Abstract
Context:
Persistent muscle weakness after anterior cruciate ligament (ACL) reconstruction may be due to underlying activation failure and arthrogenic muscle inhibition (AMI). Knee-joint cryotherapy has been shown to improve quadriceps function transiently in those with AMI, thereby providing an opportunity to improve quadriceps muscle activation and strength in patients with a reconstructed ACL.
Objective:
To compare quadriceps muscle function in patients with a reconstructed ACL who completed a 2-week intervention including daily cryotherapy (ice bag), daily exercises, or both.
Design:
Cross-sectional study.
Setting:
Laboratory.
Patients or Other Participants:
A total of 30 patients with reconstructed ACLs who were at least 6 months post-index surgery and had measurable quadriceps AMI.
Intervention(s):
The patients attended 4 supervised visits over a 2-week period. They were randomly assigned to receive 20 minutes of knee-joint cryotherapy, 1 hour of therapeutic rehabilitation exercises, or cryotherapy followed by exercises.
Main Outcome Measure(s):
We measured quadriceps Hoffmann reflex, normalized maximal voluntary isometric contraction torque, central activation ratio using the superimposed-burst technique, and patient-reported outcomes before and after the intervention period.
Results:
After the 2-week intervention period, patients who performed rehabilitation exercises immediately after cryotherapy had higher normalized maximal voluntary isometric contraction torques (P = .002, Cohen d effect size = 1.4) compared with those who received cryotherapy alone (P = .16, d = 0.58) or performed exercise alone (P = .16, d = 0.30).
Conclusions:
After ACL reconstruction, patients with AMI who performed rehabilitation exercises immediately after cryotherapy experienced greater strength gains than those who performed cryotherapy or exercises alone.
Key Words: knee, arthrogenic muscle inhibition, disinhibition, therapeutic exercises
Key Points
Applying ice to the knee joint for 20 minutes before therapeutic exercises facilitated strength gains in the quadriceps muscles of patients after anterior cruciate ligament reconstructions.
Patients with persistent muscle weakness secondary to joint injury may benefit from the strategy of treating arthrogenic muscle inhibition before performing therapeutic exercises.
This is a small clinical study, and the findings are generalizable only to patients with anterior cruciate ligament reconstructions who have chronic quadriceps muscle dysfunction.
Quadriceps muscle weakness and dysfunction are common in patients with anterior cruciate ligament (ACL) injuries and can persist for years after reconstruction.1 Rehabilitation strategies for maximizing strength gains are important as patients recover and return to their preinjury lifestyles. Chronic quadriceps weakness, activation failure, and altered gait patterns occur frequently after ACL reconstruction1–5 and may persist for years after injury and release from formal postoperative therapy.
A likely source of these chronic deficits in ACL-reconstructed patients is arthrogenic muscle inhibition (AMI),4,6,7 which is an ongoing reflex response to damaged joint structures that affects the periarticular musculature. In theory, for patients with knee injuries, aberrant sensory information from the joint mechanoreceptors results in a reduction in the efferent drive to the quadriceps muscle, thereby reducing the volume of motor units that are available for voluntary recruitment.7,8 Thus, a clinically important consequence of AMI is the inability to completely activate the quadriceps due to the reduced availability of quadriceps motor units. This impairment plays a primary role in posttraumatic quadriceps weakness and central activation failure and may indirectly contribute to the altered gait patterns often observed in individuals with an injured ACL.3,4 Interventions aimed at treating the underlying causes of clinically evident impairments may facilitate recovery from joint injury.
Rehabilitation exercise programs aimed at improving muscle function may be limited by the presence of AMI. When patients attempt to voluntarily contract a muscle during a rehabilitation exercise, they are potentially recruiting from a diminished pool of available motoneurons. Therefore, treating underlying AMI before exercise may offer a unique strategy when prescribing rehabilitation programs for injured and inhibited patients. The simple application of cryotherapy to the knee joint can resolve AMI. This has been observed when the quadriceps Hoffmann reflex (H-reflex) increases after knee-joint cryotherapy in persons with inhibition due to artificially effused knee joints.9,10 Knee-joint cryotherapy has also resulted in transient increases in quadriceps central activation ratios (CARs) in patients with tibiofemoral osteoarthritis.11 These findings suggest that cryotherapy applied to an injured joint may provide a window of time during which patients experience quadriceps motoneuron-pool “disinhibition.” During this period of disinhibition, in theory, patients would be recruiting from an increased motoneuron pool, thereby facilitating strength gains.
Therefore, the purpose of our study was to compare the quadriceps H-reflex, normalized maximal voluntary isometric contraction torque (MVIC), CAR, and self-reported outcomes in patients with ACL reconstruction who completed 2 weeks of directed rehabilitation exercises immediately after ice-bag treatments to the knee joint. We hypothesized that quadriceps function and self-reported outcomes would improve in individuals with a reconstructed ACL who used cryotherapy before performing rehabilitation exercises.
METHODS
A total of 33 patients with a history of primary, unilateral ACL reconstruction were recruited and screened for enrollment. All participants had undergone the reconstruction at least 6 months before the recruitment period, and all had evidence of chronic quadriceps dysfunction (ie, a quadriceps CAR of 90% or less). All patients had been released by their surgeons to full activity. Those with multiple ligament injuries or postoperative complications such as infection or graft failure and those who could not tolerate cryotherapy were excluded. Three patients were excluded at the time of screening: 1 had no measurable quadriceps H-reflex, 1 had CAR >90%, and 1 had a history of multiple ligament injuries. Therefore, 30 patients (10 men, 20 women) were enrolled (Table 1). For descriptive purposes, we recorded patient-reported outcomes, including the International Knee Documentation Committee (IKDC) subjective knee-evaluation form,12,13 Tegner Activity Level Scale,14 and pain rating using a visual analog scale.14 We also performed an instrumented knee examination using the KT-1000 knee arthrometer (MEDmetric Corp, San Diego, CA; side-to-side differences in anterior translation were measured at 150 N). This study was approved by our university's institutional review board, and we obtained informed consent from each participant.
Table 1.
Patient Characteristics (Mean ± SD)
| Characteristic |
Group |
All Participants |
||
| Cryotherapy |
Exercise |
Cryotherapy + Exercise |
||
| Age, y | 27.6 ± 12.2 | 24.5 ± 10.4 | 29.8 ± 12.2 | 27.3 ± 11.4 |
| Height, cm | 168.1 ± 9.1 | 166.9 ± 10.5 | 167.4 ± 7.6 | 167.4 ± 8.8 |
| Mass, kg | 74.8 ± 17.5 | 72.0 ± 8.8 | 73.2 ± 8.8 | 73.3 ± 12.2 |
| KT-1000 measurement, mma | 1.3 ± 1.4 | 1.5 ± 1.8 | 1.5 ± 0.7 | 1.4 ± 1.3 |
| International Knee Documentation Committee form, % | 74.8 ± 10.7 | 74.0 ± 12.4 | 82.2 ± 12.3 | 77.0 ± 12.0 |
| Visual analog scale (range, 1–10) | 1.2 ± 1.3 | 1.4 ± 0.7 | 0.9 ± 1.0 | 1.2 ± 1.0 |
| Tegner Activity Level Scale (range, 0–10) | 6 ± 2 | 5 ± 1 | 6 ± 2 | 6 ± 1 |
| Time since reconstruction, mo | 44 ± 59 | 27 ± 31 | 31 ± 35 | 34 ± 42 |
Measured as side-to-side difference.
Quadriceps Activation
Quadriceps activation was measured at baseline and after the 2-week intervention. We used the superimposed-burst technique, described previously.1 Two nonadhesive, carbon-impregnated electrodes (8 × 14 cm) were coated with aqueous conduction gel and secured to the superolateral and distal-medial anterior thigh with an elastic wrap. Participants were seated in a chair with hips and knees flexed to approximately 90°. The participants were secured to the chair of a multi-mode dynamometer system (System 3; Biodex Inc, Shirley, NY) using a lap belt and were asked to maintain good seated posture (back straight, shoulders against the chair back) with their hands in their lap. After a brief period of familiarization and several practice trials, participants performed 2 to 3 MVICs with continuous oral encouragement by the tester. When each participant reached the maximal torque plateau, the tester manually triggered an electrical stimulus to the thigh using a square-wave stimulus generator (Grass-Telefactor, West Warwick, RI). The stimulus consisted of a train of 10 pulses; each was 0.6 ms in duration and delivered at 100Hz with an intensity of 125 V. The electrical stimulus caused a transient increase in knee-extension torque, above that of the MVIC; this is called the superimposed-burst torque. The MVIC torque was calculated as the mean torque value during a 200-millisecond time period immediately before the electrical stimulation. The CAR was calculated between the MVIC torque and peak superimposed-burst torque and reported as a percentage of complete (100%) activation.15
Hoffmann Reflex Testing
We collected the quadriceps H-reflex as described previously16 on all participants' reconstructed limb before and after rehabilitation. Two round, self-adhesive silver–silver chloride pregelled surface electromyography recording electrodes were placed over the vastus medialis obliquus, parallel to the fiber orientation, with an interelectrode distance of 2 cm. The ground electrode was placed on the distal anterior tibia. Participants rested comfortably and quietly in a supine position with the knee supported at 12° to 20° of flexion. A square-wave, current-controlled simulator (Biopac Inc, Goleta, CA) delivered short (1-millisecond) pulses to the proximal anterior thigh via a small (5-mm) electrode placed in the inguinal fold superficial to the femoral nerve. Serial pulses (with a minimum 15-second pause between pulses) were applied at progressively increasing intensities until maximum H-reflex and muscle (M) responses were identified.16 The average of 3 H-reflexes and 3 M-responses were used to calculate the H:M ratio. All measures were recorded by the same experienced investigator. Excellent intersession reliability for the H-reflex technique has been previously reported.17
Blinding and Randomization
One investigator performed all baseline and postrehabilitation measures and was blinded to group assignment. Participants were randomized to 1 of 3 treatment groups after the baseline testing procedures were completed. Randomization occurred in a 1:1:1 ratio a priori using a random-number generator.
Intervention
All participants completed a 2-week intervention, including 4 supervised treatment sessions with an unblinded certified athletic trainer, and daily home treatments (see Supplemental Material, available online at http://dx.doi.org/10.4085/1062-6050-49.03.39.S1). The first day of treatment was a supervised session for all participants to ensure proper technique and provide instructions for the home exercise program. Participants were asked to complete a daily log for home treatments that included the names of the exercises as well as the number of sets and repetitions completed.
Cryotherapy Group
For those in the cryotherapy group (CRYO), 2 bags, each with approximately 1.5 L of crushed ice, were applied to the anterior and posterior aspects of the knee joint and secured with elastic wrap for 20 minutes. Participants were instructed to perform 1 knee-joint cryotherapy treatment every day and were provided ice bags and elastic wraps along with their home treatment log.
Exercise Group
Participants in the exercise group (EXERC) completed a program including lower extremity muscle stretching, progressive strengthening exercises, and balance training. Supervised sessions lasted roughly 1 hour and consisted of a general stretching warm-up, traditional open chain exercises with resistance, and progressive closed chain quadriceps and hamstrings strengthening exercises (Table 2). The number of repetitions and resistance used for each exercise were progressed as appropriate by the unblinded investigator. Each participant's daily home treatments were recorded in a treatment log.
Table 2.
Overview of Supervised (Clinic) and Home-Based Treatments for Study Groups
| Treatment |
Group |
|||||
| Cryotherapy |
Exercise |
Cryotherapy + Exercise |
||||
| Clinic |
Home |
Clinic |
Home |
Clinic |
Home |
|
| Cryotherapy treatment | X | X | X | X | ||
| Stretching | X | X | X | X | ||
| Quadriceps sets | X | X | X | X | ||
| Straight-leg raise | X | X | X | X | ||
| Calf raises | X | X | X | X | ||
| Resisted knee extension | X | X | ||||
| Resisted knee flexion | X | X | ||||
| Lunges | X | X | X | X | ||
| Lateral step-down | X | X | ||||
| Wall squats | X | X | ||||
| Balance training | X | X | ||||
Cryotherapy and Exercise Group
Participants in the combined group (CRYO+EXERC) completed the same treatment program as the EXERC group. Before every supervised and home treatment session, participants performed the same cryotherapy treatment as the CRYO group. Each participant's treatments were recorded in a treatment log.
Statistical Analysis
Sample size was based on anticipated changes in H-reflex amplitude that we estimated from a previously published study9 using cryotherapy as a disinhibitory modality. We estimated that 10 participants per group would be sufficient to detect significant differences. A total of 4 participants (2 from the EXERC group and 2 from the CRYO group) withdrew from the study before completion due to time or travel commitments. Given the small number of participants in this study, we used the group mean to replace the missing values.18 Another participant from the CRYO group was removed because her reconstruction had occurred more than 30 years before the study.
We used a 3 × 2 (group-by-time) repeated-measures analysis of variance for normalized MVIC torque, quadriceps CAR, and H:M ratio. Post hoc dependent t tests were performed in the event of an interaction to identify changes over time in each group. Effect-size calculations were made as pre-post differences normalized to the pooled standard deviation. We also compared patient demographics and self-reported outcomes among treatment groups with a 1-way analysis of variance. All statistical analyses were performed with SPSS for Windows (version 17.0; SPSS Inc, Chicago, IL). A priori α was set at P < .05.
RESULTS
Participant demographics and self-reported outcomes are presented in Table 1. No statistically significant differences among treatment groups were noted for any of these variables. Participants exhibited considerable activation failure at baseline (average CAR = 75.8% ± 9.7%). Treatment compliance was excellent for those completing the intervention. Three participants missed 1 session (2 from the EXERC group, 1 from the EXERC+CRYO group), and 4 participants reported missing 2 days of home treatment. Baseline and posttherapy data with percentage changes over time are shown in Table 3.
Table 3.
Baseline and Posttherapy Means Extended on Next Page
| Variable |
Group |
|||||||
| Cryotherapy |
Exercise |
|||||||
| Baseline |
Posttherapy |
Pa |
Effect Size (95% CI) |
Baseline |
Posttherapy |
Pa |
Effect Size (95% CI) |
|
| Maximal voluntary isometric contraction, Nm/kg | 1.5 ± 0.3 | 1.7 ± 0.4 | .16 | 0.58 (−0.31, 1.48) | 1.4 ± 0.6 | 1.6 ± 0.7 | .16 | 0.30 (−0.58, 1.18) |
| Central activation ratio, % | 78.1 ± 4.4 | 80.4 ± 10.5 | NA | 0.29 (−0.59, 1.17) | 73.3 ± 12.6 | 83.4 ± 8.4 | NA | 0.94 (0.02, 1.86) |
| H-reflex:M-response ratio | 0.21 ± 0.19 | 0.20 ± 0.15 | NA | −0.05 (−0.93, 0.82) | 0.22 ± 0.13 | 0.30 ± 0.12 | NA | 0.73 (−0.18, 1.63) |
Abbreviations: CI, confidence interval; NA, not applicable because no group × time interaction was observed.
P value for post hoc baseline-posttreatment therapy comparison within each group.
Table 3.
Extended From Previous Page
| Group | |||
| Cryotherapy + Exercise | |||
| Baseline |
Posttherapy |
Pa |
Effect Size (95% CI) |
| 1.6 ± 0.4 | 2.2 ± 0.7 | .002 |
1.4 (0.42, 2.4) |
| 76.0 ± 10.6 | 88.2 ± 5.5 | NA | 1.4 (0.45, 2.4) |
| 0.40 ± 0.20 | 0.40 ± 0.19 | NA | −0.02 (−0.89, 0.86) |
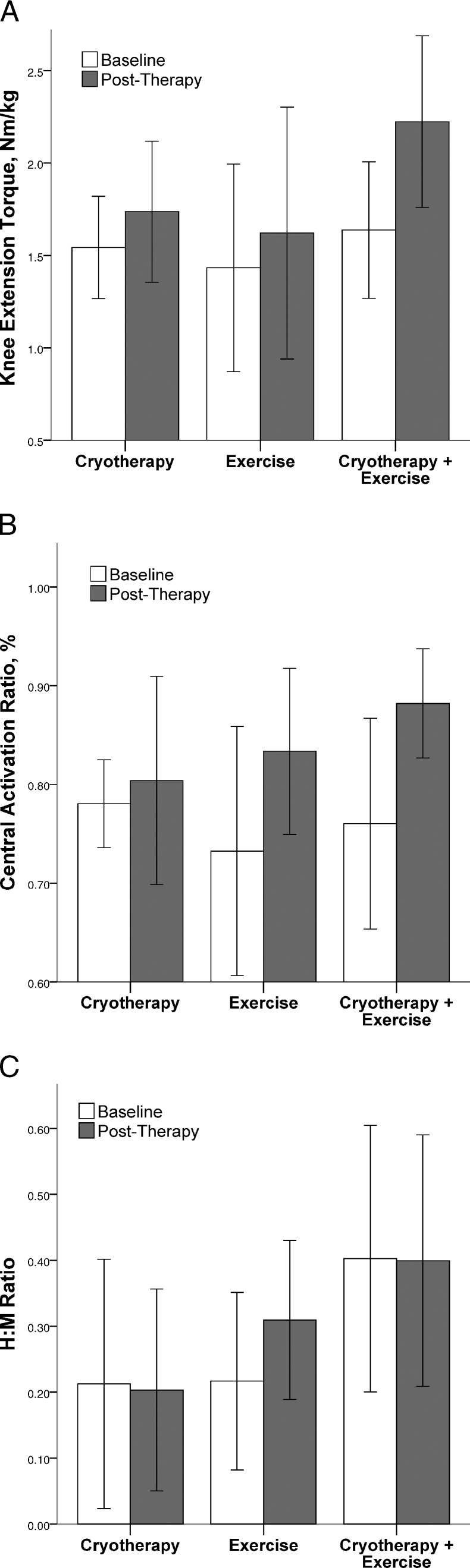
We found a significant interaction for normalized knee-extension MVIC torque (F2,26 = 3.3, P = .05, 1 − β = 0.57) but not for quadriceps CAR (F2,26 = 2.7, P = .08, 1 − β = 0.49) or H:M ratio (F2,26 = 1.1, P = .36, 1 − β = 0.22). Post hoc analysis revealed that participants in the CRYO+EXERC group experienced an increase in knee-extension torque (P = .002), whereas participants in the CRYO (P = .16) and EXERC (P = .16) groups did not (Figure).
Figure.
Baseline and posttherapy values for normalized knee extension torque (A), quadriceps central activation ratio (B), and quadriceps H:M ratio (C) for each treatment group. Bars represent the mean value, error bars represent ± 1 standard deviation.
DISCUSSION
Our primary finding was that the use of knee-joint cryotherapy before therapeutic exercises resulted in greater strength gains compared with either intervention alone. These results indicate that strategic implementation of a modality that is aimed at improving motoneuron-pool excitability while an individual performs rehabilitation exercises can facilitate strength gains in patients with chronic quadriceps dysfunction. In the context of the current study, we used cryotherapy not to treat pain but to treat an underlying source of AMI. These findings suggest an alternative and strategic use of cryotherapy when treating chronic muscle dysfunction in patients with a history of chronic joint injury.
Clinicians may be concerned about having patients perform exercises immediately after cryotherapy because of the possibility that joint and extremity function will be compromised due to the loss of peripheral sensory input associated with the modality. For example, the results of 1 study19 seemed to match anecdotal reports that a joint will stiffen more after cryotherapy. In addition, shoulder-joint function and performance during throwing were impaired after cryotherapy20; yet, another study21 indicated that cryotherapy did not affect shoulder-joint position sense. The findings regarding joint function after cryotherapy are conflicting and inconclusive.22 Knee-joint position sense was impaired immediately after cryotherapy23; however, biomechanics during single-legged landings did not change after knee-joint cryotherapy.24 The most profound changes in joint and extremity function were reported in studies22,24 that used cooling modalities other than ice bags, such as cold-water immersion and commercial ice packs. Unfortunately, whether therapeutically cooling a joint or muscle tissue influences injury risk is unknown. In the current study, participants performed controlled rehabilitation exercises in a safe environment and were not experiencing current pain or instability. One author10 reported reductions in lower extremity kinetics after quadriceps AMI induced by an artificial knee-joint effusion. The application of knee-joint cryotherapy negated these changes; however, it is important to note that the exercises were performed on a recumbent leg-press machine at a controlled cadence. In our study, the use of cryotherapy as a disinhibitory modality was intended to enhance motoneuron-pool availability during controlled rehabilitation exercises. Clinicians should consider underlying pain and instability due to joint injury when prescribing functional exercises that follow a disinhibitory modality such as cryotherapy.
In the presence of knee-joint injury or ligament reconstruction, quadriceps AMI is common. The AMI may limit patients' ability to progress during rehabilitation or strengthening exercises because of the reduced or “closed” quadriceps motoneuron pool from which to recruit motor units during volitional contractions. Aberrant sensory information arising from pain, instability, and distention is likely conveyed via activation of the nociceptors and mechanoreceptors in periarticular tissues,7 resulting in reflexive closing of the associated motoneuron pool. Therefore, strategies aimed at treating AMI would either try to regulate the amount of sensory information conveyed to the central nervous system or provide a stimulus that would potentially be preferentially interpreted (ie, the gate control theory of pain control).
Our findings agree with those of previous researchers,9–11,25–28 who identified the ability of cryotherapy to “open” or disinhibit an inhibited motoneuron pool. We did not observe differences in the H:M ratio over time. This is not surprising given that prior authors used within-session designs and the H-reflex as an outcome measure. Unfortunately, the variability in our quadriceps H:M ratios was high, as were the percentage changes over time. One potential explanation is that participants were still experiencing the same level of spinal-reflex excitability before and after the rehabilitation session. This, too, is not surprising, because our reason for using cryotherapy was not to permanently resolve AMI. Cryotherapy will not undo the damage to a joint. We do not know of any therapy that will completely remove AMI forever; therefore, individuals with major knee-joint damage may always be at risk for persistent muscle weakness due to AMI.29 The goal of using cryotherapy is to provide a transient period after application during which quadriceps muscle function is enhanced. For example, in prior research, H-reflex amplitudes that fell below baseline measures after an artificial knee-joint effusion increased above baseline levels for about an hour after ice-bag application to the knee joint.9,30 In theory, altered or incomplete motor-unit activation during voluntary contractions is a consequence of motoneuron-pool inhibition. This is a possible explanation for why some people experience persistent muscle weakness after a joint injury that seems resistant to rehabilitation. Therefore, during rehabilitation exercises, patients with knee injuries would benefit from a more complete motoneuron pool because it is possible to recruit from more available (ie, not inhibited) motor units during voluntary contractions. In the current study, we observed an increase in isometric strength but not in our estimates of quadriceps motoneuron excitability. This makes sense because our intervention was really a rehabilitation strategy: Remove AMI and then perform exercises with a more complete motoneuron pool. This strategy worked and isometric knee-extension strength increased more in the CRYO+EXERC group than in the EXERC group. Therefore, it makes sense that H:M ratios did not change over time, because our treatment was not capable of making permanent changes in the excitability of the motoneuron pool.
We also did not observe a statistical interaction for the quadriceps CAR. The changes in our outcome measures over time, which exceeded 15% on average for the treatment groups that included exercise, are shown in the Figure. The CRYO group had only a marginal increase. Although not statistically significant, the large changes in CAR with 95% confidence intervals that do not cross zero (Table 3) suggest the possibility of a type II error in interpreting this interaction as a non–statistically significant finding because the trend indicates a clinically important change.
The treatment groups in our study were designed to use cryotherapy to open the quadriceps motoneuron pool. The exercise intervention was a method of exploiting the available motoneurons during voluntary exercise contractions. The fact that our CRYO+EXERC group was the only group to experience significant knee-strength improvements indicates that the “open, then exploit” strategy is superior to either intervention alone. This also suggests that the application of a disinhibitory modality, such as joint cryotherapy, alone will not necessarily benefit the patient with AMI beyond pain control. Disinhibition provides an opportunity to perform exercises aimed at improving muscle function so that patients will be able to recruit motor units from a more complete (ie, disinhibited) motoneuron pool. Furthermore, we can only make practical recommendations about the mode of cryotherapy used for a disinhibitory effect. We cannot comment on the extent of skin or articular temperature changes due to cryotherapy because we did not perform these measurements.
Generalizability of our findings may be a limitation of this study because our numbers were low and our patient population was diverse. The ranges of ages and times since surgery was high. However, these ranges may represent the type of patient who presents clinically: the active individual with a history of major knee-joint injury and persistent quadriceps dysfunction or the patient who was noncompliant with postoperative rehabilitation and needs a way to efficiently get back on track. Whether it is 6 months or 30 years postreconstruction, if the patient is suffering from weakness, inhibition, or another poor outcome, rehabilitation is indicated. Quadriceps weakness has been associated with altered knee-joint movement patterns during walking31 and has been implicated after ACL reconstruction when patients who have weak and asymmetric quadriceps strength were less likely to pass traditional return-to-play criteria.32 Because muscle strength is paramount to joint function, the results of this study provide evidence that traditional rehabilitation can be enhanced by treating AMI before exercising. In addition, the duration of our rehabilitation program was short. We made this decision before the start of the study to maximize compliance and to complete the project within the timeline of the supportive grant. The likelihood of hypertrophic changes in muscle after a 2-week intervention is low but not impossible. Early strength gains during a resistance-training program have been attributed to neuromuscular adaptations.33 Our results demonstrate that the adaptations experienced by the patients treated with cryotherapy before exercise were accelerated, resulting in greater force-generating capability over a very short period of time in patients with quadriceps dysfunction.
CONCLUSIONS
For individuals with ACL reconstruction who have chronic quadriceps AMI, performing rehabilitation exercises immediately after cryotherapy enhanced quadriceps function and resulted in higher-magnitude strength gains when compared with performing either intervention alone. The strategic implementation of knee-joint cryotherapy immediately before controlled, low-intensity rehabilitation exercises appeared to facilitate strength gains in patients with inhibited quadriceps muscles after ACL reconstruction.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by a grant from the American Orthopaedic Society for Sports Medicine. Our institutional review board for health sciences research reviewed and approved this study (University of Virginia IRB-HSR 13010).
REFERENCES
- 1.Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45(1):87–97. doi: 10.4085/1062-6050-45.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart JM, Ko JW, Konold T, Pietrosimone B. Sagittal plane knee joint moments following anterior cruciate ligament injury and reconstruction: a systematic review. Clin Biomech (Bristol, Avon) 2010;25(4):277–283. doi: 10.1016/j.clinbiomech.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Ingersoll CD, Grindstaff TL, Pietrosimone BG, Hart JM. Neuromuscular consequences of anterior cruciate ligament injury. Clin Sports Med. 2008;27(3):383–404. doi: 10.1016/j.csm.2008.03.004. vii. [DOI] [PubMed] [Google Scholar]
- 4.Palmieri-Smith RM, Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009;37(3):147–153. doi: 10.1097/JES.0b013e3181aa6669. [DOI] [PubMed] [Google Scholar]
- 5.Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27(3):405–424. doi: 10.1016/j.csm.2008.02.001. vii–ix. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins JT, Ingersoll CD. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9(2):135–159. [Google Scholar]
- 7.Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum. 2010;40(3):250–266. doi: 10.1016/j.semarthrit.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins J, Ingersoll C. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9(2):135–159. [Google Scholar]
- 9.Hopkins JT, Ingersoll CD, Edwards J, Klootwyk TE. Cryotherapy and transcutaneous electric neuromuscular stimulation decrease arthrogenic muscle inhibition of the vastus medialis after knee joint effusion. J Athl Train. 2002;37(1):25–31. [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins JT. Knee joint effusion and cryotherapy alter lower chain kinetics and muscle activity. J Athl Train. 2006;41(2):177–184. [PMC free article] [PubMed] [Google Scholar]
- 11.Pietrosimone BG, Hart JM, Saliba SA, Hertel J, Ingersoll CD. Immediate effects of transcutaneous electrical nerve stimulation and focal knee joint cooling on quadriceps activation. Med Sci Sports Exerc. 2009;41(6):1175–1181. doi: 10.1249/MSS.0b013e3181982557. [DOI] [PubMed] [Google Scholar]
- 12.Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 13.Irrgang JJ, Ho H, Harner CD, Fu FH. Use of the International Knee Documentation Committee guidelines to assess outcome following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1998;6(2):107–114. doi: 10.1007/s001670050082. [DOI] [PubMed] [Google Scholar]
- 14.Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890–897. doi: 10.1177/0363546508330143. [DOI] [PubMed] [Google Scholar]
- 15.Hart JM, Turman KA, Diduch DR, Hart JA, Miller MD. Quadriceps muscle activation and radiographic osteoarthritis following ACL revision. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):634–640. doi: 10.1007/s00167-010-1321-z. [DOI] [PubMed] [Google Scholar]
- 16.Palmieri RM, Ingersoll CD, Hoffman MA. The Hoffmann reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train. 2004;39(3):268–277. [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins JT, Wagie NC. Intrasession and intersession reliability of the quadriceps Hoffmann reflex. Electromyogr Clin Neurophysiol. 2003;43(2):85–89. [PubMed] [Google Scholar]
- 18.Hawthorne G, Elliott P. Imputing cross-sectional missing data: comparison of common techniques. Aust N Z J Psychiatry. 2005;39(7):583–590. doi: 10.1080/j.1440-1614.2005.01630.x. [DOI] [PubMed] [Google Scholar]
- 19.Uchio Y, Ochi M, Fujihara A, Adachi N, Iwasa J, Sakai Y. Cryotherapy influences joint laxity and position sense of the healthy knee joint. Arch Phys Med Rehabil. 2003;84(1):131–135. doi: 10.1053/apmr.2003.50074. [DOI] [PubMed] [Google Scholar]
- 20.Wassinger CA, Myers JB, Gatti JM, Conley KM, Lephart SM. Proprioception and throwing accuracy in the dominant shoulder after cryotherapy. J Athl Train. 2007;42(1):84–89. [PMC free article] [PubMed] [Google Scholar]
- 21.Dover G, Powers ME. Cryotherapy does not impair shoulder joint position sense. Arch Phys Med Rehabil. 2004;85(8):1241–1246. doi: 10.1016/j.apmr.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Costello JT, Donnelly AE. Cryotherapy and joint position sense in healthy participants: a systematic review. J Athl Train. 2010;45(3):306–316. doi: 10.4085/1062-6050-45.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliveira R, Ribeiro F, Oliveira J. Cryotherapy impairs knee joint position sense. Int J Sports Med. 2010;31(3):198–201. doi: 10.1055/s-0029-1242812. [DOI] [PubMed] [Google Scholar]
- 24.Hart JM, Leonard JL, Ingersoll CD. Single-leg landing strategy after knee-joint cryotherapy. J Sport Rehabil. 2005;14(4):313–320. [Google Scholar]
- 25.Del Balso C, Cafarelli E. Adaptations in the activation of human skeletal muscle induced by short-term isometric resistance training. J Appl Physiol (1985) 2007;103(1):402–411. doi: 10.1152/japplphysiol.00477.2006. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins JT, Stencil R. Ankle cryotherapy facilitates soleus function. J Orthop Sports Phys Ther. 2002;32(12):622–627. doi: 10.2519/jospt.2002.32.12.622. [DOI] [PubMed] [Google Scholar]
- 27.Palmieri-Smith RM, Leonard-Frye JL, Garrison CJ, Weltman A, Ingersoll CD. Peripheral joint cooling increases spinal reflex excitability and serum norepinephrine. Int J Neurosci. 2007;117(2):229–242. doi: 10.1080/00207450600582702. [DOI] [PubMed] [Google Scholar]
- 28.Rice D, McNair PJ, Dalbeth N. Effects of cryotherapy on arthrogenic muscle inhibition using an experimental model of knee swelling. Arthritis Rheum. 2009;61(1):78–83. doi: 10.1002/art.24168. [DOI] [PubMed] [Google Scholar]
- 29.Kuenze C, Hart JM. Cryotherapy to treat persistent muscle weakness after joint injury. Phys Sportsmed. 2010;38(3):38–44. doi: 10.3810/psm.2010.10.1806. [DOI] [PubMed] [Google Scholar]
- 30.Hopkins JT, Ingersoll CD, Krause BA, Edwards JE, Cordova ML. Effect of knee joint effusion on quadriceps and soleus motoneuron pool excitability. Med Sci Sports Exerc. 2001;33(1):123–126. doi: 10.1097/00005768-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2002;17(1):56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 32.Hartigan EH, Zeni J, Jr, Di Stasi S, Axe MJ, Snyder-Mackler L. Preoperative predictors for non-copers to pass return to sports criteria after ACL reconstruction. J Appl Biomech. 2012;28(4):366–373. doi: 10.1123/jab.28.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cannon J, Marino FE. Early-phase neuromuscular adaptations to high- and low-volume resistance training in untrained young and older women. J Sports Sci. 2010;28(14):1505–1514. doi: 10.1080/02640414.2010.517544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.