Abstract
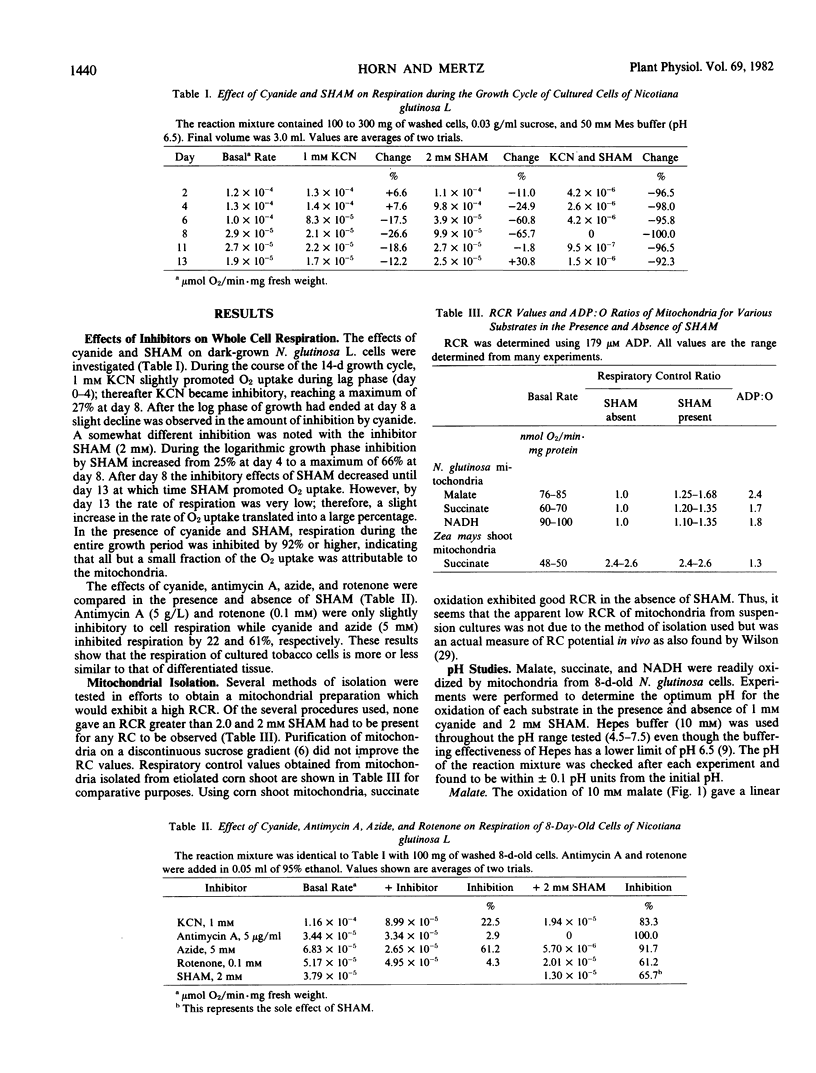
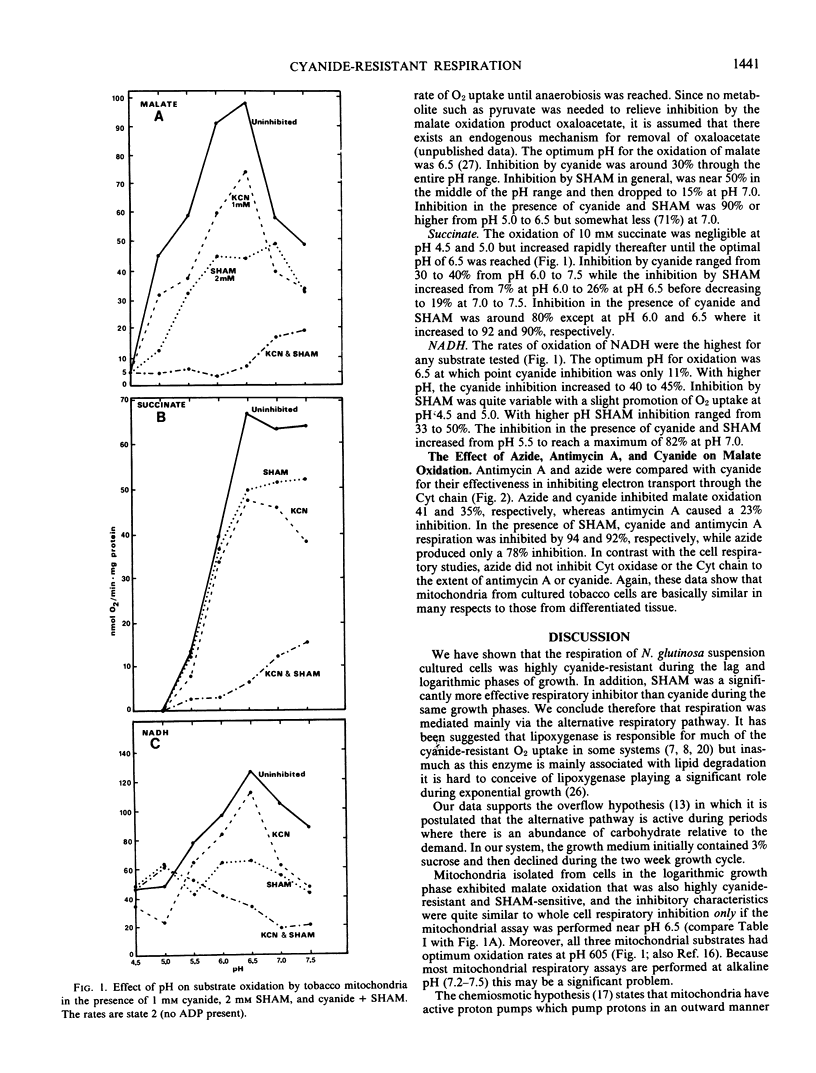
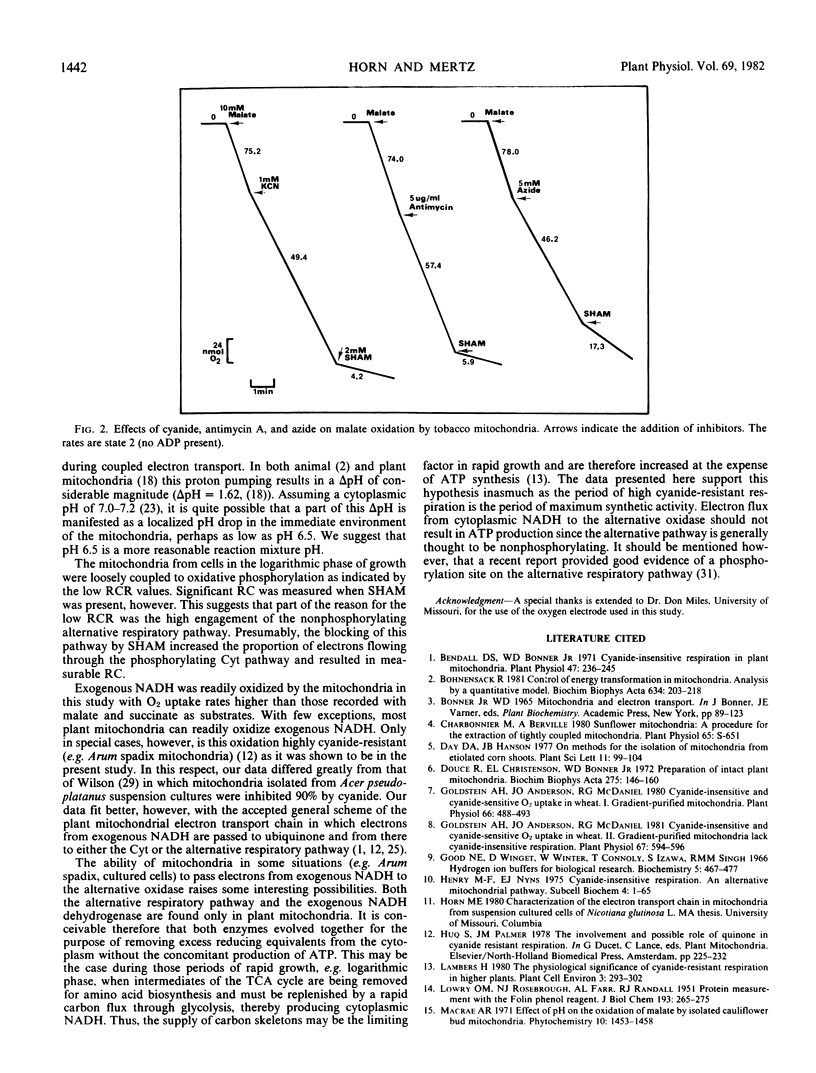
The respiration of dark-grown Nicotiana glutinosa L. cells in liquid suspension culture was found to be highly cyanide resistant and salicylhydroxamic acid (SHAM) sensitive, indicative of an active alternative respiratory pathway. This was especially true during the lag and logarithmic phases of the 14-day growth cycle. Mitochondria isolated from logarithmically growing cells exhibited active oxidation of malate, succinate, and exogenous NADH. Oxidation of all three substrates had an optimum pH of 6.5 and all were highly resistant to inhibited by cyanide and sensitive to SHAM. Respiratory control was exhibited by all three substrates but only if SHAM was present to block the alternative pathway and divert electrons to the phosphorylating cytochrome pathway. The cyanide-resistant oxidation of exogenous NADH has previously only been associated with Arum spadix mitochondria. Coemergence during evolution of the alternative respiratory pathway and the exogenous NADH dehydrogenase in plant mitochondria as a possible mechanism for removal of cytoplasmic NADH is proposed. Evidence is presented which suggests that mitochondrial assays should be performed at pH 6.5.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendall D. S., Bonner W. D. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971 Feb;47(2):236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnensack R. Control of energy transformation of mitochondria. Analysis by a quantitative model. Biochim Biophys Acta. 1981 Jan 14;634(1):203–218. doi: 10.1016/0005-2728(81)90139-0. [DOI] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Goldstein A. H., Anderson J. O., McDaniel R. G. Cyanide-insensitive and Cyanide-sensitive O(2) Uptake in Wheat: I. GRADIENT-PURIFIED MITOCHONDRIA. Plant Physiol. 1980 Sep;66(3):488–493. doi: 10.1104/pp.66.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. H., Anderson J. O., McDaniel R. G. Cyanide-insensitive and Cyanide-sensitive O(2) Uptake in Wheat: II. GRADIENT-PURIFIED MITOCHONDRIA LACK CYANIDE-INSENSITIVE RESPIRATION. Plant Physiol. 1981 Mar;67(3):594–596. doi: 10.1104/pp.67.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Bonner W. D., Jr A comparison of the phosphorylation potential and electrochemical proton gradient in mung bean mitochondria and phosphorylating sub-mitochondrial particles. Biochim Biophys Acta. 1981 Jan 14;634(1):117–128. doi: 10.1016/0005-2728(81)90132-8. [DOI] [PubMed] [Google Scholar]
- Parrish D. J., Leopold A. C. Confounding of alternate respiration by lipoxygenase activity. Plant Physiol. 1978 Sep;62(3):470–472. doi: 10.1104/pp.62.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. Respiratory Chain of Plant Mitochondria: XVIII. Point of Interaction of the Alternate Oxidase with the Respiratory Chain. Plant Physiol. 1976 Oct;58(4):521–525. doi: 10.1104/pp.58.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin A., Djerdjour B., Journet E., Neuburger M., Douce R. Effect of NAD on Malate Oxidation in Intact Plant Mitochondria. Plant Physiol. 1980 Aug;66(2):225–229. doi: 10.1104/pp.66.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. B. Energy conservation associated with cyanide-insensitive respiration in plant mitochondria. Biochim Biophys Acta. 1970 Dec 8;223(2):383–387. doi: 10.1016/0005-2728(70)90195-7. [DOI] [PubMed] [Google Scholar]
- Wilson S. B. Energy conservation by the plant mitochondrial cyanide-insensitive oxidase. Some additional evidence. Biochem J. 1980 Aug 15;190(2):349–360. doi: 10.1042/bj1900349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. B. Studies on the cyanide insensitive oxidase of plant mitochondria. FEBS Lett. 1971 Jun 2;15(1):49–52. doi: 10.1016/0014-5793(71)80077-7. [DOI] [PubMed] [Google Scholar]


