Abstract
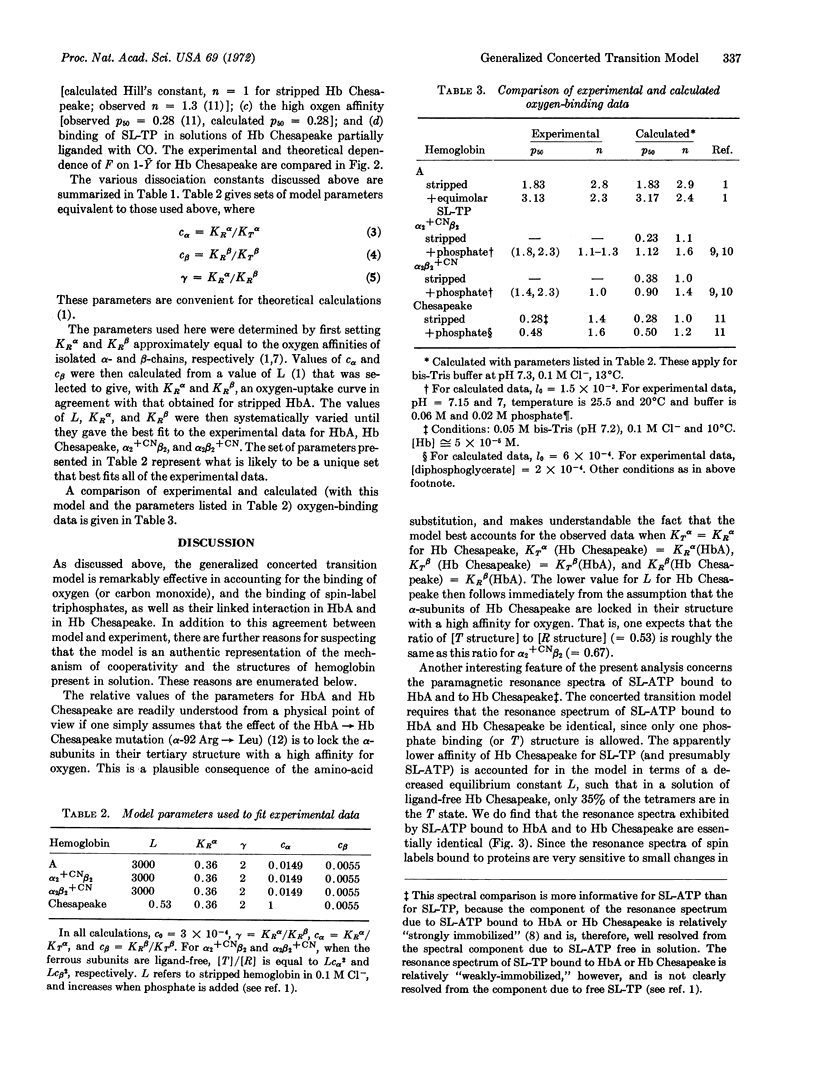
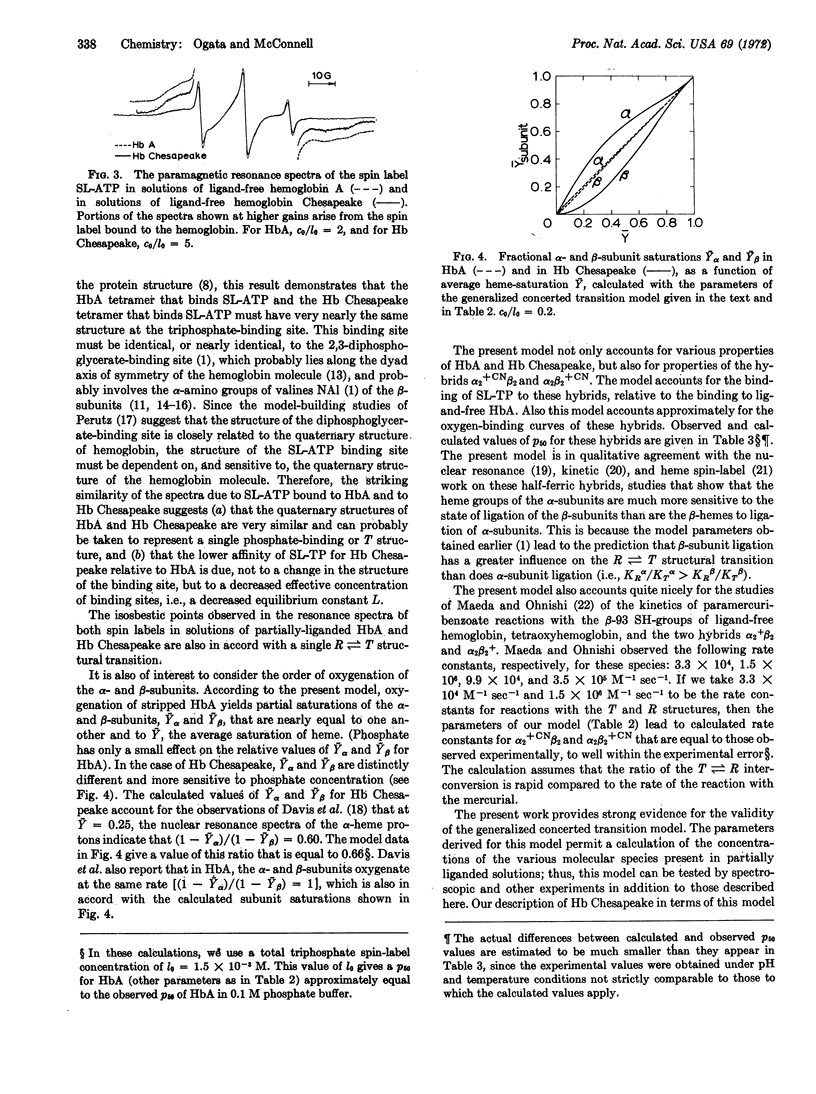
Evidence is presented that a generalized concerted transition model provides a quantitative understanding of (a) the molecular species that are present in solutions of partially liganded hemoglobin and (b) the macromolecular mechanism of cooperativity. Model parameters for hemoglobin A and for hemoglobin Chesapeake were determined from studies of the binding of spin-label triphosphates to ligand-free and partially liganded hemoglobin solutions, and to the hybrids α2+CNβ2 and α2β2+CN. This model is the same as that proposed originally by Monod, Wyman, and Changeux [J. Mol. Biol. (1965) 12, 88] for hemoglobin, except that the α-subunits are treated as nonequivalent to the β-subunits.
Keywords: spin-labeled triphosphate, concerted transition model, hemoglobin Chesapeake
Full text
PDF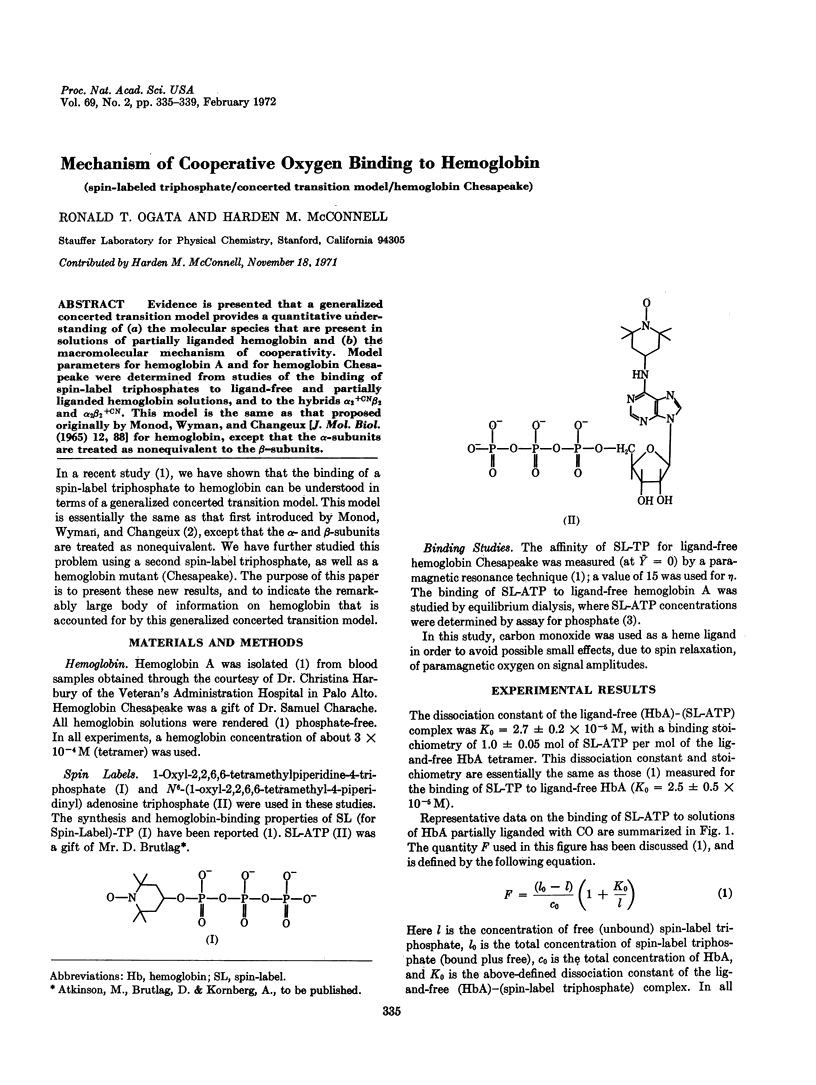


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Asakura T., Drott H. R. Evidence of heme-heme interaction in heme-spin-labeled hemoglobin. Biochem Biophys Res Commun. 1971 Sep;44(5):1199–1204. doi: 10.1016/s0006-291x(71)80213-9. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. Intracellular organic phosphates as regulators of oxygen release by haemoglobin. Nature. 1969 Feb 15;221(5181):618–622. doi: 10.1038/221618a0. [DOI] [PubMed] [Google Scholar]
- Bolton W., Perutz M. F. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature. 1970 Nov 7;228(5271):551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- Bonaventura J., Riggs A. Hemoglobin Kansas, a human hemoglobin with a neutral amino acid substitution and an abnormal oxygen equilibrium. J Biol Chem. 1968 Mar 10;243(5):980–991. [PubMed] [Google Scholar]
- Brunori M., Amiconi G., Antonini E., Wyman J. Artificial intermediates in the reaction of haemoglobin. Functional and conformational properties of the cyanmet intermediates. J Mol Biol. 1970 Apr 28;49(2):461–471. doi: 10.1016/0022-2836(70)90257-3. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Briehl R. W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970 Jun;49(6):1088–1095. doi: 10.1172/JCI106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. R., Nagel R. L., Jaffe E. R. The effect of oxygen, carbon dioxide, pH and cyanate on the binding of 2,3-diphosphoglycerate to human hemoglobin. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1504–1509. doi: 10.1016/s0006-291x(71)80256-5. [DOI] [PubMed] [Google Scholar]
- Cassoly R., Gibson Q. H., Ogawa S., Shulman R. G. Effects of phosphate upon CO binding kinetics and NMR spectra of hemoglobin valency hybrids. Biochem Biophys Res Commun. 1971 Sep;44(5):1015–1021. doi: 10.1016/s0006-291x(71)80187-0. [DOI] [PubMed] [Google Scholar]
- Charache S., Weatherall D. J., Clegg J. B. Polycythemia associated with a hemoglobinopathy. J Clin Invest. 1966 Jun;45(6):813–822. doi: 10.1172/JCI105397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. G., Lindstrom T. R., Mock N. H., Baldassare J. J., Charache S., Jones R. T., Ho C. Nuclear magnetic resonance studies of hemoglobins. VI. Heme proton spectra of human deoxyhemoglobins and their relevance to the nature of co-operative oxygenation of hemoglobin. J Mol Biol. 1971 Aug 28;60(1):101–111. doi: 10.1016/0022-2836(71)90450-5. [DOI] [PubMed] [Google Scholar]
- Edelstein S. J. Extensions of the allosteric model for haemoglobin. Nature. 1971 Mar 26;230(5291):224–227. doi: 10.1038/230224a0. [DOI] [PubMed] [Google Scholar]
- Haber J. E., Koshland D. E., Jr Evidence for beta--beta interactions during the binding of oxygen to hemoglobin. Biochim Biophys Acta. 1969 Nov 11;194(1):339–341. doi: 10.1016/0005-2795(69)90215-3. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Maeda T., Onishi S. I. Kinetic evidence for propagation of conformational changes in the alpha subunit to the beta subunit of hemoglobin. Biochemistry. 1971 Mar 30;10(7):1177–1180. doi: 10.1021/bi00783a013. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., McFarland B. G. Physics and chemistry of spin labels. Q Rev Biophys. 1970 Feb;3(1):91–136. doi: 10.1017/s003358350000442x. [DOI] [PubMed] [Google Scholar]
- Muirhead H., Greer J. Three-dimensional Fourier synthesis of human deoxyhaemoglobin at 3.5 Angstrom units. Nature. 1970 Nov 7;228(5271):516–519. doi: 10.1038/228516a0. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Shulman R. G. Observation of allosteric transition in hemoglobin. Biochem Biophys Res Commun. 1971 Jan 8;42(1):9–15. doi: 10.1016/0006-291x(71)90354-8. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- REISSMANN K. R., RUTH W. E., NOMURA T. A human hemoglobin with lowered oxygen affinity and impaired heme-heme interactions. J Clin Invest. 1961 Oct;40:1826–1833. doi: 10.1172/JCI104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S., Riggs A. Studies of the interaction of 2,3-diphosphoglycerate and carbon dioxide with hemoglobins from mouse, man, and elephant. J Biol Chem. 1971 Feb 10;246(3):547–554. [PubMed] [Google Scholar]


