Abstract
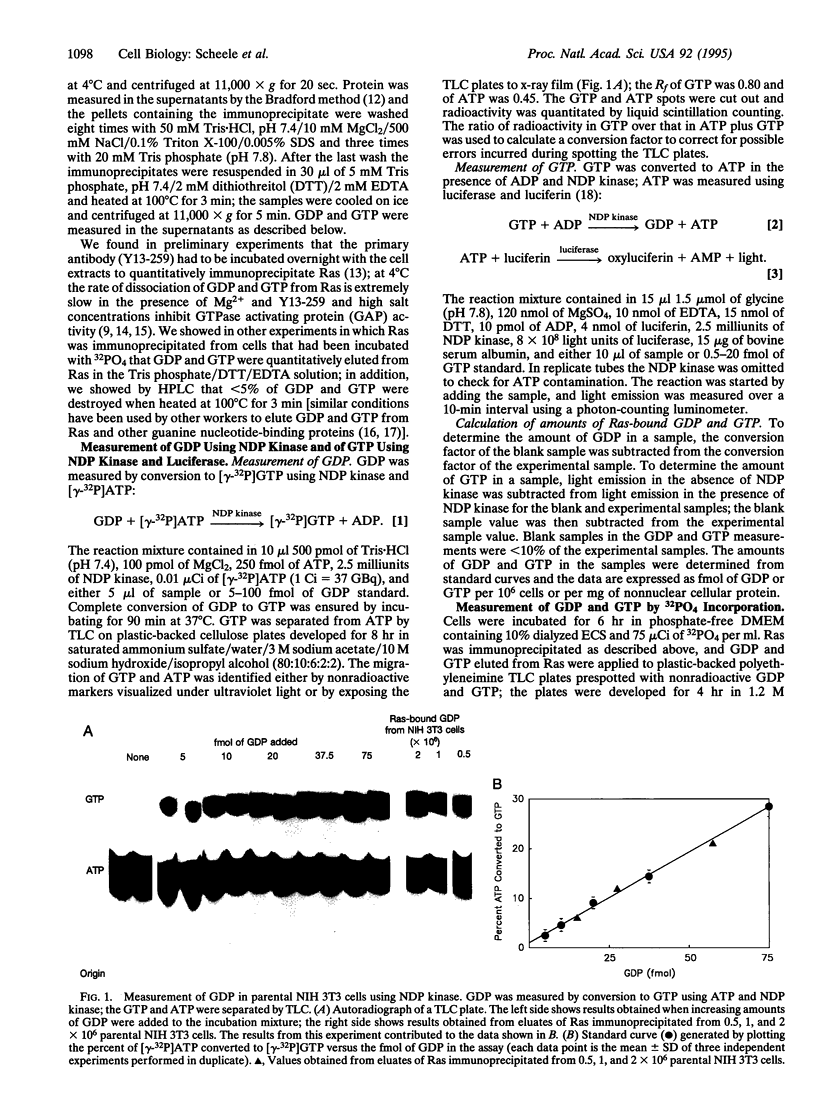
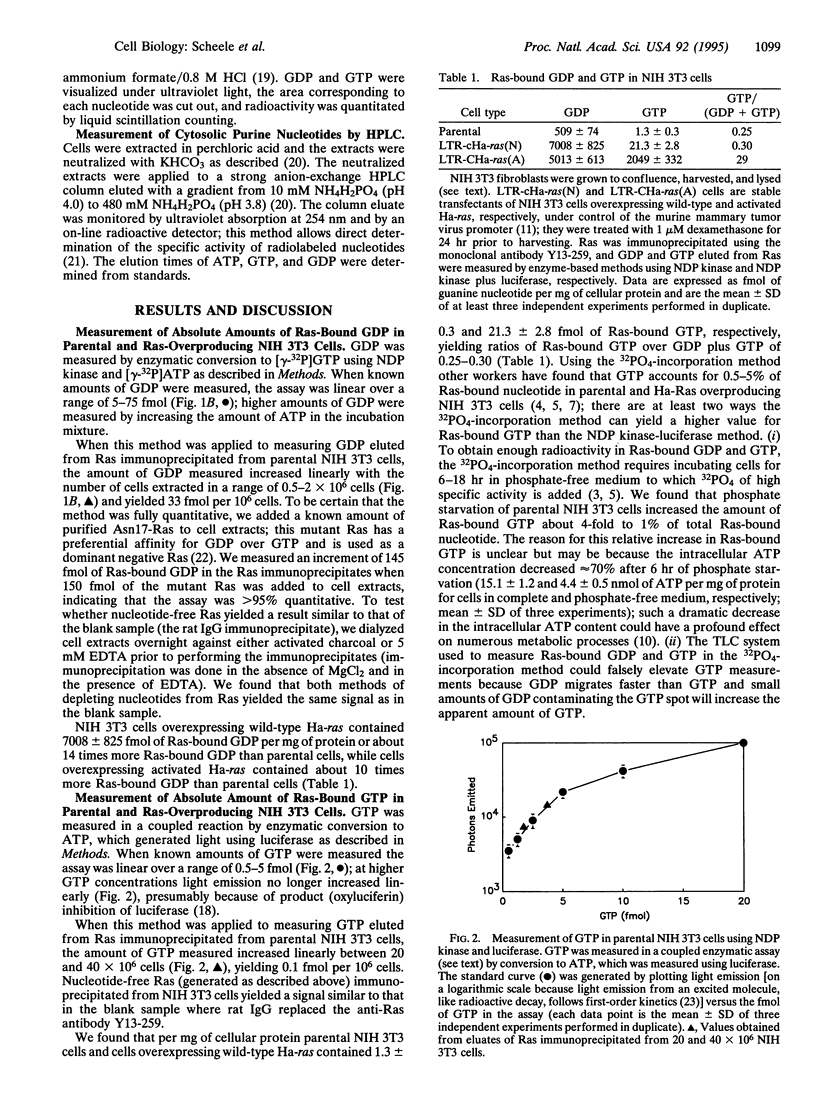
We devised enzyme-based methods to measure fmol amounts of GDP and GTP and applied these methods to measure absolute amounts of Ras-bound GDP and GTP in NIH 3T3 fibroblasts. We found that parental NIH 3T3 cells contained 509 and 1.3 fmol of Ras-bound GDP and GTP, respectively, per mg of cellular protein and that stable transfectants of NIH 3T3 cells overexpressing wild-type Ha-Ras contained 7008 and 21.3 fmol of Ras-bound GDP and GTP, respectively, per mg of cellular protein; thus, in both cell types < 0.3% of Ras was in the active GTP-bound state. In contrast, NIH 3T3 cells overexpressing an activated form of Ha-Ras contained 5013 and 2049 fmol of Ras-bound GDP and GTP, respectively, per mg of protein, yielding 29% of Ras in the GTP-bound state. Since intracellular Ras is probably all in a guanine-nucleotide bound state, this method allows one to calculate the number of Ras molecules in each cell: parental NIH 3T3 cells and the Ha-Ras overproducing cells contain approximately 20,000 and approximately 275,000 Ras molecules per cell, respectively. We also incubated parental NIH 3T3 cells with 32PO4 and determined the radioactivity in Ras-bound GDP and GTP and the specific activity of cytosolic GDP and GTP; these experiments indicated that Ras-bound GTP may not be in equilibrium with the total cytosolic GTP pool.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Boss G. R., Pilz R. B. Phosphoribosylpyrophosphate synthesis from glucose decreases during amino acid starvation of human lymphoblasts. J Biol Chem. 1985 May 25;260(10):6054–6059. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgering B. M., Medema R. H., Maassen J. A., van de Wetering M. L., van der Eb A. J., McCormick F., Bos J. L. Insulin stimulation of gene expression mediated by p21ras activation. EMBO J. 1991 May;10(5):1103–1109. doi: 10.1002/j.1460-2075.1991.tb08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig L. A., Cooper G. M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988 Aug;8(8):3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Ferguson K. M., Higashijima T., Smigel M. D., Gilman A. G. The influence of bound GDP on the kinetics of guanine nucleotide binding to G proteins. J Biol Chem. 1986 Jun 5;261(16):7393–7399. [PubMed] [Google Scholar]
- Feuerstein J., Goody R. S., Wittinghofer A. Preparation and characterization of nucleotide-free and metal ion-free p21 "apoprotein". J Biol Chem. 1987 Jun 25;262(18):8455–8458. [PubMed] [Google Scholar]
- Freissmuth M., Casey P. J., Gilman A. G. G proteins control diverse pathways of transmembrane signaling. FASEB J. 1989 Aug;3(10):2125–2131. [PubMed] [Google Scholar]
- Gibbs J. B., Marshall M. S., Scolnick E. M., Dixon R. A., Vogel U. S. Modulation of guanine nucleotides bound to Ras in NIH3T3 cells by oncogenes, growth factors, and the GTPase activating protein (GAP). J Biol Chem. 1990 Nov 25;265(33):20437–20442. [PubMed] [Google Scholar]
- Gibbs J. B., Schaber M. D., Marshall M. S., Scolnick E. M., Sigal I. S. Identification of guanine nucleotides bound to ras-encoded proteins in growing yeast cells. J Biol Chem. 1987 Aug 5;262(22):10426–10429. [PubMed] [Google Scholar]
- Hattori S., Clanton D. J., Satoh T., Nakamura S., Kaziro Y., Kawakita M., Shih T. Y. Neutralizing monoclonal antibody against ras oncogene product p21 which impairs guanine nucleotide exchange. Mol Cell Biol. 1987 May;7(5):1999–2002. doi: 10.1128/mcb.7.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen B. T., Sadée W. Compartmentation of guanine nucleotide precursors for DNA synthesis. Biochem J. 1986 Mar 1;234(2):263–269. doi: 10.1042/bj2340263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nånberg E., Westermark B. Platelet-derived growth factor increases the turnover of GTP/GDP on ras in permeabilized fibroblasts. J Biol Chem. 1993 Aug 25;268(24):18187–18194. [PubMed] [Google Scholar]
- Osterop A. P., Medema R. H., Bos J. L., vd Zon G. C., Moller D. E., Flier J. S., Möller W., Maassen J. A. Relation between the insulin receptor number in cells, autophosphorylation and insulin-stimulated Ras.GTP formation. J Biol Chem. 1992 Jul 25;267(21):14647–14653. [PubMed] [Google Scholar]
- Pilz R. B., Willis R. C., Boss G. R. The influence of ribose 5-phosphate availability on purine synthesis of cultured human lymphoblasts and mitogen-stimulated lymphocytes. J Biol Chem. 1984 Mar 10;259(5):2927–2935. [PubMed] [Google Scholar]
- Poe M., Scolnick E. M., Stein R. B. Viral Harvey ras p21 expressed in Escherichia coli purifies as a binary one-to-one complex with GDP. J Biol Chem. 1985 Apr 10;260(7):3906–3909. [PubMed] [Google Scholar]
- Santos E., Nebreda A. R. Structural and functional properties of ras proteins. FASEB J. 1989 Aug;3(10):2151–2163. doi: 10.1096/fasebj.3.10.2666231. [DOI] [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakafuku M., Akiyama T., Yamamoto T., Kaziro Y. Accumulation of p21ras.GTP in response to stimulation with epidermal growth factor and oncogene products with tyrosine kinase activity. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7926–7929. doi: 10.1073/pnas.87.20.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakafuku M., Nakamura S., Kaziro Y. Platelet-derived growth factor stimulates formation of active p21ras.GTP complex in Swiss mouse 3T3 cells. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5993–5997. doi: 10.1073/pnas.87.15.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakamura S., Kaziro Y. Analysis of guanine nucleotide bound to ras protein in PC12 cells. FEBS Lett. 1988 Aug 15;236(1):185–189. doi: 10.1016/0014-5793(88)80311-9. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Torti M., Marti K. B., Altschuler D., Yamamoto K., Lapetina E. G. Erythropoietin induces p21ras activation and p120GAP tyrosine phosphorylation in human erythroleukemia cells. J Biol Chem. 1992 Apr 25;267(12):8293–8298. [PubMed] [Google Scholar]




