Abstract
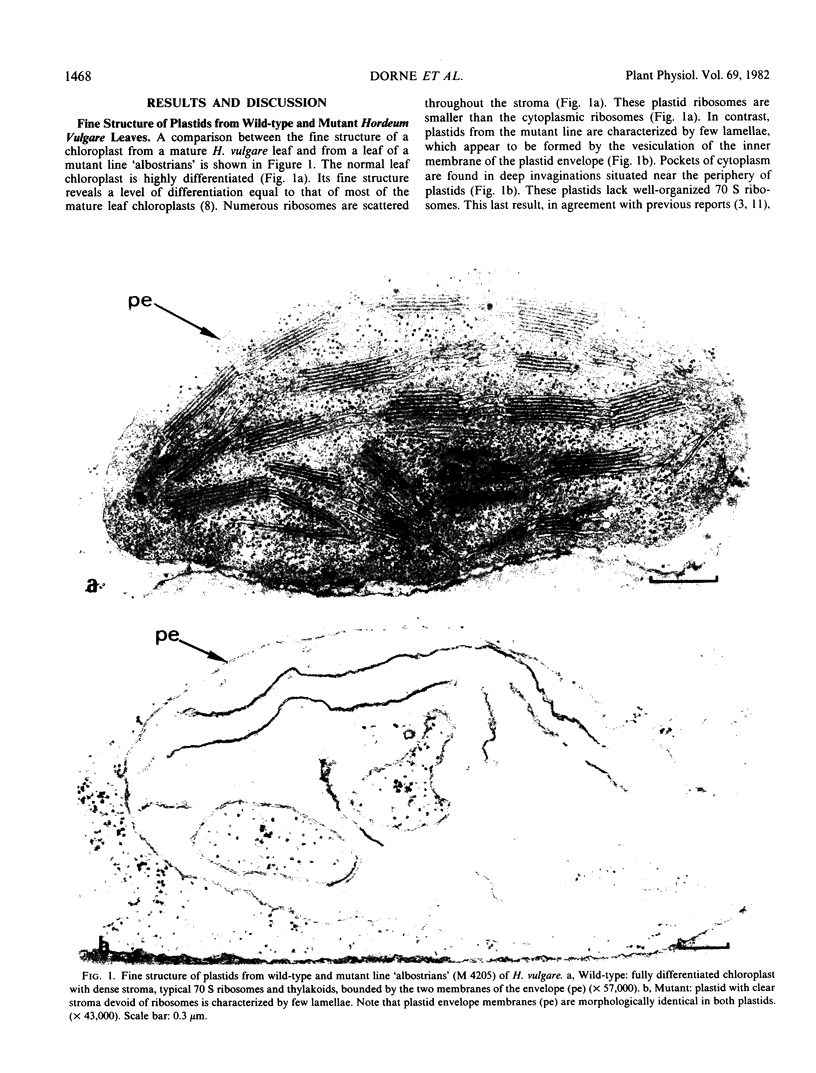
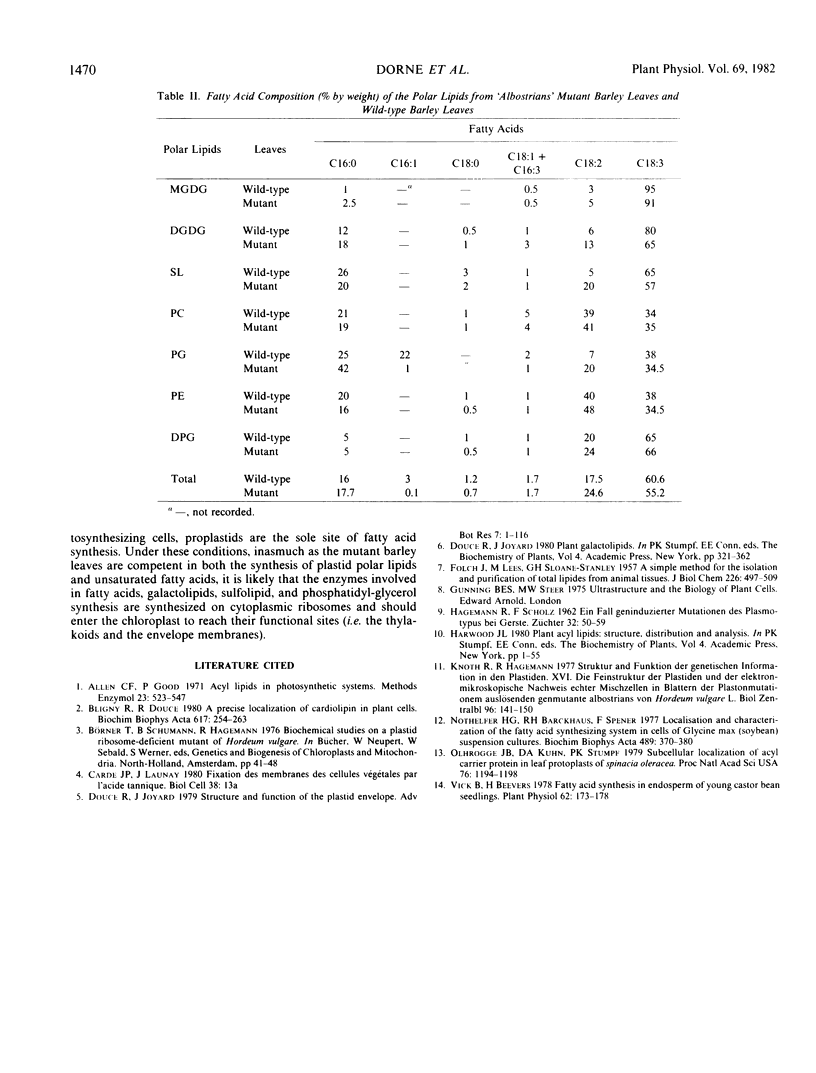
Green and white leaves of the barley mutant line `albostrians' were compared for their polar lipid content and fatty acid composition. The mutant plastids of the white leaves have a double-layered envelope, but in contrast with the normal chloroplasts, lack 70 S ribosomes and thylakoids. In the green leaves, the amount of monogalactosyldiacylglycerol (MGDG) consistently exceeds the amount of digalactosyldiacylglycerol (DGDG) and the amount of galactolipids exceeds the amount of phospholipids. In contrast, in white leaves the amount of DGDG exceeds the amount of MGDG and the amount of phospholipids exceeds the amount of galactolipids. In white leaves, the galactolipid composition reflects the plastid envelope composition which is rich in DGDG, whereas in green leaves the galactolipid composition reflects the thylakoid composition which is rich in MGDG. These results demonstrate the likelihood that all the enzymes involved in galactolipid, sulfolipid and fatty acid synthesis are coded by the nuclear genome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker W. M., Leaver C. J., Weir E. M., Riezman H. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: I. Developmental Changes in Cotyledonary Protein, RNA, and Enzyme Activities during Germination. Plant Physiol. 1978 Oct;62(4):542–549. doi: 10.1104/pp.62.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligny R., Douce R. A precise localization of cardiolipin in plant cells. Biochim Biophys Acta. 1980 Feb 22;617(2):254–263. doi: 10.1016/0005-2760(80)90168-x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Nothelfer H. G., Barckhaus R. H., Spener F. Localisation and characterization of the fatty acid synthesizing system in cells of Glycine max (soubean) suspension cultures. Biochim Biophys Acta. 1977 Dec 21;489(3):370–380. doi: 10.1016/0005-2760(77)90157-6. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S., Saltvedt E., Pihl A. Isolation and comparison of galactose-binding lectins from Abrus precatorius and Ricinus communis. J Biol Chem. 1974 Feb 10;249(3):803–810. [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Protein bodies of castor bean endosperm: isolation, fractionation, and the characterization of protein components. Plant Physiol. 1976 Dec;58(6):710–716. doi: 10.1104/pp.58.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B., Beevers H. Fatty Acid synthesis in endosperm of young castor bean seedlings. Plant Physiol. 1978 Aug;62(2):173–178. doi: 10.1104/pp.62.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Association of lysosomal activity with aleurone grains in plant seeds. Arch Biochem Biophys. 1968 Mar 20;124(1):466–471. doi: 10.1016/0003-9861(68)90354-8. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Huang A. H. Albumin storage proteins in the protein bodies of castor bean. Plant Physiol. 1978 Jan;61(1):13–16. doi: 10.1104/pp.61.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle R. J., Huang A. H. Protein Bodies from the Endosperm of Castor Bean: Subfractionation, Protein Components, Lectins, and Changes during Germination. Plant Physiol. 1976 Dec;58(6):703–709. doi: 10.1104/pp.58.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]



