeum vulgare L. cv. Gateway) and its virescens mutant were studied. The rate of chlorophyll accumulation per plastid was faster in the wild-type than in the mutant seedlings upon illumination after 6 days of etiolation, but was not different after 8 days. Although the protein content per plastid did not vary during greening, there was a change in the sodium dodecyl sulfate-polyacrylamide gel polypeptide profiles. High molecular weight proteins of 96,000 and 66,000 decreased whereas those at 34,000, 27,000 and 22,000 increased in relative quantity as a function of greening. The fully greened mutant seedlings were not deficient in the light-harvesting chlorophyll protein complex (LHC) or the reaction centers of photosystem I and photosystem II. Photosystem I-associated photochemical activities appeared within the first hour of plastid development and photosystem II associated activities and O2 evolution within the next 6 hours. In all cases, the developmental rates per unit protein were slower in the mutant following 6 days of etiolation, but no differences between the two genotypes could be seen after 8 days due to a decrease in the developmental rate of the wild-type chloroplasts. An increase in photosynthetic unit size associated with plastid morphogenesis was faster in the wild-type seedlings after 6 days, but again the difference was negligible after 8 days. It was concluded that no single measured photochemical parameter is affected by this mutation, but rather, all aspects of chloroplast development are affected similarly by an overall reduction in the rate of chloroplast morphogenesis. This mutant, therefore, undergoes the normal pattern of proplastid to chloroplast development, but at a markedly reduced rate.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberte R. S., Hesketh J. D., Hofstra G., Thornber J. P., Naylor A. W., Bernard R. L., Brim C., Endrizzi J., Kohel R. J. Composition and activity of the photosynthetic apparatus in temperature-sensitive mutants of higher plants. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2414–2418. doi: 10.1073/pnas.71.6.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsen K. W., Boardman N. K. Development of Photochemical Activity and the Appearance of the High Potential Form of Cytochrome b-559 in Greening Barley Seedlings. Plant Physiol. 1973 Jun;51(6):1117–1126. doi: 10.1104/pp.51.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron H. A., Mauzerall D. The development of photosynthesis in a greening mutant of chlorella and an analysis of the light saturation curve. Plant Physiol. 1972 Jul;50(1):141–148. doi: 10.1104/pp.50.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A. Developmental Physiology of Bean Leaf Plastids II. Negative Contrast Electron Microscopy of Tubular Membranes in Prolamellar Bodies. Plant Physiol. 1968 Nov;43(11):1769–1780. doi: 10.1104/pp.43.11.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leese B. M., Leech R. M. Sequential changes in the lipids of developing proplastids isolated from green maize leaves. Plant Physiol. 1976 May;57(5):789–794. doi: 10.1104/pp.57.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. A., Zalik S. Effect of Light Quality, Light Intensity and Temperature on Pigment Accumulation in Barley Seedlings. Plant Physiol. 1965 May;40(3):569–574. doi: 10.1104/pp.40.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesnicar M., Bendall D. S. The photochemical activities and electron carriers of developing barley leaves. Biochem J. 1973 Nov;136(3):803–812. doi: 10.1042/bj1360803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson L. W., Zalik S. Acyl lipids, pigments, and gramine in developing leaves of barley and its virescens mutant. Plant Physiol. 1981 Apr;67(4):646–654. doi: 10.1104/pp.67.4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellburn A. R., Hampp R. Appearance of photochemical function in prothylakoids during plastid development. Biochim Biophys Acta. 1979 Aug 14;547(2):380–397. doi: 10.1016/0005-2728(79)90019-7. [DOI] [PubMed] [Google Scholar]
- Wessels J. S., Borchert M. T. Polypeptide profiles of chlorophyll . protein complexes and thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta. 1978 Jul 6;503(1):78–93. doi: 10.1016/0005-2728(78)90163-9. [DOI] [PubMed] [Google Scholar]