Abstract
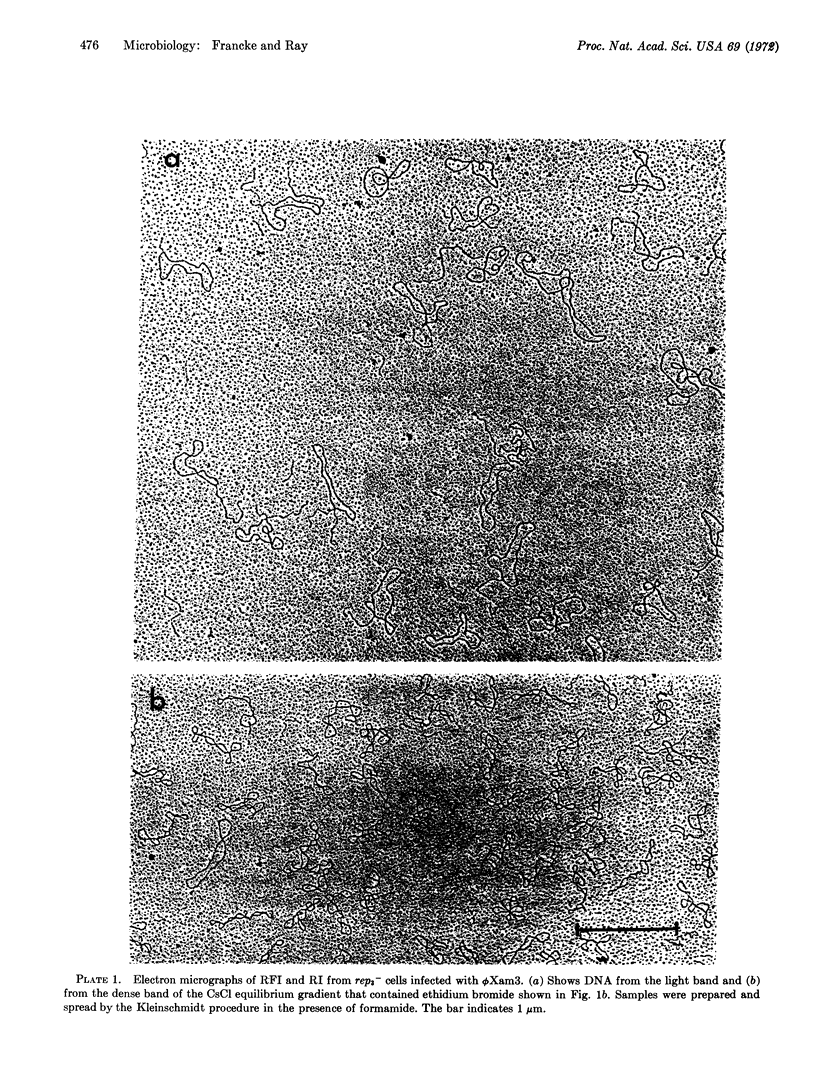
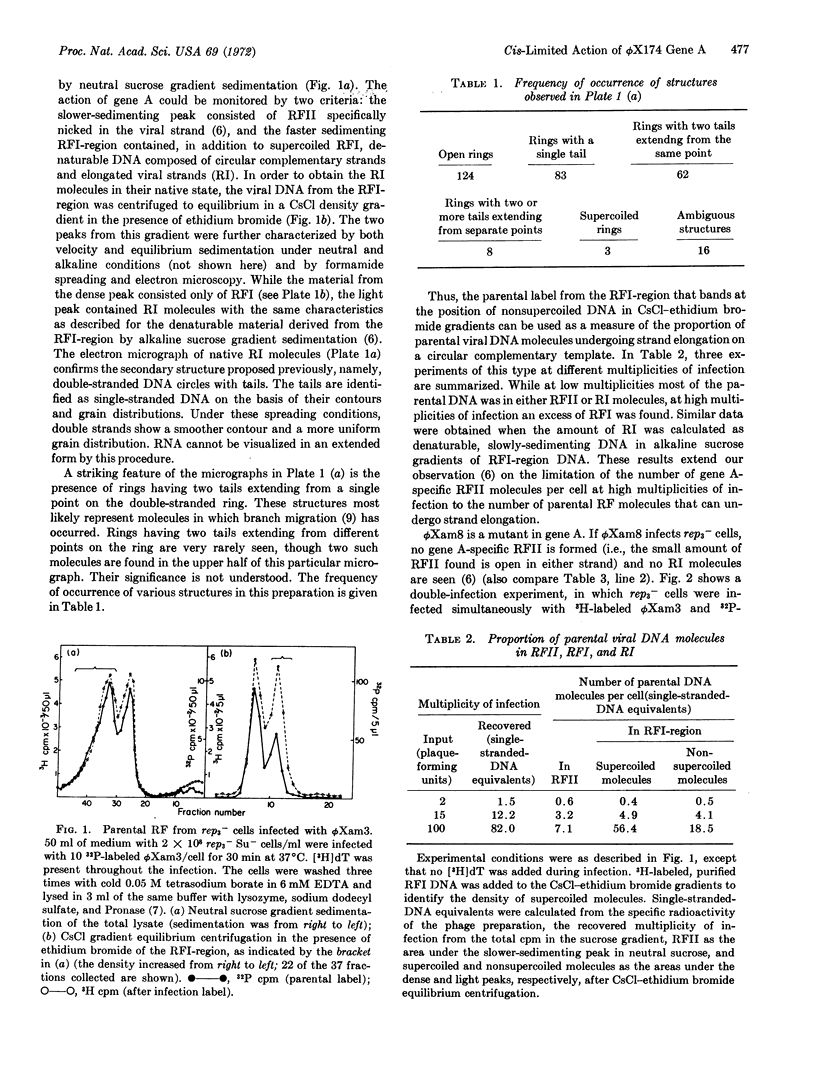
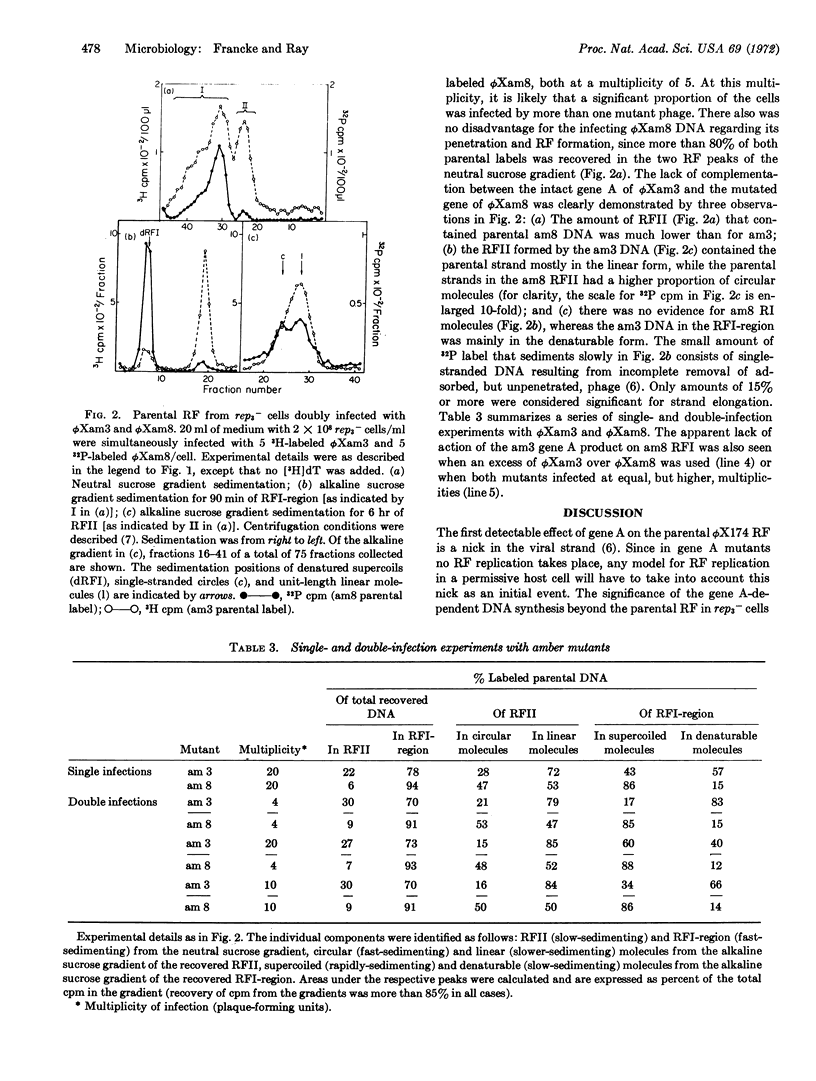
Parental replicative-form (RF*) DNA of bacteriophage ϕX174 in a replication-deficient host cell (rep3-) exhibits two characteristic features that correlate the function of viral gene A with the initiation of viral DNA replication: a specific discontinuity in the viral strand of a constant number of RF molecules and elongation of the viral strand to yield replicative-intermediate DNA forms with single-stranded tails. At high multiplicities of infection, these initiation events are limited to an average of four specifically nicked RFII molecules per cell. The limiting factor from the host cell may be related (or identical) to the essential bacterial sites known to limit the participation of parental genomes in RF replication. Double-infection experiments with wild-type phage and phage carrying an amber mutation in gene A show that the formation of gene A-specific RFII and RI is cis-limited to only the wild-type DNA. These results provide a basis at the DNA level for the known asymmetric complementation of gene A.
Keywords: E. coli, electron microscopy, cis-acting protein, specifically-nicked RF
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair D. G., Clewell D. B., Sheratt D. J., Helinski D. R. Strand-specific supercoiled DNA-protein relaxation complexes: comparison of the complexes of bacterial plasmids ColE1 and ColE2. Proc Natl Acad Sci U S A. 1971 Jan;68(1):210–214. doi: 10.1073/pnas.68.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Dressler D. H., Hathaway A. THE ABORTIVE REPLICATION OF PhiX174 DNA IN A RECOMBINATION-DEFICIENT MUTANT OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):813–820. doi: 10.1073/pnas.57.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Fate of parental phi X174-DNA upon infection of starved thymine-requiring host cells. Virology. 1971 Apr;44(1):168–187. doi: 10.1016/0042-6822(71)90163-2. [DOI] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Formation of the parental replicative form DNA of bacteriophage phi-X174 and initial events in its replication. J Mol Biol. 1971 Nov 14;61(3):565–586. doi: 10.1016/0022-2836(71)90065-9. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Goebel W., Helinski D. R. Nicking activity of an endonuclease. I. Transfer ribonucleic acid complex of Escherichia coli. Biochemistry. 1970 Nov 24;9(24):4793–4801. doi: 10.1021/bi00826a025. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Davis R. W., Davidson N. A physical study by electron microscopy of the terminally reptitious, circularly permuted DNA from the coliphage particles of Escherichia coli 15. J Mol Biol. 1970 Feb 28;48(1):1–22. doi: 10.1016/0022-2836(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXV. Studies with bacteriophage phiX174 mutants blocked in progeny replicative form DNA synthesis. J Mol Biol. 1969 Feb 14;39(3):619–639. doi: 10.1016/0022-2836(69)90149-1. [DOI] [PubMed] [Google Scholar]
- Loos L. J., Salivar W. O. The replication of bacteriophage phi-X174 in a mutant of Escherichia coli strain C. I. Characterization of the physiological defect in viral reproduction. Virology. 1971 Mar;43(3):541–553. doi: 10.1016/0042-6822(71)90279-0. [DOI] [PubMed] [Google Scholar]
- Loos L. J., Salivar W. O. The replication of bacteriophage phi-X174 in a mutant of Escherichia coli strain C. II. Evidence that the C-SM17ts mutation is not at the rep locus. Virology. 1971 Mar;43(3):554–560. doi: 10.1016/0042-6822(71)90280-7. [DOI] [PubMed] [Google Scholar]
- TESSMAN E. S. COMPLEMENTATION GROUPS IN PHAGE S13. Virology. 1965 Feb;25:303–321. doi: 10.1016/0042-6822(65)90208-4. [DOI] [PubMed] [Google Scholar]
- Tessman E. S. Mutants of bacteriophage S13 blocked in infectious DNA synthesis. J Mol Biol. 1966 May;17(1):218–236. doi: 10.1016/s0022-2836(66)80104-3. [DOI] [PubMed] [Google Scholar]
- Yarus M. J., Sinsheimer R. L. The process of infection with bacteriophage phiX174. 8. Evidence for an essential bacterial "site". J Virol. 1967 Feb;1(1):135–144. doi: 10.1128/jvi.1.1.135-144.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]




