Abstract
The model genetic organism Drosophila melanogaster, commonly known as the fruit fly, uses many of the same neurotransmitters as mammals and very similar mechanisms of neurotransmitter storage, release and recycling. This system offers a variety of powerful molecular-genetic methods for the study of transporters, many of which would be difficult in mammalian models. We review here progress made using Drosophila to understand the function and regulation of neurotransmitter transporters and discuss future directions for its use.
Keywords: Drosophila, neurotransmitter transporter, vesicular transporter, VMAT, VGAT, VGLUT, VAChT, EAAT, DAT, SERT, GAT, inebriated, SLC6, SLC1, SLC18, SLC17, dopamine, serotonin, GABA, octopamine, glutamate, portabella, acetylcholine, ChT1, VNUT
Introduction
Several features of the model genetic system Drosophila melanogaster make it attractive for the study of neurotransmitter transporters. These include a powerful molecular-genetic toolset, a short lifespan, low cost and the availability of an essentially limitless supply of “test subjects”. Here we provide an overview of current studies on neurotransmitter transporters in Drosophila and discuss potential uses for this system in the future. By including additional background, we hope to provide a general introduction to the field for both Drosophilists and non-Drosophilists alike. Conversely, for “fly people” with other primary interests, we also provide an overview of neurotransmitter transporters in general and some of the outstanding questions that remain unanswered.
Neurotransmitter Transporters
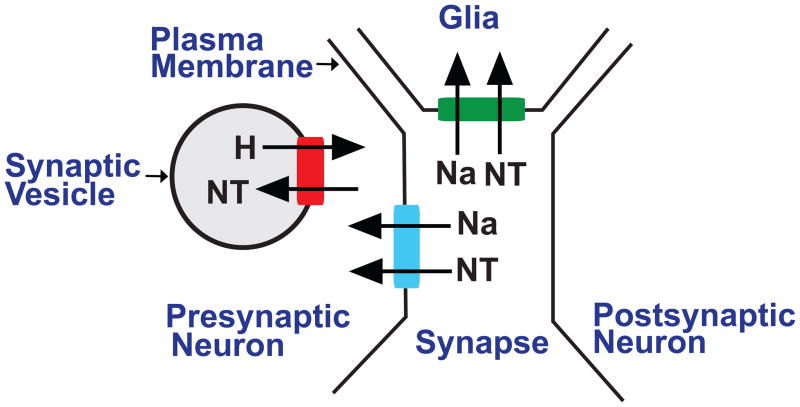
Neurotransmitter transporters are responsible for the movement across biological membranes of classical neurotransmitters-- the biogenic amines and acetylcholine-- and the amino acid neurotransmitters-- GABA, glutamate and glycine. Plasma membrane and vesicular neurotransmitter transporters represent two distinct activities (Figure 1). Plasma membrane neurotransmitter transporters are responsible for the termination of synaptic transmission and recycling neurotransmitters after they are released (Blakely and Edwards, 2012) (Figure 1).
Figure 1. Vesicular and plasma membrane neurotransmitter transporters.
Vesicular and plasma membrane neurotransmitter transporters differ in several respects, including their localization and bioenergetics. In general, vesicular transporters localize to secretory vesicles, including synaptic vesicles in the presynaptic neurons that synthesize and release neurotransmitter. Depending on their subtype (see text) plasma membrane transporters may localize to either presynaptic neurons or surrounding glia. The movement of neurotransmitter via vesicular and plasma membrane transporters are coupled to proton and sodium gradients respectively. Vesicular transporters move protons (H) and neurotransmitter (NT) in opposite directions (antiport), using the high concentration of lumenal protons to drive transport. At the plasma membrane, sodium (Na) and neurotransmitters move in the same direction (symport).
Vesicular neurotransmitter transporters localize to the membranes of secretory vesicles and are responsible for transport and storage of neurotransmitters into the vesicle lumen (Blakely and Edwards, 2012) (Figure 1). Vesicular transporters are required for the storage of neurotransmitters in synaptic vesicles (SVs) as well as large dense core vesicles (LDCVs), which also store and release peptide neurotransmitters (Fei et al., 2008). However, peptides are not transported into LDCVs via vesicular transporters, but rather packaged into the lumen of the vesicle as it is being formed (Dikeakos and Reudelhuber, 2007). Peptides also do not undergo transport at the plasma membrane. Similarly, “novel” neurotransmitters such as nitrous oxide do not require specific transport proteins since they are synthesized on demand and pass relatively freely through lipid membrane barriers (Boehning and Snyder, 2003). It remains unclear whether lipid-based signaling molecules such as anandamide require specific transporters for movement across biological membranes (Fowler, 2013).
All known plasma membrane and vesicular transporters are members of the Solute Carrier (SLC) family of proteins and share several characteristic features (Blakely and Edwards, 2012). All contain multiple transmembrane domains and are responsible for moving relatively small (~75–200 Daltons) hydrophilic molecules across lipid membranes. As “active” transporters, all use energy to drive the movement of neurotransmitter against a concentration gradient, and as “secondary” active transporters, the energy is supplied by an ion gradient rather than by direct hydrolysis of ATP. By contrast, the activities of “facilitated” transporters allow solute movement down a concentration gradient and do not require additional energy (e.g. (Richter and Hargreaves, 2013)), while primary active transporters directly hydrolyze ATP (e.g. (Dermauw and Van Leeuwen, 2014)
At the plasma membrane, transporters exploit the steep sodium gradient experienced by most eukaryotic cells. The sodium gradient drives the movement of the transporter and is coupled to neurotransmitter flux in a stoichiometrically defined manner, and in some cases with additional ions facilitating transport (Grewer et al., 2014; Kanai et al., 2014; Pramod et al., 2013; Rudnick et al., 2014). Since both sodium and the neurotransmitter move in the same direction at the plasma membrane, this is by definition a “symport” mechanism. Vesicular transporters use a distinct but related “antiport” mechanism in which a proton gradient is used to drive the movement of neurotransmitter in the opposite direction and into the lumen of the secretory vesicle (Parsons, 2000) (Figure 1).
The manner in which the transporters couple the movement of ions and neurotransmitter is not yet fully understood, but biochemical experiments and more recent crystallographic and modeling studies have begun to unravel the underlying mechanisms (Penmatsa and Gouaux, 2014; Zhao et al., 2011). Plasma membrane transporters may also function in an uncoupled or loosely coupled mode characterized by relatively large ionic currents (Fairman et al., 1995; Galli et al., 1997; Schicker et al., 2013). The potential function of these currents in synaptic physiology is not yet clear. Efflux represents an additional functional mode in which neurotransmitter moves out of rather than into the cell (Fleckenstein et al., 2007; Sulzer, 2011). The mechanism by which this occurs is a very active area of investigation, and includes work in Drosophila described in more detail below (Pizzo et al., 2013; Pizzo et al., 2012).
Vesicular transporters generally localize to secretory vesicles in the presynaptic neurons that synthesize and release neurotransmitter (Blakely and Edwards, 2012). Plasma membrane transporters for biogenic amines also localize to presynaptic neurons (Blakely and Edwards, 2012). By contrast plasma membrane glutamate and GABA transporters may be expressed in either neurons or glia (Figure 1) depending on their subtype (Zhou and Danbolt, 2013). We discuss the localization of specific Drosophila transporters below.
A circumscribed set of phylogenetic families encodes the plasma membrane and vesicular transporters (see Tables 1 and 2). Glutamate or Excitatory Amino Acid Transporters (EAATs) are members of SLC1; this family also contains neutral amino acid transporters (Grewer et al., 2014; Kanai et al., 2014; Sheldon and Robinson, 2007; Yang et al., 2009). All other known plasma membrane transporters, including those for GABA and the biogenic amines, are members of SLC6 (Pramod et al., 2013; Rudnick et al., 2014). SLC6 also includes a number of amino acid transporters, some of which are specific for insects, as well as a few remaining “orphans”, whose substrate is not known (Boudko, 2012; Boudko et al., 2005; Caveney et al., 2006). Acetylcholine is hydrolyzed before reuptake and there is no plasma membrane acetylcholine transporter. Rather, following ACh hydrolysis, choline is transported into the presynaptic neuron by a high affinity choline transporter, a member of SLC5 (Ferguson et al., 2004; Ribeiro et al., 2006).
Table 1. Mammalian and Drosophila vesicular transporters.
In both mammals and flies, known vesicular transporters are members of the SLC17, 18 or 32 subfamilies. See text for details.
| SLC17 | SLC18 | SLC32 | |||
|---|---|---|---|---|---|
| Mammals | Drosophila | Mammals | Drosophila | Mammals | Drosophila |
| VGLUT1 | dVGLUT | VMAT1 | dVMAT | VGAT | dVGAT |
| VGLUT2 | VMAT2 | ||||
| VGLUT3 | VAChT | dVAChT | |||
| VNUT (SLC17A9) | CG15438 | prt | |||
Table 2. Mammalian and Drosophila plasma membrane transporters.
In both mammals and flies, known plasma membrane neurotransmitter transporters are members of the SLC1 and 6 subfamilies. As described in the text, dEAAT2 may not function as a glutamate transporter. The choline transporter is a member of SLC5, and although it is not a neurotransmitter transporter per se, it is responsible for reuptake of choline into the presynaptic neuron after acetylcholine is metabolized in the synapse. The predicted fly gene CG11880 appears to be most similar to mammalian choline transporters, but has not been characterized. CG5549 is listed as a putative glycine transporter based on its similarity to mammalian glycine (and proline) transporters, but its function is not known; it also remains unclear whether glycine functions as a neurotransmitter in the fly. The gene CG7075, recently named Neurotransmitter Transporter-Like (Ntl) is also a putative glycine transporter but found only in male germline cells (Chatterjee et al., 2011). SLC6 also includes amino acid transporters required for nutrient uptake and several additional members whose functions are not known (not shown) as well as inebriated, which may transport a metabolite of histamine (see text).
| SLC1 | SLC6 | (SLC5) | |||
|---|---|---|---|---|---|
| Mammals | Drosophila | Mammals | Drosophila | Mammals | Drosophila |
| EAAT1 |
dEAAT1 dEAAT2 |
DAT | dDAT | ChT1 | CG11880? |
| EAAT2 | SERT | dSERT | |||
| EAAT3 | GAT1 | dGAT | |||
| EAAT4 | GAT2 | ||||
| EAAT5 | GAT3 | ||||
| GlyT1 | (CG7075) CG5549? | ||||
| GlyT2 | |||||
| NET | |||||
| (Inebriated) | |||||
Vesicular transporter subfamilies include SCL32, of which VGAT is the lone member (Schioth et al., 2014). SLC18 includes vesicular transporters for the biogenic amines and acetylcholine (Lawal and Krantz, 2013) and SLC17 includes the vesicular glutamate transporters as well as other transporters with divergent functions such as organic anion uptake at the plasma membrane (Reimer, 2013). Another member of SLC17 was recently identified in mammals as a vesicular ATP or nucleotide transporter (VNUT) (Sawada et al., 2008); a possible VNUT ortholog is present in the fly genome but has not yet been characterized (see Table 1).
Current Research Topics
Current research on neurotransmitter transporters includes investigations into the precise mechanisms by which they move solutes across membranes. Although some of the binding sites for ions and the neuorotransmitters have been mapped, much remains unclear. At present, we primarily have a static structural view of the binding sites, with only modeling able to capture the moment-to-moment movement of substrates (Penmatsa and Gouaux, 2014; Zhao et al., 2011). A surprisingly large number of commonly used drugs are known to bind to plasma membrane neurotransmitter transporters, yet their mechanism of action also remains incompletely understood. Many currently used antidepressants are Strongly Serotonergic Reuptake Inhibitors (SSRIs; e.g. fluoxetine/Prozac) and primarily inhibit the serotonin transporter (SERT), while others target the noradrenaline transporter (NET) or both NET and SERT (Hamon and Blier, 2013). Most psychostimulants, including cocaine, amphetamines and MDMA/ecstasy also interact with neurotransmitter transporters acting either as inhibitors or, in the case of amphetamines, to promote efflux (Fleckenstein et al., 2007; Sulzer, 2011). Importantly, drugs that act to block transport use a mechanism different from those that cause efflux and the nature of these differences remains a very important area of investigation (Fleckenstein et al., 2007; Foster et al., 2013; Kahlig et al., 2005; Sulzer, 2011). The downstream effects of both antidepressants and psychostimulants and the way they exert their therapeutic and/or addictive effects remains obscure. The developmental regulation of transport function and the potential effects of pharmacologically disrupting this activity is another fascinating topic (Daubert and Condron, 2010). The rapid development of the fly and the relative simplicity of its nervous system may be advantageous for these studies (Daubert and Condron, 2010).
A number of current investigations focus on the interplay between transporters and regulatory pathways. Transporters are essential elements of a network of interacting genes required for neurotransmitter homeostasis and behavior (see e.g. (Talkowski et al., 2008)), and a few elegant biochemical studies have highlighted direct protein-protein interactions and relatively direct regulatory processes (Bauer et al., 2012; Binda et al., 2008; Brunk et al., 2006; Sager and Torres, 2011; Winter et al., 2005; Yang et al., 2009). However, compared to our knowledge of the complex web of proteins implicated in receptor-mediated signaling, our understanding of transporter interactions and their regulation is limited.
Pathways critical for regulating the function of neurotransmitter transporters include those required for trafficking within the cell. Localization of vesicular transporters to different subsets of secretory vesicles has the potential to dramatically influence the site and amount of presynaptic neurotransmitter release. Some of the signals and machinery responsible for this process have been determined, but much remains unclear (Colgan et al., 2007; Foss et al., 2013; Grygoruk et al., 2010; Hua et al., 2011; Santos et al., 2013; Yao and Hersh, 2007). Current questions include the physiological function of vesicular transporter co-localization to the same vesicles in some cells. This phenomenon has important ramifications for both the possible co-release of neurotransmitter as well as the regulation of the electrochemical gradients that drive transport activity (Guzman et al., 2011; Hnasko and Edwards, 2011). Drosophila VMAT and VGLUT are co-expressed in at least some nerve terminals (Greer et al., 2005) but co-release has not been investigated in the fly. The trafficking of plasma membrane transporters through the secretory pathway and their regulated internalization is a particularly active and exciting area of study, and a number of the intrinsic signals required for internalization as well as the protein kinases that regulate these processes have been identified (Bauer et al., 2012; Chen et al., 2013b; Gabriel et al., 2013; Madsen et al., 2012; Sakrikar et al., 2012; Vina-Vilaseca and Sorkin, 2010). The increasingly refined understanding of these events may help determine the mechanism of action of psychostimulants, as well as providing fundamental information about synaptic physiology.
Some research questions are particularly well suited to fly models. These include structure-function analyses in vivo and the relationship between transporter function and disease processes. Variation in transporter expression may function as markers for disease and in some cases may play a pathogenenic role (Hahn and Blakely, 2007; Lawal and Krantz, 2013; Yang et al., 2009). In addition, risk alleles for human disease have been associated with some neurotransmitter transporters (Adamczyk et al., 2011; Barwick et al., 2012; Hahn and Blakely, 2007; Hamilton et al., 2013; Jen et al., 2005; Mazei-Robison et al., 2008; Veenstra-VanderWeele et al., 2012), however the mechanisms by which these variants cause downstream phenotypic changes remains poorly understood. More generally, it remains unclear how variations in transport activity or regulatory processes may effect synaptic transmission and behavior. The powerful molecular genetic tools available in the fly coupled with their surprisingly complex array of behaviors provide an excellent platform to address these questions.
Although we focus here on Drosophila, many of the same principles apply to C. elegans, an equally important invertebrate model with a similarly large array of powerful molecular and genetic tools. As highlighted below, comparative studies in other insects including locusts, bees and moths represent another valuable source of information about the function and regulation of neurotransmitter transporters.
A Brief Fly Neurobiology Primer
To discuss the molecular genetics of fly transporters it is useful to first briefly describe a few characteristics of fly neurobiology and terms that may be obscure to those outside the Drosophila research community. After fertilization and egg-laying, embryos develop rapidly over the course of 24 hours, through 20 relatively distinct stages (Hartenstein and Wodarz, 2013; Menon et al., 2013). After hatching from the egg, larvae mature over ~5 days through three successive stages known as instars. Following a ~5 day pupal stage, the adult or “imago”, ecloses from the pupa. A dramatic reorganization of the nervous system and body plan occurs during pupation and includes the addition of adult-specific organs from discreet packets of stem cells known as imaginal discs. Both neurons and glia are present in both larval and adult nervous systems. These include cells that make up the glial sheath, which surrounds the nervous system at both stages and, acts as both a diffusion barrier and a site for active xenobiotic removal, analogous to the blood brain barrier (BBB) in mammals (DeSalvo et al., 2011; Mayer et al., 2009). The BBB and the glial sheath also express a similar complement of transporters for sugars, mono carboxylic acids and neutral amino acids, supporting the idea that they serve similar functions (R. Bainton, personal communication).
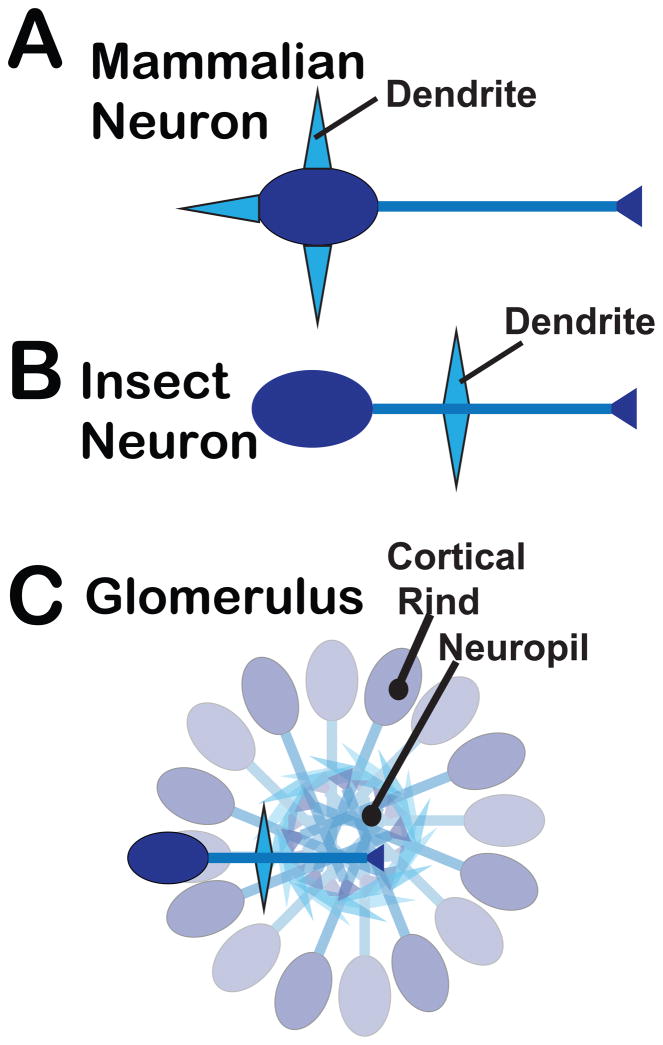
The structure of most neurons in flies and other insects differs somewhat from their canonical form in mammals. In flies, a single process emanates from the soma and gives rise to both dendrites and axons. This structure facilitates the organization of insect neurons into glomeruli or ganglia in which both axons and dendrites project into a central neuoropil, with the somata forming a surrounding cortical rind (Figure 2). Fly neurons support action potentials and calcium mediated release at the nerve terminal similar to mammals, but most terminals in the fly possess a characteristic presynaptic specialization known as a T-bar, around which synaptic vesicles (SVs) cluster. Large dense core vesicles (LDCVs), which release aminergic neurotransmitters and neuropeptides do not cluster near T-bars and few if any T-bars are found in terminals that contain primarily LDCVs either centrally (Karsai et al., 2013) or peripherally (Atwood et al., 1993; Jia et al., 1993).
Figure 2. The morphology and organization of mammalian and insect neurons.
A canonical mammalian (A) and insect neuron (B) are indicated, highlighting differences in the location of dendrites: on the cell body of mammalian neurons versus a single process emanating from the soma of insect neurons that also gives rise to the axon. This morphology allows the characteristic organization of insect neurons into glomeruli (C), or larger but similarly organized ganglia, which include a cortical rind and neuropil as indicated.
Neuroanatomy
A complete description of fly neuroanatomy is beyond the scope of this review. However, a brief description of some particularly salient features may be useful for understanding the effects of mutations in neurotransmitter transporters. Although variation within individual cell clusters exist (Sykes et al., 2004), neuronal somata and processes are generally localized to stereotypic positions. The embryonic and larval central nervous systems include the segmented ventral nerve cord and the bulbous, anterior structure variously referred to as “the larval brain,” or the “fused subesophageal and supraesophageal ganglia”. Since the central nervous systems of both the larva and adult are small (on the order of 100 microns), most immunolabeling studies employ confocal imaging of the entire brin. Another common preparation for immunolabeling and electrophysiological studies is the larval fillet, which exposes the abdominal musculature and the neuromuscular junction (NMJ) (Jan and Jan, 1976).
Excellent resources for learning the neuroanatomy of the adult fly are available online http://www.virtualflybrain.org/site/vfb_site/home.htm. Here we describe a few well-characterized structures that may be encountered by the reader in studies of neurotransmitter transporters (Figure 3). These include the optic ganglia (lamina, medulla, lobula and lobula plate) (Melnattur and Lee, 2011; Menon et al., 2013) and the antennal lobes (a critical component of the olfactory system) (Masse et al., 2009; Mu et al., 2012; Wilson, 2013). The function and anatomy of two anatomically distinct structures in the “central brain” have been extensively studied: the mushroom bodies (MBs) shown to be critical for learning, memory and the integration of sensory information (Heisenberg, 2003), and the central complex (including the fan shaped body, ellipsoid body, protocerebral bridge and noduli), important for the coordination of movement (Strauss, 2002) and an increasing number of other behaviors (Lebestky et al., 2009; Liu et al., 2012a; Seelig and Jayaraman, 2013; Ueno et al., 2012).
Figure 3. The neuroanatomy of the adult fly brain.
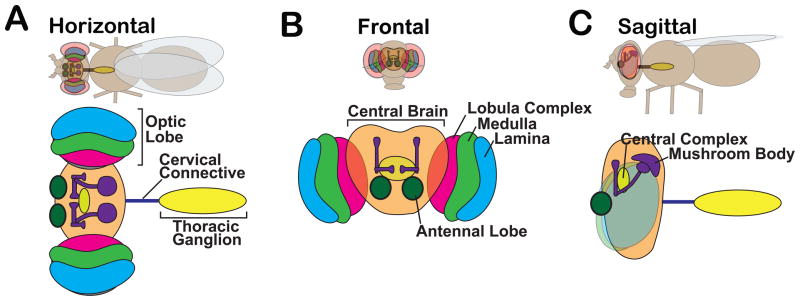
Cartoons show (A) horizontal, (B) frontal and (C) sagittal views of the fly and corresponding diagrams of the central nervous system. For simplicity, structures are labeled in one panel but color-coded to allow identification in all panels. Labeled structures include the central brain (orange), bilaterally symmetric optic lobes, the thoracic ganglion (yellow) and the cervical connective (blue), which contains both ascending and descending fibers. Ganglia within the optic lobes include the lamina (blue) and medulla (green) both of which receive projections from photoreceptors in the retina. Cells in both the lamina and medulla project to the lobula and lobula plate, indicated here as the lobular complex (pink). Major structures within the central brain include the mushroom bodies (purple), required for olfactory learning and memory and the central complex (yellow ellipsoid), which regulates some motor behaviors.
A Brief Fly Genetics Primer
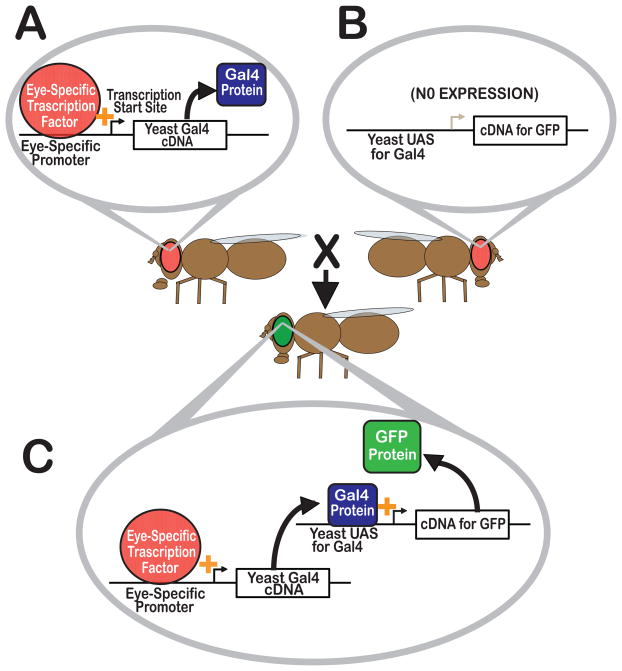
The growing number of molecular-genetic tools available in Drosophila are described in detail elsewhere (Bier, 2005; Frank et al., 2013). Here we provide a brief description of the Gal4/UAS expression system (Brand and Perrimon, 1993), a tool used in a number of studies on neurotransmitter transporters (see below). In the Gal4/UAS system, a promoter sequence of choice drives expression of the yeast-derived Gal4 transcriptional activator (Figure 4) (Brand and Perrimon, 1993). The Gal4 protein binds to its cognate Upstream Activating Sequence (UAS) to express a cDNA of choice (e.g. for GFP, Figure 4). Expression of the Gal4 protein in a specific temporal and spatial pattern dictated by the chosen promoter will thus express the transgene in a highly specific manner. RNAi interference constructs can be similarly expressed in specific cell types using this system. The bipartite nature of the system allows any lines containing a Gal4 “driver” transgene to be paired with any other line containing a UAS transgene. Thousands of Gal4 and UAS lines are available including those representing specific neurotransmitter systems. These include dVGLUT-Gal4 (Daniels et al., 2008), dVGAT-Gal4 (Fei et al., 2010), TH-Gal4 (Friggi-Grelin et al., 2003), Tdc2-Gal4 (Cole et al., 2005), TrH-Gal4 (Alekseyenko et al., 2010; Park et al., 2006) and Ddc-Gal4 (Li et al., 2000) for glutamate, GABA, dopamine, serotonin, octopamine+tyramine, and dopamine+serotonin neurons respectively.
Figure 4. The Gal4/UAS System.
The bipartite Gal4/UAS System employs separate transgenes to (A) drive expression and (B) allow specific cDNAs to be expressed, e.g. for Green Fluorescent Protein (GFP) as shown here. The Gal4 “driver” consists of a cell or tissue specific promoter e.g. for cells in the eye as shown in (A) immediately upstream of the cDNA encoding the yeast Gal4 transcription factor. Mating these lines results in a fly that contains both the Gal4 and UAS transgenes (C). In these progeny, Gal4 is expressed and binds to its UAS, which in turn drives expression of GFP in cells defined by the Gal4 promoter.
Additional, specialized UAS transgenes may be used to either activate or disrupt the function of specific populations of cells. For example, the fly ortholog of dynamin, shibire (shi), is required for endocytosis as well as other vesicle budding events (Kitamoto, 2001; Koenig and Ikeda, 1989; Poodry et al., 1973). The temperature sensitive (ts) mutant shits can be shifted to a non-permissive temperature to block endocytosis and deplete the pool of SVs (Kitamoto, 2001). Another useful transgene encodes tetanus toxin (TNT), which cleaves SNARE proteins and thus blocks exocytosis (Frank et al., 2013). Optogenetic and temperature sensitive probes capable of activating or inactivating electrical activity in subpopulations of neurons are also available (Frank et al., 2013). Importantly, both optogenetic probes and TNT will affect exocytosis of both SVs as well as LDCVs, the latter containing both classical and peptide neurotransmitters. By contrast, transgenes or mutations that alter the function of neurotransmitter transporters or synthetic enzymes do not share this potentially confounding effect. Genetic reagents based on neurotransmitter transporters may become increasingly useful to specifically assess the affects of amines, GABA and glutamate in the fly “connectome.”
Thousands of mutations and useful chromosomal abnormalities are available in the fly (St Johnston, 2013). Remarkably, many of the first reported Drosophila mutants such as white are still in use. Point mutations have been traditionally generated using chemical mutagens such as the DNA alkylating agent Ethyl Methyl Sulfate (EMS) (St Johnston, 2013). Inversions, duplications and larger deletions have been traditionally generated using X-rays. More recently, mutations as well as small deletions have been generated using mobile, transposable elements. Several types of mobile elements have been used, including “P” elements, the first mobile elements to be identified in the fly (Bachmann and Knust, 2008; Engels, 1992). Mobile elements can cause mutations either by “hopping” into a gene, or by hopping out. Hopping out can be precise, leaving no trace of the insertion. Mutations are generated when transposons hop out via “imprecise excision”, either removing a portion of genomic DNA or leaving behind a portion of the transposable element. Of note, some types of mobile elements only generate precise excisions, and mutations can be generated only when they hop into a gene. Knock-out technology is also available in the fly (Rong and Golic, 2000), but the large number of existing mutations (and perhaps newer technologies such as CRISPR/Cas9 (Gratz et al., 2013)) may alleviate the need for de novo knockouts.
The nomenclature for Drosophila mutations is somewhat idiosyncratic. Alleles with no detectable function are labeled nulls whereas mutants that possess small or large amounts of residual activity are known as strong and weak “hypomorphs” respectively (Muller, 1932). The historic precedent of using the phenotype of the mutation to name the gene can also be confusing to those more familiar with other genetic systems. In the current molecular age, it may seem odd that a gene required for the transport of red and brown eye pigments is named white.
Drosophila Neurotransmitters and Their Metabolism
Drosophila use many of the same neurotransmitters as mammals including GABA, glutamate, acetylcholine and some of the same monoamines including dopamine, serotonin and histamine. Similar to mammals, GABA is synthesized via Glutamic Acid Decarboxylase (GAD) and GABA functions as an inhibitory transmitter (Lee et al., 2003). Ach is synthesized from choline and an activated acetyl moiety via Choline Acetyl Transferase (ChAT) (Kitamoto et al., 1998). The genomic organization of the cholinergic locus is phylogenetically conserved, with the vesicular acetylcholine transporter (VAChT) contained within an intron of ChAT (Kitamoto et al., 1998). Both glutamate and acetylcholine act as excitatory transmitters, but unlike mammals, glutamate rather than ACh is released at the fly NMJ (Jan and Jan, 1976). In addition, glutamate in flies can also function as an inhibitory transmitter through activation of a glutamate-gated chloride channels (Liu and Wilson, 2013; Rohrbough and Broadie, 2002). Unlike mammals, flies and other insects do not appear to further process dopamine to noradrenalin or adrenalin, but rather use the structurally similar neurotransmitters tyramine and octopamine (OA) (Roeder, 2005), both classified as trace amines in mammals (Borowsky et al., 2001). OA and tyramine contain one rather than two hydroxyl (OH) groups on the phenyl ring and thus are classified as phenolamines rather than catecholamines. Unlike DA, synthesis of OA and tyramine does not employ tyrosine hydroxylase or Dopa decarboxylase. Rather, both are converted from tyrosine by Tyrosine decarboxylase (Tdc) (Cole et al., 2005) and in case of OA, an additional hydroxylation step catalyzed by Tyramine β Hydroxylase (TβH) (Monastirioti et al., 1996).
Neurotransmitter metabolism in the fly has not been documented as thoroughly as in mammals. In particular, the role of monoamine oxidation remains unclear. Although a monoamine oxidase (MAO) homolog is absent from the fly genome (Roelofs and Van Haastert, 2001), MAO-like activity has been reported (Chaudhuri et al., 2007; Wang et al., 2011) and some drugs active as MAO inhibitors in mammals also show activity in the fly (Yellman et al., 1997).
Unlike mammals, conjugation to β-alanine plays a role in histamine and dopamine metabolism in the fly. Interestingly, some of same biosynthetic and conjugation pathways used for cuticle formation and pigmentation are also used in neurotransmitter synthesis and degradation (Wright, 1987). One of the earliest identified fly mutants was ebony, which generates a darker cuticle than wild type (Wittkopp et al., 2003). Ebony has since been shown to encode a β-alanyl-dopamine synthetase and to include both histamine and DA as substrates (Borycz et al., 2002; Hovemann et al., 1998; Richardt et al., 2002). In addition to metabolism of DA in the cuticle, ebony may also metabolize DA released as a neurotransmitter in the central nervous system (Suh and Jackson, 2007). In the visual system, ebony conjugates histamine to alanine to generate carcinine (Borycz et al., 2002). Since ebony is expressed in glia, histamine and its metabolites are presumably transported in and out of both glia and neurons, but the details of this process are poorly understood (Edwards and Meinertzhagen, 2010).
Based on the localization of the plasma membrane transporters, exocytotically released glutamate and GABA are likely to be taken up primarily into glia in flies, similar to the prominent role of glia in recycling glutamate and GABA in mammals (see below). Localization of DA and 5HT plasma membrane transporters on aminergic neurons suggests, that as is in mammals, these amines are recycled in part into presynaptic cells, although other, less specific transport pathways for amines are also possible in flies as in mammals (Daws, 2009).
Drosophila Vesicular Neurotransmitter Transporters
dVAChT
VAChT was the first vesicular neurotransmitter transporter to be molecularly identified in the fly (Kitamoto et al., 2000). dVAChT mutants include dVAChT1 which is embryonic lethal, and the weaker allele dVAChT2 which survives through the second larval stage but locomotes slower than wild type animals (Kitamoto et al., 2000). Similar to VAChT knock-down mice, heterozygous dVAChT mutants survive to adulthood and show subtle phenotypic differences from controls. In particular, electrophysiological analysis of adult dVAChT heterozygotes suggest that during periods of sustained vesicle release, at least one circuit in the adult CNS fails to maintain normal levels of ACh release (Kitamoto et al., 2000). This phenomenon may be related to changes in ACh release observed during periods of sustained release at the cholinergic NMJ of frogs (Naves and Van der Kloot, 1996; Van der Kloot, 2003) and in VAChT knock-down mice (Lima et al., 2010; Rodrigues et al., 2013). The mechanisms underlying these changes may include the presence of variable numbers of VAChT molecules per SV and a consequent reduction in the amount of transmitter stored in each vesicle (Prado et al., 2013). In addition, it is likely that under conditions of reduced VAChT expression, some vesicles are essentially empty (Prado et al., 2013). The contribution of each mechanism may vary depending on the rate of SV recycling and remains an outstanding unanswered question (Prado et al., 2013).
dVGLUT
Prior to its molecular characterization, biochemical studies suggested vesicular glutamate transport activity was present in the fly (Tabb and Ueda, 1991). Unlike mammals, which have three distinct VGLUT genes, Drosophila contains only a single VGLUT ortholog, dVGLUT (Daniels et al., 2004). dVGLUT is expressed in all glutamatergic neurons in the larva and adult fly including the glutamatergic motoneurons innervating the larval NMJ (Daniels et al., 2004; Daniels et al., 2008). The membrane topology of dVGLUT has been examined using a series of epitope tags predicted to reside on the lumenal and cytosolic loops between transmembrane domains as well as the cytosolic N- and C-termini (Fei et al., 2007). Deletion of the cytosolic C-terminus abrogates lethality caused by dVGLUT over-expression, clearly implicating an important functional role for this domain (Grygoruk et al., 2010) and some critical trafficking motifs reside in the C-termini of mammalian VGLUTs (Foss et al., 2013; Voglmaier et al., 2006). However, the baseline localization of dVGLUT to SVs appears to be surprisingly unaffected by deletion of the C-terminus (Grygoruk et al., 2010) and recent studies of mammalian VGLUTs have identified trafficking motifs in both the N- and C-termini (Foss et al., 2013; Voglmaier et al., 2006). It is possible that the contribution of specific domains to VGLUT trafficking could vary between cell types or between species.
Post-synaptic excitatory potentials representing individual vesicles (minis) have been used to examine neurotransmitter content in those vesicles capable of glutamate storage and release in embryonic hypomorphic dVGLUT mutants (Daniels et al., 2006). Mini frequency was significantly lowered, suggesting that empty vesicles can fuse with the plasma membrane, consistent with other mammalian studies. More surprisingly, the size of the remaining minis-- an indication of SV filling-- was not altered in the mutant in comparison to controls, thus suggesting that a reduced number of VGLUT molecules, and perhaps only one, may suffice to fill a vesicle under these conditions (Daniels et al., 2006). Using other techniques, recent estimates of VGLUT copies per vesicle in wild type mammalian preparations have ranged from 4 to 14 (Mutch et al., 2011; Takamori et al., 2006). One electrophysiological study in VGLUT1 knock-out mice suggested a reduction in mini size (Wojcik et al., 2004), while another did not (Fremeau Jr et al., 2004). Unlike flies, the presence of multiple VGLUT isoforms in mammals complicates these analyses (Takamori et al., 2006). Although there is no evidence for oligomerization of vesicular transporters, it is possible that a single functional unit is composed of multiple molecules. It also possible that multiple copies of vesicular transporters may be needed to increase the rate of filling during rapid recycling, with fewer copies sufficient for neurotransmitter release under some conditions (Blakely and Edwards, 2012). These questions remain an important topic in studies of vesicular transporters.
Over-expression of dVGLUT at high levels using relatively strong Gal4 drivers is larval lethal. Over-expression using a weaker driver allows survival through adulthood (Daniels et al., 2011). Interestingly, the surviving adults show large lacunae in their CNS, possibly the result of glutamate-mediated excitotoxicity (Daniels et al., 2011). Consistent with the observation that dVGLUT over-expression causes functional changes in SV homeostasis, electrophysiological studies of dVGLUT over-expression in larva show an increase in quantal size, as well as large spontaneous events at the larval NMJ (Daniels et al., 2004; Daniels et al., 2011), a phenotype similar to that seen with over-expression of vesicular transporters in mammals (Edwards, 2007; Wilson et al., 2005).
dVMAT
In contrast to the mammalian genome which contains two distinct VMAT genes (Liu et al., 1992), flies contains only one (Greer et al., 2005). The primary structures of dVMAT and mammalian VMATs are similar throughout the 12 predicted transmembrane domains, the regions likely to be responsible for substrate recognition and transport (Greer et al., 2005). Indeed, neurotransmitter substrate specificity and relative affinity of dVMAT are generally similar to mammalian VMATs. For example, dVMAT is also inhibited by the drug reserpine at sub-micromolar concentrations (Greer et al., 2005).
dVMAT encodes at least two splice variants, including dVMAT-A and –B which differ at their C-termini (Greer et al., 2005). dVMAT-A is expressed in both larvae and adults in all dopaminergic, serotonergic and octopaminergic cells (Greer et al., 2005). Neither dVMAT-A nor B appear to be expressed in the otherwise well-characterized histaminergic photoreceptor cells of the adult eye (Chang et al., 2006), and the vesicular transporter responsible for the storage of histamine in the SVs of histaminergic photoreceptors is not known. It also is not known whether dVMAT is used for DA storage in other non-neuronal tissue, such as “crystal cells”, which are present in Drosophila hemolymph (blood) and release DA for melanotic scab formation (Galko and Krasnow, 2004; Rizki and Rizki, 1984).
The cytosolic C-terminus of dVMAT-A is similar to mammalian orthologs, whereas the C-terminus of dVMAT-B is divergent (Greer et al., 2005). The C-terminus of dVMAT-A, but not dVMAT-B, also contains endocytosis motifs active in vitro in S2 cells and in vivo ((Greer et al., 2005; Grygoruk et al., 2010) and manuscript under review). Both dVMAT-A and B contain a large amino terminal domain that is not conserved in mammals (Greer et al., 2005). Interestingly, this region contains a number of polyproline motifs that could conceivably play a role in protein-protein interactions, but this has not yet been tested.
In contrast to dVMAT- A and other VMAT orthologs, dVMAT-B is expressed in glia rather than neurons, and more specifically, in a small subset of glia in the adult optic lobes where its function appears to include the storage of histamine (Romero-Calderón et al., 2008). Although mammalian glia likely take up amines via low affinity mechanisms (Dahlin et al., 2007; Yoshikawa et al., 2013), the expression of a specific amine transporter in glia is clearly unusual. Further studies of dVMAT-B may help determine the role of glia in the recycling of histamine and perhaps other biogenic amines.
The dVMAT mutant shows a number of behavioral deficits consistent with the loss of exocytotic amine release in the nervous system (Chen et al., 2013a; Simon et al., 2009). Similar to mouse knockouts, mutant tissue levels of amines are dramatically reduced, presumably due to the degradation of amines that are not sequestered in secretory vesicles (Fon et al., 1997; Simon et al., 2009). Under standard fly culture conditions, loss of dVMAT is lethal, but reducing the density of the culture results in 100% viability, thus allowing behavioral assays. dVMAT mutants show a dramatic decrease in baseline larval locomotion (Simon et al., 2009), mirroring previously demonstrated effects of reserpine (Pendleton et al., 2000). dVMAT mutants also show a reduced startle response, reduced male courtship (Simon et al., 2009) and increased sleep (Nall and Sehgal, 2013). Treatment of adult mutant flies with reserpine also increases sleep (Nall and Sehgal, 2013). In contrast, amphetamines decrease sleep in flies (Andretic et al., 2005), presumably via release of DA (Pizzo et al., 2013). In each case, changes in sleep were distinguished from changes in locomotion using previously established criteria (Hendricks et al., 2000; Shaw et al., 2000).
The Gal/UAS system was used to express dVMAT in subpopulations of aminergic neurons and thereby determine which aspects of the mutant phenotype result from loss of specific aminergic circuits (Chen et al., 2013a). Consistent with other reports on the function of OA signaling in flies and other insects (Adamo et al., 1995; Bacon et al., 1995; Fox et al., 2006; Koon et al., 2011; Lee et al., 2009; Monastirioti et al., 1996), expression of dVMAT in OA cells was required for rescue of baseline larval locomotion, female fertility, and the initial phase of the startle response (Chen et al., 2013a). In contrast, the later recovery phase of the startle response depends on serotonergic signaling since expression of dVMAT in 5HT cells rescued this deficit (Chen et al., 2013a). Male courtship could be rescued via expression in DA cells, consistent with recent studies using other genetic reagents to determine the function of DA circuits in the fly (Alekseyenko et al., 2010). The restoration of dVMAT function in smaller subsets of neurons in a null background may be used to map the identity of aminergic neurons responsible for other complex behaviors. Importantly, relatively broad Gal4 drivers can be used for these studies since, in general, dVMAT transgenes will only be active in cells in which amines are endogenously synthesized.
Current molecular-genetic techniques in Drosophila are particularly well-suited to in vivo over-expression studies, making it possible to determine how increased expression of a vesicular transporter may affect behavior. Flies over-expressing dVMAT in DA and 5HT cells (Li et al., 2000) survive through adulthood (Chang et al., 2006). The resulting changes in behavior, presumably caused by an increase in DA and 5HT release, include an increase in motor activity and a blunted behavioral response to cocaine (Chang et al., 2006). Similarly, administration of cocaine results in increased motor activity in the fly (Bainton et al., 2000; McClung and Hirsh, 1998; Torres and Horowitz, 1998). Similar to amphetamines (Andretic et al., 2005), over-expression of dVMAT in 5HT+DA cells also increases male courtship behavior (Chang et al., 2006).
Importantly, the effects of dVMAT and dVGLUT over-expression demonstrate that the electrophysiological changes previously seen with over expression of vesicular transporters in vitro (Pothos et al., 2000; Song et al., 1997; Wilson et al., 2005) can have significant downstream behavioral sequelae in an intact organism. This observation has important ramifications for drugs potentially able to increase vesicular transporter activity (see Future Directions below).
Over-expression of dVMAT as well as loss of function alleles have been used to explore the role of DA homeostasis in vivo in neurodegenerative processes relevant to Parkinson’s disease (PD) (Lawal et al., 2010; Sang et al., 2007). DA has a high oxidative potential and may also conjugate to proteins implicated in genetic forms of PD (Conway et al., 2001; Hastings et al., 1996). Thus, it has been suggested that an increase in VMAT activity may have neuroprotective effects (Guillot and Miller, 2009). This has been tested in vitro in cell cultures using mammalian VMAT and dVMAT (Mosharov et al., 2009; Park et al., 2007), and in vivo in the fly (Inamdar et al., 2013; Lawal et al., 2010; Sang et al., 2007). Over-expression of dVMAT rescues the loss of DA neurons caused by either a genetic or chemical insult (Inamdar et al., 2013; Lawal et al., 2010; Sang et al., 2007). Conversely, loss of dVMAT activity increases the toxicity of both genetic and chemical insults to DA cells (Inamdar et al., 2013; Lawal et al., 2010; Sang et al., 2007). Parallel experiments have not yet been reported in rodents. However, a mouse VMAT2 knockdown showed baseline loss of DA cells (Caudle et al., 2007) and some hVMAT2 alleles may decrease the risk of PD in patients (Glatt et al., 2006). It is therefore possible that over-expression of VMAT2, or drugs that increase VMAT2 activity, could show neuroprotective effects in mammalian models and perhaps patients at risk for PD.
dVMAT-A serves as a useful model for membrane trafficking to secretory vesicles. Mutation of signals in the C-terminus blocks endocytosis in cultured cells, and also disrupts trafficking to SVs in vivo (Grygoruk et al., 2010). Ongoing experiments will determine how these and other signals affect trafficking to SVs and LDCVs in neurons. Genetic rescue experiments in which these and other dVMAT mutant transgenes are expressed in a mutant dVMAT background provide a convenient method for analyzing the in vivo effects of these alleles without generating a large number of knock-in lines. Importantly, this type of in vivo structure-function analysis provides an opportunity to determine the effects of altered dVMAT trafficking on behavior. Similar studies on Drosophila dopamine transporter (dDAT) point mutants have been used to determine the behavioral effects of N-terminal phosphorylation and an hDAT mutant linked to autism (Hamilton et al., 2013; Pizzo et al., 2013; Pizzo et al., 2014). This method of analysis is likely to useful for the study of a variety of other transporters (see Future Directions below).
dVGAT
Similar to mammals, the Drosophila genome contains a single vesicular GABA transporter gene (dVGAT) (Fei et al., 2010). dVGAT appears to be expressed in all GABAergic neurons in the larva, since it precisely co-localizes with GABA in the ventral nerve cord, and is also expressed in most, if not all adult GABAergic neurons (Enell et al., 2007; Fei et al., 2010). It is not clear whether Drosophila, like mammals, use glycine as a neurotransmitter and whether dVGAT could also serve to store glycine. It is also possible that dVGAT could play a role in β-alanine storage or metabolism in the fly although this has not been tested. The structure of β-alanine is intermediate between glycine and GABA and mammalian VGAT has been shown to transport β-alanine in vitro (Juge et al., 2013). As noted above, conjugation of β-alanine to histamine is required for histamine recycling in the fly (Borycz et al., 2002), and conjugation of β-alanine is also important for DA metabolism in both the cuticle and the central nervous system (Suh and Jackson, 2007; Wright, 1987).
Mutation of dVGAT causes developmental lethality (Fei et al., 2010). However, inducible expression of a dVGAT transgene has been used to restore dVGAT function during development and thereby allow adult behavioral studies. One adult phenotype demonstrated in the conditional rescue line was a surprisingly specific defect in the detection of small objects in the fly’s visual field (Fei et al., 2010). Other aspects of reduced GABAergic signaling in this line remain to be explored.
portabella
Drosophila and some other insects express portabella, an additional vesicular transporter than appears to be absent from mammalian genomes (Brooks et al., 2011; Lawal and Krantz, 2013). The gene name was based on its robust expression in MBs (See Neuroanatomy above) (Brooks et al., 2011). Prt is expressed in the intrinsic neurons of the MBs, the Kenyon cells (KCs) (Brooks et al., 2011). Surprisingly, the neurotransmitter stored and released by KCs is not known. The primary structure of the prt protein is most similar to DVMAT and it is possible that the substrate is also similar to known monoamines; however, the biosynthetic enzymes for DA, 5HT, OA and histamine are not expressed in KCs, suggesting that prt may transport a novel neurotransmitter (Brooks et al., 2011). In the absence of an identified substrate, a genetic approach was used to test the hypothesis that prt functions as a transporter. Mutation of residues conserved in prt and VMAT ablated the ability of a prt transgene to rescue the prt mutant phenotype (Brooks et al., 2011). These data support the idea that prt is likely to function as a bone fide transporter and that the substrate may be similar to known biogenic amines.
Mutation of prt results in a peculiar defect in sexual behavior, characterized primarily by an inability of males to maintain their position during copulation (Brooks et al., 2011). The mutants are nonetheless fertile (Brooks et al., 2011). Expression of prt using a Gal4 driver that is prominently expressed in MBs restores these behavioral defects (Brooks et al., 2011). MBs are best known for their role in learning and memory and the prt mutant shows a defect in olfactory learning, however the phenotype is relatively subtle (Brooks et al., 2011; Kahsai and Zars, 2011; Roman and Davis, 2001). It is possible that compensatory changes in the prt mutant mitigate its effect on learning. Alternatively, the release of a peptide, rather than a classical neurotransmitter, may be more important for some functions of KCs (Iliadi et al., 2008; Knapek et al., 2013).
Plasma membrane transporters
dSERT
The Drosophila serotonin transporter (dSERT) was the first plasma membrane neurotransmitter transporter to be identified in the fly (Corey et al., 1994; Demchyshyn et al., 1994). These studies revealed that the substrate specificity of dSERT diverged from mammalian orthologs, including a lower affinity for some antidepressants such as citalopram but a higher affinity for the mammalian DAT antagonist mazindol (Corey et al., 1994; Demchyshyn et al., 1994). Transport via dSERT also appeared to show a less stringent requirement for chloride than hSERT (Demchyshyn et al., 1994). Although one report suggested that the affinity of cocaine for dSERT was significantly higher than hSERT (Corey et al., 1994), another suggested similar affinities for both orthologs (Demchyshyn et al., 1994). Interestingly, the SERT ortholog that is expressed in the tobacco hornworm Manduca sexta is more similar to dSERT than hSERT but has an extremely low affinity for cocaine and most antidepressants (Sandhu et al., 2002).
Extensive structure-function comparisons of hSERT and dSERT have been performed using heterologous expression of hSERT/dSERT chimeras in cultured cells. These studies revealed the importance of a tyrosine residue (Y95 in hSERT, F90 in dSERT) in TM1 for some pharmacologic differences including the differential binding to citalopram (Adkins et al., 2001; Barker et al., 1998) Other sites in transmembrane domains 5–9 contribute to the apparent inability of dSERT to transport the neurotoxin MPP+ (Rodriguez et al., 2003). Additional modeling studies have suggested an interaction between Y95 and E444 (Combs et al., 2011).
dSERT RNA is expressed late in embryonic developmental (stage 15) just before 5HT can be detected (stage 17) (Demchyshyn et al., 1994). In situ hybridization of larva shows a pattern consistent with the stereotyped localization of serotonergic neurons in larva (Giang et al., 2011) and adult brain (Thimgan et al., 2006). The dSERT gene is predicted to be less than 4 kB and to encode two non-coding splice variants http://flybase.org/cgi-bin/gbrowse/dmel/?Search=1;name=FBgn0010414. Northern blots confirm the presence of at least two bands likely to represent mature mRNA species (Romero-Calderón et al., 2007).
A specific antiserum to dSERT has been used to map protein expression in embryo, larvae and adults (Giang et al., 2011). All cell bodies in the larva and adult that express dSERT also co-label with an antiserum to 5HT and vice versa (Giang et al., 2011). However, some processes in the adult brain, including those in the MB lobes, express dSERT but do not detectably label for 5HT. The function of dSERT at these sites is not clear but could conceivably involve uptake of a transmitter other than 5HT (Larsen et al., 2011), and perhaps the substrate of prt (see above).
Immunocytochemistry and fast scan cyclic voltametry have helped demonstrate the importance of reuptake mechanisms for 5HT clearance in Drosophila (Borue et al., 2010; Borue et al., 2009). Pharmacologic inhibition of amine uptake increased the time required for 5HT clearance (Borue et al., 2010; Borue et al., 2009) in larvae and reduced the pool available for release following repetitive stimulation (Borue et al., 2010; Borue et al., 2009).
A mutation in dSERT has been generated by Henrike Scholz’s lab (University of Cologne, Germany). A phenotype characterization is now in progress and will determine how a potential increase in extracellular 5HT might affect fly behavior. Pharmacologic manipulation of 5HT release using amphetamine derivatives (fenfluramine and 3,4-methylenedioxymethamphetamine a.k.a. MDMA or ‘ecstasy’) has already demonstrated that increased extracellular 5HT causes enlarged varicosities in 5HT processes (Daubert et al., 2010). By contrast, direct application of exogenous 5HT decreases the number of varicosities in 5HT processes in the central neuropil (Sykes and Condron, 2005), and loss of 5HT (and DA) synthesis in Ddc mutants results in increased branching of serotonergic processes (Budnik et al., 1989). The mechanisms underlying these phenomena remain unclear, but support the idea that both excessive and inadequate levels of extracellular 5HT, and possibly dSERT, activity could have significant developmental effects (Daubert and Condron, 2010; Lesch and Waider, 2012). Further studies using dSERT transporter transgenes and mutants are likely to be useful to explore this issue. Importantly, the availability of a dSERT mutant will now allow in vivo structure-function studies in which specific mutations can be expressed in a loss of function background.
Further studies may also help to determine whether proteins other than neurotransmitter transporters are required for amine uptake and storage in 5HT cells. Mammalian thalamocortical projections appear to take up and release exogenous 5HT in the absence of cell-autonomous synthesis (Lebrand et al., 1996; Lebrand et al., 2006). In contrast, in the fly, exogenously applied 5HT did not appear to be taken up or stored in non-aminergic cells ectopically expressing dSERT (and dVMAT) (Giang et al., 2011; Park, 2006). Although speculative, it is possible that additional factors beyond the transporters themselves are required for amine uptake and storage.
dDAT
The Drosophila dopamine transporter (dDAT) was identified via homology-based cloning and exhibits a kinetic profile similar to that of hDAT (Porzgen et al., 2001). In situ hybridization experiments showed that dDAT expression matched the pattern in larva previously established for DA neurons (Porzgen et al., 2001). Northern blots show a single mRNA species of 3–4 kB (Kume et al., 2005; Romero-Calderón et al., 2007) and in situs of in adult heads partially match the localization DA cells, although not all known DA cells were detectably labeled (Thimgan et al., 2006). As with all other DAT orthologs, dDAT-mediated transport is sodium dependent and able to support efflux as well as sodium-coupled transport (Porzgen et al., 2001). The affinity of dDAT for cocaine was noted to be relatively low compared to hDAT and dSERT (Porzgen et al., 2001). However, in vivo studies have shown that dDAT is likely to be required for at least some of the behavioral effects of cocaine (Bainton et al., 2000) and inhibition of dDAT by cocaine prolongs DA uptake in vivo (Makos et al., 2010; Vickrey et al., 2013).
Real time, in vivo measurements of DA have been made with carbon fiber electrodes using both fast scan cyclic voltametry in larvae (Vickrey et al., 2009) and amperometry in adults (Berglund et al., 2013; Makos et al., 2010). Both cocaine and nisoxetine block reuptake of endogenous DA in larvae (Vickrey et al., 2009). In the adult fly, clearance of exogenously applied dopamine was decreased by cocaine, amphetamine, methamphetamine, and methylphenidate (Ritalin). Additional experiments using a dDAT mutant demonstrated that these effects on DA uptake were mediated by dDAT, rather than another transporter such as dSERT (Makos et al., 2010).
The dDAT mutant was originally isolated in a screen for sleep defects and the dDAT gene was renamed fumin (fmn), meaning sleepless in Japanese (Kume et al., 2005). Whereas wild type flies follow a diurnal circadian rhythm, fmn mutants are active almost continuously over each 24-hour period. Moreover, after sleep deprivation they show no rebound need for rest (Kume et al., 2005; Ueno et al., 2012; Wu et al., 2008). fmn mutants also show an enhanced response to mechanical stimuli (Kume et al., 2005). The pathways mediating the sleep defect effect in fmn have been mapped using genetic interactions with the DA receptor, DopR (Ueno et al., 2012), which is similar to mammalian DA1 receptors (Kim et al., 2007; Liu et al., 2012b). These data indicate that that DA processes innervating the adult central complex help promote sleep (Ueno et al., 2012).
Mutations in dDAT have also been used to study the mechanism of action of amphetamines (Pizzo et al., 2013; Pizzo et al., 2014). By disrupting the function of membrane rafts in DA cells, the authors elegantly showed that localization of dDAT to these sites is required for efflux but not for transport in vivo (Pizzo et al., 2013) consistent with in vitro studies (Cremona et al., 2011). These studies also demonstrated that the behavioral effects of amphetamines in the fly are mediated through DA neurons rather than another aminergic cell type (Pizzo et al., 2013; Pizzo et al., 2014). Further genetic rescue experiments used expression of a dDAT phosphoacceptor site mutant in the dDAT loss of function background to show that phosphorylation of the dDAT N-terminus is required for efflux in vivo (Fog et al., 2006; Khoshbouei et al., 2004; Pizzo et al., 2013). In addition, a dominant negative form of CamKII was used to demonstrate the specific requirement of this kinase for phosphorylation-mediated efflux through dDAT (Pizzo et al., 2014).
Another elegant study of dDAT used genetic rescue of the mutant to test the behavioral effects of a rare de novo, non-synonymous mutation (T356M) detected in a patient with autism (Neale et al., 2012). Importantly, the rarity of some de novo mutations in many genes can make it difficult to conclusively evaluate their phenotypic impact on the basis of human genetic studies alone. Thus, additional models to test the potential effect(s) of such mutants are quite useful. When expressed in cultured cells, the T356M mutation caused sustained constitutive efflux in the absence of amphetamines. When expressed in vivo in a wild type dDAT background, the mutant caused a genetically dominant increase in locomotion in adult flies similar to the effects of the dDAT loss of function fmn mutant (Hamilton et al., 2013). These data strongly suggest that physiologically significant effects can result from the T356M mutation and that this allele is unlikely to represent an innocuous polymorphism unrelated to disease.
Risk alleles associated with other transporters may also be investigated in this way. To our knowledge, only one mouse model containing a risk allele in hSERT for Attention Deficit Hyperactive Disorder (ADHD) has been generated (Veenstra-VanderWeele et al., 2012). The phenotypic effects of other possible risk alleles in SERT are not known (Ye and Blakely, 2011) and might be explored in the fly.
Octopamine uptake and metabolism in flies vs. other insects
OA is sometimes referred to as the invertebrate noradrenaline, since its structure differs by only a single ring oxygen and it serves some similar functions (Roeder, 2005). OA is a substrate of dVMAT (Greer et al., 2005) and the fly expresses an adrenergic-like OA receptor gene OAMB and three β-adrenergic-like receptors Oct β1R, Oct β2R and Oct β3R (Evans and Maqueira, 2005). However, a plasma membrane OA transporter has not been identified in the fly. Surprisingly, a high affinity OA transporter has been identified in the cabbage looper Trichoplusia ni (T. ni) and several other insect species (Donly et al., 2007; Malutan et al., 2002), but is absent in Drosophila and other Dipteran species as well as bees (Hymenoptera) (Donly and Caveney, 2005). T. ni also express a high affinity DA transporter (Gallant et al., 2003). TriDAT and TriOAT show similar affinities for DA, but OA and tyramine inhibit TrnOAT to a greater extent than TrnDAT (Gallant et al., 2003). In the absence of an OAT ortholog, it remains unclear how OA is recycled in Drosophila. It is conceivable that dDAT functions as both an OA and DA transporter in the fly but this remains speculative.
dEAAT1
Drosophila EAATs were independently identified by three different groups (Besson et al., 1999; Kawano et al., 1999; Seal et al., 1998). Drosophila EAAT1 (CG3747) shares 41 and 35% amino acid identity, respectively, with human EAAT1 and EAAT2 (Besson et al., 1999). dEAAT2 is also similar, but current data suggest that it may not function as a glutamate transporter as we discuss below. We note that the naming of the dEAATs was based on their order of identification rather than correspondence to specific mammalian orthologs. dEAAT1 is sodium-dependent and shows high affinity for L-glutamate, as well as L- and D- aspartate (Seal et al., 1998). The sodium:glutamate stoichiometry of the homologous TriEAAT1 is 2: 1 (Donly et al., 1997), similar to mammalian EAATs (Wadiche et al., 1995). Also, similar to some mammalian EAATs (Fairman et al., 1995) dEAAT1 shows loosely coupled chloride channel-like activity (Seal et al., 1998).
Four non-coding dEAAT1 mRNA splice variants are predicted, but their functional relevance is not known http://flybase.org/cgi-bin/gbrowse/dmel/?Search=1;name=FBgn0026439. In the embryo and larva, dEAAT1 mRNA appears to be confined to the nervous system and robustly expressed in glia, with little if any expression in neurons (Besson et al., 1999; Freeman et al., 2003; Soustelle et al., 2002; Stacey et al., 2010). dEAAT1 is co-expressed with the well-characterized glial marker repo but some repo-positive cells do not express dEAAT1 (Soustelle et al., 2002). dEAAT1 expression in adult glia has been confirmed using a dEAAT1 antiserum (Rival et al., 2006) in addition to a dEAAT1-Gal4 driver (Rival et al., 2004; Stacey et al., 2010). dEAAT1-Gal4 expressing cells co-label for the marker Prospero, indicating that a large majority of dEAAT1-expressing cells are of the anterior LG subtype of glia (Stacey et al., 2010). Surprisingly, dEAAT1 is not expressed in glia (or neurons) at the larval glutamatergic NMJ (Rival et al., 2006). Rather, uptake of glutamate at the larval NMJ is likely to be mediated by the cystine/glutamate exchanger encoded by the gene genderblind (Grosjean et al., 2008).
The reduction of dEAAT1 expression in adult glia using RNA interference (RNAi) was reported to shorten life span, increase sensitivity to oxidative stress and cause neural degeneration (Rival et al., 2004), however, RNAi experiments in a later report showed less severe effects (Rival et al., 2006). In these later experiments, electrophysiological recordings from adult flight muscles showed broadening of peaks representing individual depolarization events, consistent with the idea that decreased glutamate uptake can have significant post-synaptic effects in flies as well as mammals (Rival et al., 2006).
Mutations in the dEAAT1 gene were recently reported and are lethal at approximately 48 hours post-hatching (Stacey et al., 2010). Two mutant alleles were identified, including dEAAT1SM1 and dEAAT1SM2, both likely to be null alleles since the start codon and the first two transmembrane domains were deleted and no expression of dEAAT1 mRNA was detected. Additionally, the phenotypes of the homozygous mutants were equivalent to each other and to heterozygotes containing a mutant allele over a large chromosomal deletion (Stacey et al., 2010). We note that this type of genetic assay, rather than RNA or protein expression, is the gold standard for determining whether a Drosophila mutant is a true null allele.
The survival of the dEAAT1 mutants as 1st instar larvae allowed a further phenotypic analysis (Stacey et al., 2010). Unlike wild type larvae that move almost continuously, dEAAT1 homozygous mutants were nearly immobile with occasional slow contractions of the body wall musculature (Stacey et al., 2010). In contrast to findings in RNAi studies (see above), no obvious morphologic or neurodegenerative defects were detected in the mutant (Stacey et al., 2010).
Additional functional studies of dEAAT1 may help determine how glutamatergic signaling is regulated and the potential effects of dysregulation. Mutations in human EAAT1 have been identified in a rare form of episodic ataxia (de Vries et al., 2009; Jen et al., 2005) and in collaboration with another lab (Joanna Jen, UCLA) we are currently testing the potential pathogenic effects of these mutations in Drosophila.
dEAAT2
Despite the similarity between dEAAT1 and dEAAT2, the latter may not function as a high affinity glutamate transporter (Besson et al., 2000; Besson et al., 2005; Besson et al., 2011). Similar to other EAAT orthologs, dEAAT2 transports aspartate, but its comparative affinity for glutamate is relatively low (Besson et al., 2000). Surprisingly, EAAT2 also transports taurine (aminoethane sulfonic acid), a β-amino acid whose physiological role in all species remains poorly understood (Besson et al., 2005; Besson et al., 2011). Some structures in the insect brain such as the MBs are labeled by antisera to taurine (Bicker, 1991; Strausfeld et al., 2003) and a role for taurine as a neurotransmitter has been suggested, but this remains incompletely defined (Wu and Prentice, 2010). Rather, taurine may function primarily as an osmolyte in the CNS and other tissue (Chesney et al., 2010; Schaffer et al., 2010). In mammals, taurine uptake is mediated by a distinct taurine transporter (Pramod et al., 2013), which appears to be absent in the fly and other insects (Besson et al., 2011). Consistent with an in vivo role for dEAAT2 in taurine uptake, mutations of dEAAT2 exhibit dramatically decreased tissue content of taurine (Besson et al., 2011). The mutants also showed mild defects in the behavioral response to saline and propionic acid, but phototaxis and locomotion were similar to wild type controls; levels of aspartate and glutamate were also similar to controls (Besson et al., 2011).
In the embryo, dEAAT2 mRNA is expressed in sensory organs in the peripheral nervous system and midline glia (Freeman et al., 2003; Soustelle et al., 2002). An antibody to dEAAT2 is not available but a dEAAT2-GAL4 construct has been used to localize expression in conjunction with a UAS-GFP marker. In contrast to in situ data, expression using the Gal4 driver was predominantly neuronal (Besson et al., 2011). However, the authors acknowledged that the 4.5-kb promoter fragment used to make the dEAAT2-GAL4 transgene could be incomplete and additional studies might uncover expression in some subsets of glia.
dGAT
A Drosophila GABA transporter (GAT) cDNA was identified prior to the sequencing of the fly genome (Neckameyer and Cooper, 1998) and corresponds to the single GAT ortholog present in the genome (CG1732). In parallel, similar GAT orthologs were identified in the tobacco hornworm Manduca sexta (Mbungu et al., 1995) (MasGAT) and the cabbage looper moth Trichoplusia ni. (TrnGAT) (Gao et al., 1999). All three insect orthologs are similar in primary structure to mammalian GAT-1. The pharmacology of cloned MasGAT and TrnGAT have been investigated in vitro using heterologous expression systems and, similar to mammalian orthologs, both are sensitive to the mammalian GAT inhibitor DL-2,4-diaminobutyric acid (DABA) (Gao et al., 1999; Mbungu et al., 1995). In contrast to mammalian GATs, neither MasGAT nor TrnGAT were significantly inhibited by β-alanine. Nipecotic acid showed partial inhibition at high concentrations but significantly lower affinity for insect versus mammalian GATs (Gao et al., 1999; Mbungu et al., 1995).
The effects of both DABA and nipecotic acid have been tested in Drosophila membranes derived from homogenized embryos, larvae, pupae and adult flies (Leal et al., 2004; Leal and Neckameyer, 2002). The homogenates showed saturable uptake of tritiated GABA and inhibition by DABA and nipecotic acid; however, the required concentrations of inhibitors were higher than those used in assays of cloned transporters (Leal et al., 2004; Leal and Neckameyer, 2002). Feeding DABA to larva and adults or feeding nipecotic acid to adults disrupted several aspects of motor behavior (Leal et al., 2004; Leal and Neckameyer, 2002). At least some affects of DABA and nipecotic acid, such as disruption of negative geotaxis in the adult were reversible over the course of 2 days, which might prove useful for further behavioral studies. Although most insect GABA receptors (including RDL) are insensitive to the GABAA receptor antagonist bicuculline (Buckingham et al., 2005) the behavioral effects of DABA could be reversed with this drug (Leal and Neckameyer, 2002). It was also surprising that the GABA analog gabapentin rescued the effects of DABA (Leal and Neckameyer, 2002), since the phenotype caused by blockade of GABA reuptake might be expected to be enhanced by a GABAergic agonist. A dGAT mutant would be useful to further explore these effects, but has not yet been identified.
dGAT expression is likely to be restricted to glia. In the adult fly, in situ hybridization showed an mRNA pattern similar to that of the glial marker repo (Thimgan et al., 2006). Two possible splice variants have been predicted but both are the same predicted size http://flybase.org/reports/FBgn0039915.html. To our knowledge, a specific antiserum to Drosophila GAT is not available. Antisera to mammalian GATs 1–3 have been used in Drosophila as probes and showed three distinct patterns, but their relationship to the expression of dGAT is not clear. By contrast, a highly specific antiserum to MasGAT has been generated (Oland et al., 2010; Umesh and Gill, 2002) and used for both light microscopy and ultrastructural studies. A detailed study of the antennal lobe showed that MasGAT localizes to neuronal processes early in development (Oland et al., 2010) but labeling appeared to be confined to glia in the adult. Consistent with the idea that insect GATs in general may primarily localize to glia in adults, uptake of GABA into the optic ganglia of both Drosophila and larger flies (Musca) appeared to be primarily glial (Campos-Ortega, 1974) and in situ hybridization of dGAT also appeared to localize to glia rather than neurons (Thimgan et al., 2006).
Choline Transport in other insects
Unlike most other neurotransmitters, acetylcholine is degraded extracellularly to choline prior to transport. Choline is then recycled by a specific high-affinity transporter that has been extensively studied in vertebrates (Parikh et al., 2013; Traiffort et al., 2013). A possible ortholog of the mammalian choline transporter (ChT1) is present in the Drosophila genome (see Table 2) but has not been investigated. An ortholog from the cabbage lopper (TrnChT) has been characterized and unlike mammalian orthologs, is weakly inhibited by Hemicholinium-3 (McLean et al., 2005). Interestingly, mammalian ChT resides primarily on synaptic vesicles at baseline (Blakely and Edwards, 2012). It is not known if this is also true in insects.
Additional SLC6 members
The Drosophila SLC6 family of sodium-dependent plasma membrane transporters contains several additional members whose function remains unclear. These include the gene inebriated (ine) (Soehnge et al., 1996; Stern and Ganetzky, 1992). A mutation at this locus was independently isolated in a screen for defects in the electroretinogram (ERG), the electrical field potential across the retina and downstream neurons in the optic ganglia (Pak, 1975; Wu and Wong, 1977). The characteristic oscillations in the mutant led to its designation as “receptor oscillation A”, or rosA (Burg et al., 1996; Pak, 1975; Wu and Wong, 1977). rosA/ine mutants also show reduced on and off transients in the ERG suggesting impaired signaling of photoreceptors to neurons immediately downstream in the optic ganglia (Burg et al., 1996; Pak, 1975; Wu and Wong, 1977). However, electrophysiological defects in rosA/ine are not confined to the eye and include potentiation of larval motoneuron depolarization (an increase in long term facilitation) (Huang and Stern, 2002). These observations are consistent with the idea that rosA/ine may generally function to regulate neuronal excitability.
A homolog of ine has been cloned from Manduca sexta (Chiu et al., 2000) and expression in oocytes was used to test a variety of potential substrates but to date, the substrates of Manduca and Drosophila ine remain unclear (Chiu et al., 2000) (however see below). Ine mRNA and protein appear to be broadly expressed in both Drosophila and Manduca and unlike some other members of SLC6, expression is not confined to the nervous system but also prominent in the gut and malpighian tubule, the “fly equivalent” of the mammalian kidney (Burg et al., 1996; Chiu et al., 2000). In these structures, ine has been proposed to function as an osmolyte transporter (Huang et al., 2002).
It has been suggested that ine could have a function in recycling histamine via transport of the histamine metabolite carcinine (Stuart et al., 2007). Carcinine is generated by ebony, which is expressed in a subset glia that surround photoreceptor terminals and functions to conjugate histamine to β-alanine (Stuart et al., 2007). Several tantalizing findings support the idea that ine could be involved in recycling histamine and perhaps function as a carcinine transporter (Gavin et al., 2007). For example, feeding flies carcinine phenocopies ine (Gavin et al., 2007). These effects are consistent with the idea that loss of ine increases extracellular carcinine and that excess extracellular carcinine is the cause of the ine/rosA mutant phenotype.
Since histamine is released from photoreceptor cells but metabolized in glia, recycling is likely to follow a fairly circuitous path, similar to that proposed for glutamate recycling through glia. Therefore, similar to System A and N in the glutamate:glutamine cycle, additional transporters are likely to be involved in histamine recycling. Some of these may be novel members of SLC6. In addition to ine, insects express other distinct subfamilies of SLC6 including 6 insect-specific Nutrient Amino Acid Transporters (iNATs) (Boudko et al., 2005). Another 5 genes whose function remains obscure form another distinct subfamily of transporters in SLC6 (Romero-Calderón et al., 2007). mRNA expression of two members in this group (CG13794 and 5) as well as CG4476 are down-regulated by the loss of eye tissue, consistent with a possible function in the visual system (Romero-Calderón et al., 2007). Furthermore, mutation of CG4776 causes a defect in phototactic behavior (Romero-Calderón et al., 2007). We suspect that CG4476, CG13794 CG13795 and perhaps other novel members of SLC6 could participate in recycling histamine in the fly and other insects, but additional experiments are clearly needed to test this idea.
Future Directions
Structure-function studies in vivo
As described elsewhere in this issue, experiments using cultured cells have been invaluable for exploring the functional consequences of specific mutations in neurotransmitter transporters. In parallel, mouse knockouts and mutations in model organisms such as C. elegans and Drosophila have demonstrated the physiological importance of specific neurotransmitter transporters for a variety of processes. We suggest that flies and other model organisms will be very important for combining these strategies and that structure-function studies of neurotransmitter transporters in vivo will be an important future direction.
One use of this strategy is the expression of risk alleles for human disease in vivo. For rare or de novo mutations the relationship between a particular polymorphism and the disease phenotype can be difficult to ascertain. In the absence of a pedigree or the association of a phenotype with multiple de novo events, it is often impossible to determine if a given polymorphism or copy number variant has a relationship to the phenotype of the patient. In some cases, it may be possible to demonstrate molecular or cellular phenotypes for a candidate gene, as shown for disease-associated mutations in ion channels. However, it is also possible that the cellular phenotype of a disease allele may not reflect its previously defined physiological function, e.g. a cellular phenotype of a rare mutation associated with Autism Spectrum Disorders (ASDs) is dendritic retraction (Krey et al., 2013). This phenotype was not predicted from the known function of the gene as a calcium channel subunit and does not appear to be associated with alterations in calcium flux (Krey et al., 2013).
The potential pathogenic mechanisms associated with polymorphisms in neurotransmitter transporters are also likely to be complex, and it may be difficult to predict how a subtle change in the structure of a neurotransmitter transporter could increase risk for a complex illness such as autism or ADHD. As discussed above, the potential use of flies to examine these questions is exemplified by the analysis of a dDAT allele associated with autism (Hamilton et al., 2013). We anticipate that similar studies will be very useful to explore the consequences of other risk alleles such as those in SERT linked to autism. To date only one of the SERT mutants has been tested in mice (Ye and Blakely, 2011; Veenstra-VanderWeele et al., 2012). Generating mutant lines in flies or worms may be used for detailed mechanistic studies, or alternatively, large panels of potentially relevant polymorphisms may be screened in model invertebrates to determine which ones should be studied in more detail in mammals.
More generally, structure-function studies in vivo will be useful to determine the downstream consequences of other mutations in neurotransmitter transporters. Mutants predicted to affect transport activity, post-translational regulatory processes or trafficking can be easily expressed in a loss of function mutant background to determine their effects on synaptic transmission and behavior. As noted above, this strategy has already been used to determine the in vivo importance of phosphoacceptor sites in dDAT (Pizzo et al., 2013).
We believe that this strategy will be particularly useful to understand the functional importance of transporter trafficking. A large number of studies have already dissected some of the signals and pathways required for the internalization of vesicular transporters and their targeting to secretory vesicles (reviewed in (Fei et al., 2008)). Similarly, a number of studies have explored the signals that allow internalization of plasma membrane transporters (Eriksen et al., 2010; Quick, 2006; Robertson et al., 2009; Sager and Torres, 2011; Steiner et al., 2008). However, it is not known how disruption of these pathways might affect the function of the nervous system as a whole. We are currently investigating these questions using trafficking mutants in dVMAT. We have focused on dVMAT in part because it localizes to two distinct types of vesicles, SVs, required for rapid release at the nerve terminal and LDCVs, thought to be required for neuromodulatory signaling. The contribution of amine release from SV versus LDCVs for specific behaviors remains surprisingly obscure. We are using mutations that shift DVMAT between SVs and LDCVs to explore this question (manuscript under review). We suggest that a similar analysis may be useful to explore the functional significance of mutations that disrupt the internalization of plasma membrane transporters.
Forward Genetic Screens for Interacting Genes and Proteins
Most psychiatric illnesses are likely to follow a complex pattern of inheritance with large numbers of genes contributing to the phenotype, and additional environmental factors also playing a role (Sullivan et al., 2012). Genetic modifiers alter the phenotype of the ASD mutation in SERT when expressed in mice, but the identities of these interacting genes are not known (Kerr et al., 2013). We suggest that “modifier” a.k.a. “enhancer/suppressor” screens in model invertebrates such as C. elegans and Drosophila represent a powerful and essentially untapped method to identity specific genes that interact with risk alleles in SERT, and more generally to identify genes that interact with neurotransmitter transporters.
Modifier screens may be used to identify new loci that interact with any type of mutation, provided it generates a genetically tractable phenotype. In common genetic parlance, the mutation is used to first generate a “sensitized genetic background” which may consist of an anatomic or behavioral defect, or simply a decrease in survival. A panel of mutations in other genes-- so-called “second-site mutations”-- are then crossed into the sensitized genetic background. The resulting progeny are screened to determine whether the phenotype of the parent strain-- the sensitized genetic background-- has been modified by any of the additional mutations. The defect seen in the parent strain may be worsened or “enhanced”. Alternatively, the defect might improve, and the phenotypic defect “suppressed”.
Classic screens for molecules that modulate the function of the oncogene ras provide an important example of this strategy in flies (Gaul et al., 1993). Suppression or enhancement of defects in Drosophila eye development caused by mutations in ras were used to identify genes relevant to ras and ras-dependent pathways. Although eye development in flies was used as a screening phenotype, the molecules that emerged from these screens were not specific for eye development or Drosophila but rather represented general components of the ras signaling pathway. Flies and worms may similarly be used to screen for genes that modulate the function of neurotransmitter transporters. The first step will be to identify a genetically tractable phenotype caused by mutation of the transporter in order to generate a sensitized genetic background. Importantly, the nature of the phenotype need not be particularly interesting, just consistent, robust and easily scored.
In C. elegans, mutations in VAChT/unc-17 have already been used to screen for interacting loci (Mathews et al., 2013; Sandoval et al., 2006). One screen identified a mutant allele of synaptobrevin and a second screen identified the novel gene sup-1 (Mathews et al., 2013; Sandoval et al., 2006). The nature of these second site mutant alleles suggest that in both cases the interactions are likely to be mediated by direct protein-protein binding to VAChT. However, this need not always be the case. One advantage of this type of forward genetic screen is that it can detect indirect interactions. Another advantage is that it is not strictly hypothesis based. The authors of the VAChT interaction studies did not know that they would detect synaptobrevin, much less sup-1 (Mathews et al., 2013; Sandoval et al., 2006). Similarly, we suggest that identification of molecules that indirectly influence the function of neurotransmitter transporters in ways that we are unable to predict will be a very important future use for Drosophila.
Forward genetic screens in C. elegans have also been used to identify novel genes that regulate DA signaling and could potentially regulate dopamine transport. Mutations in the C. elegans ortholog DAT-1 generate a genetically tractable phenotype termed Swimming-Induced Paralysis (SWIP) (Hardaway et al., 2013; McDonald et al., 2007). Some DAT-1 mutations that cause SWIP decrease DAT-1 localization to the synapse, and the SWIP phenotype is thought to result from activation of extrasynaptic DA receptors by DA spillover (Hardaway et al., 2013; McDonald et al., 2007). Thus, screens for mutations that modify SWIP have the potential to identify novel genes required for DAT trafficking as well as other aspects of DAT activity. Although a classical modifier screen using a weak allele of DAT-1 has not yet been reported, a recent study reported a screen for genes that would mimic or phenocopy the DAT-1 mutant and cause SWIP. In addition to new alleles of DAT-1, the screen identified two novel second-site mutations, one of which appears to genetically interact with DAT-1 (Hardaway et al., 2013). It is possible that modifiers of Drosophila fmn could be similarly identified using sleep or the response to amphetamines as a screening phenotype.
Drug screens
Although still a nascent field, a number of labs have begun to use flies as an in vivo platform for drug screens (Tickoo and Russell, 2002). One strategy, based on classical forward genetic screens is to select a phenotype of interest, e.g. sleep (Nall and Sehgal, 2013) or feeding (Gasque et al., 2013), then screen for drugs that are responsible for or enhance that phenotype. Some of the identified targets of these screens may be neurotransmitter transporters, e.g. the VMAT inhibitor reserpine was identified in a screen for small molecules that increase sleep in flies (Nall and Sehgal, 2013). A more efficient strategy to identify agents that affect the function of a given neurotransmitter transporter may be to screen for drugs that modify the phenotype caused by mutation or over-expression of the transporter, akin to screens to identify genetic modifiers. Similar to genetic modifier screens, this strategy again takes advantage of the fact that all of the regulatory machinery is in place, and drug targets may be novel elements of the regulatory machinery rather than the transporter itself. We suggest that this approach might expand the way we think about drugs that affect transporters. In addition, we suggest that this represents a potential way to identify drugs that may increase transport activity. There are few if any drugs that make neurotransmitters transporters work more effectively. Targeting upstream proteins that inhibit transporter function could accomplish this.
We have taken this approach to identity new molecules that might potentiate the function of VMAT in vivo (Lawal et al., 2014). Using a partially functional dVMAT mutant background, we screened larvae for drugs that would increase signaling in the octopaminergic circuit required for baseline larval locomotion. The results of this screen yielded several molecules that a priori would not be classified as aminergic drugs and are unlikely to directly bind dVMAT (Lawal et al., 2014). Rather, we suggest that some of the identified drugs may act on upstream targets to indirectly increase VMAT activity, and we are currently testing this hypothesis. We suggest that similar screens might be used to identify drugs that modify the function of other neurotransmitter transporters.
Highlights.
Drosophila express orthologs of most mammalian neurotransmitter transporters.
Fly genetics provides a powerful tool-set to explore transporter function in vivo.
Screens for genes and drugs that modify transport function are possible in the fly.
Acknowledgments
We thank Henrike Scholz (University of Cologne, Germany), Roland Bainton (UCSF, USA) and the anonymous reviewers for helpful comments on the manuscript. This work was supported by National Institute of Mental Health R01 MH076900, National Institute of Environmental Health (NIEHS) R01 ES015747, an Independent Investigator Award from The Brain and Behavior Research Foundation, and the Joanne and George Miller and Family Endowed Chair in Depression Research at the UCLA Brain Research Institute (D.E.K.) with additional funding from an the NIEHS Center Grant (P01 ES016732; PI: M.F. Chesselet), and the NIEHS UCLA training grant in Molecular Toxicology and the Parkinson’s Disease Foundation (PDF-SFW-1336) (C.A.M.).
Abbreviations used in the text include
- 5HT
5-Hydroxytryptamine (Serotonin)
- ACh
Acetylcholine
- ASD
Autism Spectrum Disorder
- ChT
Choline Transporter
- ChAT
Choline Acetyl Transferase
- DA
Dopamine
- Ddc
Dopa decarboxylase
- EAAT
Excitatory Amino Acid Transporter
- GABA
Gamma Amino Butyric Acid
- GAT
GABA transporter
- KC
Kenyon Cell
- LDCV
Large Dense Core Vesicle
- Mas
Manduca sexta (Tobacco Hornworm)
- MAO
Monoamine Oxidase
- MB
Mushroom Body
- MDMA
3,4-methylenedioxy-N-methylamphetamine (Ecstasy)
- OA
Octopamine
- NMJ
Neuromuscular Junction
- SV
Synaptic Vesicle
- TNT
Tetanus Toxin
- T. ni or Trn
Trichoplusia ni (Cabbage Looper Caterpillar/Moth)
- TβH
Tyramine β Hydroxylase
- Tdc
Tyrosine decarboxylase
- TH
Tyrosine Hydroxylase
- VGAT
Vesicular GABA Transporter
- VGLUT
Vesicular Glutamate Transporter
- VMAT
Vesicular Monoamine Transporter
- UAS
(Yeast) Upstream Activating Sequence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamczyk A, Gause CD, Sattler R, Vidensky S, Rothstein JD, Singer H, Wang T. Genetic and functional studies of a missense variant in a glutamate transporter, SLC1A3, in Tourette syndrome. Psychiatr Genet. 2011;21:90–7. doi: 10.1097/YPG.0b013e328341a307. [DOI] [PubMed] [Google Scholar]
- Adamo SA, Linn CE, Hoy RR. The role of neurohormonal octopamine during ‘fight or flight’ behaviour in the field cricket Gryllus bimaculatus. J Exp Biol. 1995;198:1691–700. doi: 10.1242/jeb.198.8.1691. [DOI] [PubMed] [Google Scholar]
- Adkins EM, Barker EL, Blakely RD. Interactions of tryptamine derivatives with serotonin transporter species variants implicate transmembrane domain I in substrate recognition. Mol Pharmacol. 2001;59:514–23. doi: 10.1124/mol.59.3.514. [DOI] [PubMed] [Google Scholar]
- Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One. 2010;5:e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–75. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–24. doi: 10.1002/neu.480240803. [DOI] [PubMed] [Google Scholar]
- Bachmann A, Knust E. The use of P-element transposons to generate transgenic flies. Methods Mol Biol. 2008;420:61–77. doi: 10.1007/978-1-59745-583-1_4. [DOI] [PubMed] [Google Scholar]
- Bacon JP, Thompson KS, Stern M. Identified octopaminergic neurons provide an arousal mechanism in the locust brain. J Neurophysiol. 1995;74:2739–43. doi: 10.1152/jn.1995.74.6.2739. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LTY, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Barker EL, Perlman MA, Adkins EM, Houlihan WJ, Pristupa ZB, Niznik HB, Blakely RD. HIgh affinity recognition of serotonin transporter antagonists defined by species-scanning mutagenesis. J Biol Chem. 1998;273:19459–19468. doi: 10.1074/jbc.273.31.19459. [DOI] [PubMed] [Google Scholar]
- Barwick KE, Wright J, Al-Turki S, McEntagart MM, Nair A, Chioza B, Al-Memar A, Modarres H, Reilly MM, Dick KJ, Ruggiero AM, Blakely RD, Hurles ME, Crosby AH. Defective presynaptic choline transport underlies hereditary motor neuropathy. Am J Hum Genet. 2012;91:1103–7. doi: 10.1016/j.ajhg.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Jackson JG, Genda EN, Montoya MM, Yudkoff M, Robinson MB. The glutamate transporter, GLAST, participates in a macromolecular complex that supports glutamate metabolism. Neurochem Int. 2012;61:566–74. doi: 10.1016/j.neuint.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund EC, Makos MA, Keighron JD, Phan N, Heien ML, Ewing AG. Oral administration of methylphenidate blocks the effect of cocaine on uptake at the Drosophila dopamine transporter. ACS Chem Neurosci. 2013;4:566–74. doi: 10.1021/cn3002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson MT, Re DB, Moulin M, Birman S. High affinity transport of taurine by the Drosophila aspartate transporter dEAAT2. J Biol Chem. 2005;280:6621–6. doi: 10.1074/jbc.M412440200. [DOI] [PubMed] [Google Scholar]
- Besson MT, Sinakevitch I, Melon C, Iche-Torres M, Birman S. Involvement of the Drosophila taurine/aspartate transporter dEAAT2 in selective olfactory and gustatory perceptions. J Comp Neurol. 2011;519:2734–57. doi: 10.1002/cne.22649. [DOI] [PubMed] [Google Scholar]
- Besson MT, Soustelle L, Birman S. Identification and structural characterization of two genes encoding glutamate transporter homologs differently expressed in the nervous system of Drosophila melanogaster. FEBS Lett. 1999;443:97–104. doi: 10.1016/s0014-5793(98)01695-0. [DOI] [PubMed] [Google Scholar]
- Besson MT, Soustelle L, Birman S. Selective high-affinity transport of aspartate by a Drosophila homologue of the excitatory amino-acid transporters. Curr Biol. 2000;24:207–210. doi: 10.1016/s0960-9822(00)00339-0. [DOI] [PubMed] [Google Scholar]
- Bicker G. Taurine-like immunoreactivity in photoreceptor cells and mushroom bodies: a comparison of the chemical architecture of insect nervous systems. Brain Res. 1991;560:201–6. doi: 10.1016/0006-8993(91)91233-q. [DOI] [PubMed] [Google Scholar]
- Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU, Zhang M, Sen N, Colbran RJ, Gnegy ME, Gether U, Javitch JA, Erreger K, Galli A. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol Pharmacol. 2008;74:1101–8. doi: 10.1124/mol.108.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely RD, Edwards RH. Vesicular and plasma membrane transporters for neurotransmitters. Cold Spring Harb Perspect Biol. 2012;4:22199021. doi: 10.1101/cshperspect.a005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci. 2003;26:105–31. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98:8966–71. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borue X, Condron B, Venton BJ. Both synthesis and reuptake are critical for replenishing the releasable serotonin pool in Drosophila. J Neurochem. 2010;113:188–99. doi: 10.1111/j.1471-4159.2010.06588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borue X, Cooper S, Hirsh J, Condron B, Venton BJ. Quantitative evaluation of serotonin release and clearance in Drosophila. J Neurosci Methods. 2009;179:300–8. doi: 10.1016/j.jneumeth.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borycz J, Borycz JA, Loubani M, Meinertzhagen IA. tan and ebony genes regulate a novel pathway for transmitter metabolism at fly photoreceptor terminals. J Neurosci. 2002;22:10549–57. doi: 10.1523/JNEUROSCI.22-24-10549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudko DY. Molecular basis of essential amino acid transport from studies of insect nutrient amino acid transporters of the SLC6 family (NAT-SLC6) J Insect Physiol. 2012;58:433–49. doi: 10.1016/j.jinsphys.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudko DY, Kohn AB, Meleshkevitch EA, Dasher MK, Seron TJ, Stevens BR, Harvey WR. Ancestry and progeny of nutrient amino acid transporters. Proc Natl Acad Sci U S A. 2005;102:1360–5. doi: 10.1073/pnas.0405183101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brooks ES, Greer CL, Romero-Calderon R, Serway CN, Grygoruk A, Haimovitz JM, Nguyen BT, Najibi R, Tabone CJ, Steven de Belle J, Krantz DE. A putative vesicular transporter expressed in Drosophila mushroom bodies that mediates sexual behavior may define a neurotransmitter system. Neuron. 2011;72:316–29. doi: 10.1016/j.neuron.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk I, Blex C, Rachakonda S, Holtje M, Winter S, Pahner I, Walther DJ, Ahnert-Hilger G. The first luminal domain of vesicular monoamine transporters mediates G-protein-dependent regulation of transmitter uptake. J Biol Chem. 2006;281:33373–33385. doi: 10.1074/jbc.M603204200. [DOI] [PubMed] [Google Scholar]
- Buckingham SD, Biggin PC, Sattelle BM, Brown LA, Sattelle DB. Insect GABA receptors: splicing, editing, and targeting by antiparasitics and insecticides. Mol Pharmacol. 2005;68:942–51. doi: 10.1124/mol.105.015313. [DOI] [PubMed] [Google Scholar]
- Budnik V, Wu CF, White K. Altered branching of serotonin-containing neurons in Drosophila mutants unable to synthesize serotonin and dopamine. J Neurosci. 1989;9:2866–77. doi: 10.1523/JNEUROSCI.09-08-02866.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MG, Geng C, Guan Y, Koliantz G, Pak WL. Drosophila rosA gene, which when mutant causes aberrant photoreceptor oscillation, encodes a novel neurotransmitter transporter homologue. J Neurogenet. 1996;11:59–79. doi: 10.3109/01677069609107063. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA. Autoradiographic localization of 3H-gamma-aminobutyric acid uptake in the lamina ganglionaris of Musca and Drosophila. Z Zellforsch Mikrosk Anat. 1974;147:415–31. doi: 10.1007/BF00307474. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caveney S, Cladman W, Verellen L, Donly C. Ancestry of neuronal monoamine transporters in the Metazoa. J Exp Biol. 2006;209:4858–68. doi: 10.1242/jeb.02607. [DOI] [PubMed] [Google Scholar]
- Chang H-Y, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, Bainton RJ, Krantz DE. Over-expression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Molecular Psychiatry. 2006;11:99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- Chatterjee N, Rollins J, Mahowald AP, Bazinet C. Neurotransmitter Transporter-Like: a male germline-specific SLC6 transporter required for Drosophila spermiogenesis. PLoS One. 2011;6:e16275. doi: 10.1371/journal.pone.0016275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Bowling K, Funderburk C, Lawal H, Inamdar A, Wang Z, O’Donnell JM. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J Neurosci. 2007;27:2457–67. doi: 10.1523/JNEUROSCI.4239-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Ng F, Lebestky T, Grygoruk A, Djapri C, Lawal HO, Zaveri HA, Mehanzel F, Najibi R, Seidman G, Murphy NP, Kelly RL, Ackerson LC, Maidment NT, Jackson FR, Krantz DE. Dispensable, redundant, complementary, and cooperative roles of dopamine, octopamine, and serotonin in Drosophila melanogaster. Genetics. 2013a;193:159–76. doi: 10.1534/genetics.112.142042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Daining CP, Sun H, Fraser R, Stokes SL, Leitges M, Gnegy ME. Protein kinase C-beta is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. J Neurochem. 2013b;125:663–72. doi: 10.1111/jnc.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney RW, Han X, Patters AB. Taurine and the renal system. J Biomed Sci. 2010;17(Suppl 1):S4. doi: 10.1186/1423-0127-17-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Ross LS, Cohen BN, Lester HA, Gill SS. The transporter-like protein inebriated mediates hyperosmotic stimuli through intracellular signaling. J Exp Biol. 2000;203:3531–46. doi: 10.1242/jeb.203.23.3531. [DOI] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–55. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- Colgan L, Liu H, Huang SY, Liu YJ. Dileucine motif is sufficient for internalization and synaptic vesicle targeting of vesicular acetylcholine transporter. Traffic. 2007;8:512–22. doi: 10.1111/j.1600-0854.2007.00555.x. [DOI] [PubMed] [Google Scholar]
- Combs S, Kaufmann K, Field JR, Blakely RD, Meiler J. Y95 and E444 interaction required for high-affinity S-citalopram binding in the human serotonin transporter. ACS Chem Neurosci. 2011;2:75–81. doi: 10.1021/cn100066p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- Corey JL, Quick MW, Davidson N, Lester HA, Guastella J. A cocaine-sensitive Drosophila serotonin transporter: Cloning, expression, and electrophysiological characterization. Proc Natl Acad Sci USA. 1994;91:1188–1192. doi: 10.1073/pnas.91.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, Rothman JE, Galli A, Javitch JA, Yamamoto A. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–77. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin A, Xia L, Kong W, Hevner R, Wang J. Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience. 2007;146:1193–211. doi: 10.1016/j.neuroscience.2007.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Chen K, Gelfand MV, Featherstone DE, DiAntonio A. A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle. Neuron. 2006;49:11–16. doi: 10.1016/j.neuron.2005.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Gelfand MV, Dant J, Brooks ES, Krantz DE, DiAntonio A. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci. 2004;24:10466–74. doi: 10.1523/JNEUROSCI.3001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Gelfand MV, Collins CA, DiAntonio A. Visualizing glutamatergic cell bodies and synapses in Drosophila larval and adult CNS. J Comp Neurol. 2008;508:131–52. doi: 10.1002/cne.21670. [DOI] [PubMed] [Google Scholar]
- Daniels RW, Miller BR, DiAntonio A. Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration. Neurobiol Dis. 2011;41:415–20. doi: 10.1016/j.nbd.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert EA, Condron BG. Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci. 2010;33:424–34. doi: 10.1016/j.tins.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert EA, Heffron DS, Mandell JW, Condron BG. Serotonergic dystrophy induced by excess serotonin. Mol Cell Neurosci. 2010;44:297–306. doi: 10.1016/j.mcn.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries B, Mamsa H, Stam AH, Wan J, Bakker SL, Vanmolkot KR, Haan J, Terwindt GM, Boon EM, Howard BD, Frants RR, Baloh RW, Ferrari MD, Jen JC, van den Maagdenberg AM. Episodic ataxia associated with EAAT1 mutation C186S affecting glutamate reuptake. Arch Neurol. 2009;66:97–101. doi: 10.1001/archneurol.2008.535. [DOI] [PubMed] [Google Scholar]
- Demchyshyn LL, Pristupa ZB, Sugamori KS, Barker EL, Blakely RD, Wolfgang WJ, Forte MA, Niznik HB. Cloning, expression, and localization of a chloride-sensitive serotonin transporter from Drosophila melanogaster. Proc Natl Acad Sci USA. 1994;91:5158–5162. doi: 10.1073/pnas.91.11.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermauw W, Van Leeuwen T. The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem Mol Biol. 2014;45C:89–110. doi: 10.1016/j.ibmb.2013.11.001. [DOI] [PubMed] [Google Scholar]
- DeSalvo MK, Mayer N, Mayer F, Bainton RJ. Physiologic and anatomic characterization of the brain surface glia barrier of Drosophila. Glia. 2011;59:1322–40. doi: 10.1002/glia.21147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikeakos JD, Reudelhuber TL. Sending proteins to dense core secretory granules: still a lot to sort out. J Cell Biol. 2007;177:191–6. doi: 10.1083/jcb.200701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donly BC, Caveney S. A transporter for phenolamine uptake in the arthropod CNS. Arch Insect Biochem Physiol. 2005;59:172–83. doi: 10.1002/arch.20063. [DOI] [PubMed] [Google Scholar]
- Donly BC, Richman A, Hawkins E, McLean H, Caveney S. Molecular cloning and functional expression of an insect high-affinity Na+-dependent glutamate transporter. Eur J Biochem. 1997;248:535–42. doi: 10.1111/j.1432-1033.1997.t01-1-00535.x. [DOI] [PubMed] [Google Scholar]
- Donly C, Verellen L, Cladman W, Caveney S. Functional comparison of full-length and N-terminal-truncated octopamine transporters from Lepidoptera. Insect Biochem Mol Biol. 2007;37:933–40. doi: 10.1016/j.ibmb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–58. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Edwards TN, Meinertzhagen IA. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog Neurobiol. 2010;90:471–97. doi: 10.1016/j.pneurobio.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enell L, Hamasaka Y, Kolodziejczyk A, Nässel DR. gamma-Aminobutyric acid (GABA) signaling components in Drosophila: immunocytochemical localization of GABA(B) receptors in relation to the GABA(A) receptor subunit RDL and a vesicular GABA transporter. J Comp Neurol. 2007;505:18–31. doi: 10.1002/cne.21472. [DOI] [PubMed] [Google Scholar]
- Engels WR. The origin of P elements in Drosophila melanogaster. Bioessays. 1992;14:681–6. doi: 10.1002/bies.950141007. [DOI] [PubMed] [Google Scholar]
- Eriksen J, Jorgensen TN, Gether U. Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges. J Neurochem. 2010;113:27–41. doi: 10.1111/j.1471-4159.2010.06599.x. [DOI] [PubMed] [Google Scholar]
- Evans PD, Maqueira B. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci. 2005;5:111–8. doi: 10.1007/s10158-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Fei H, Chow DM, Chen A, Romero-Calderón R, Ong WS, Ackerson LC, Maidment NT, Simpson JH, Frye MA, Krantz DE. Mutation of the Drosophila vesicular GABA transporter disrupts visual figure detection. J Exp Biol. 2010;213:1717–30. doi: 10.1242/jeb.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei H, Grygoruk A, Brooks ES, Chen A, Krantz DE. Trafficking of vesicular neurotransmitter transporters. Traffic. 2008;9:1425–36. doi: 10.1111/j.1600-0854.2008.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei H, Karnezis T, Reimer RJ, Krantz DE. Membrane topology of the Drosophila vesicular glutamate transporter. J Neurochem. 2007;101:1662–1671. doi: 10.1111/j.1471-4159.2007.04518.x. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Bazalakova M, Savchenko V, Tapia JC, Wright J, Blakely RD. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc Natl Acad Sci U S A. 2004;101:8762–7. doi: 10.1073/pnas.0401667101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–98. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, Daws LC, Sitte HH, Javitch JA, Galli A, Gether U. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–29. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun B-C, Kileen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- Foss SM, Li H, Santos MS, Edwards RH, Voglmaier SM. Multiple dileucine-like motifs direct VGLUT1 trafficking. J Neurosci. 2013;33:10647–60. doi: 10.1523/JNEUROSCI.5662-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Yang JW, Moritz AE, Challasivakanaka S, Smith MA, Holy M, Wilebski K, Sitte HH, Vaughan RA. Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. J Biol Chem. 2013;287:29702–12. doi: 10.1074/jbc.M112.367706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ. Transport of endocannabinoids across the plasma membrane and within the cell. Febs J. 2013;280:1895–904. doi: 10.1111/febs.12212. [DOI] [PubMed] [Google Scholar]
- Fox LE, Soll DR, Wu CF. Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine Beta hydroxlyase mutation. J Neurosci. 2006;26:1486–1498. doi: 10.1523/JNEUROSCI.4749-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, Wang X, Collins CA, Rodal AA, Yuan Q, Verstreken P, Dickman DK. New approaches for studying synaptic development, function, and plasticity using Drosophila as a model system. J Neurosci. 2013;33:17560–8. doi: 10.1523/JNEUROSCI.3261-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–80. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular Glutamate Transporters 1 and 2 Target to Functionally Distinct Synaptic Release Sites. Science. 2004 doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–27. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Gabriel LR, Wu S, Kearney P, Bellve KD, Standley C, Fogarty KE, Melikian HE. Dopamine transporter endocytic trafficking in striatal dopaminergic neurons: differential dependence on dynamin and the actin cytoskeleton. J Neurosci. 2013;33:17836–46. doi: 10.1523/JNEUROSCI.3284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2:E239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant P, Malutan T, McLean H, Verellen L, Caveney S, Donly C. Functionally distinct dopamine and octopamine transporters in the CNS of the cabbage looper moth. Eur J Biochem. 2003;270:664–74. doi: 10.1046/j.1432-1033.2003.03417.x. [DOI] [PubMed] [Google Scholar]
- Galli A, Petersen CI, deBlaquiere M, Blakely RD, DeFelice LJ. Drosophila serotonin transporters have voltage-dependent uptake coupled to a serotonin-gated ion channel. J Neurosci. 1997;17:3401–11. doi: 10.1523/JNEUROSCI.17-10-03401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, McLean H, Caveney S, Donly C. Molecular cloning and functional characterization of a GABA transporter from the CNS of the cabbage looper, Trichoplusia ni. Insect Biochem Mol Biol. 1999;29:609–23. doi: 10.1016/s0965-1748(99)00039-9. [DOI] [PubMed] [Google Scholar]
- Gasque G, Conway S, Huang J, Rao Y, Vosshall LB. Small molecule drug screening in Drosophila identifies the 5HT2A receptor as a feeding modulation target. Sci Rep. 2013;3:srep02120. doi: 10.1038/srep02120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaul U, Chang H, Choi T, Karim F, Rubin GM. Identification of ras targets using a genetic approach. Ciba Found Symp. 1993;176:85–92. doi: 10.1002/9780470514450.ch6. discussion 92–5. [DOI] [PubMed] [Google Scholar]
- Gavin BA, Arruda SE, Dolph PJ. The role of carcinine in signaling at the Drosophila photoreceptor synapse. PLoS Genet. 2007;3:e206. doi: 10.1371/journal.pgen.0030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giang T, Ritze Y, Rauchfuss S, Ogueta M, Scholz H. The serotonin transporter expression in Drosophila melanogaster. J Neurogenet. 2011;25:17–26. doi: 10.3109/01677063.2011.553002. [DOI] [PubMed] [Google Scholar]
- Glatt C, Almonte M, Taylor T, Edwards RH, Freimer N, Tanner C. Structural variants in the vesicular monoamine transporter do not contribute to sporadic Parkinson’s disease. Mov Disord. 2006;21:426–7. doi: 10.1002/mds.20798. [DOI] [PubMed] [Google Scholar]
- Gratz SJ, Cummings AM, Nguyen JN, Hamm DC, Donohue LK, Harrison MM, Wildonger J, O’Connor-Giles KM. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013;194:1029–35. doi: 10.1534/genetics.113.152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderón R, Chang H-Y, Houshyar R, Bainton RJ, DiAntonio A, Krantz DE. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin and octopamine. J Neurobiol. 2005;64:239–258. doi: 10.1002/neu.20146. [DOI] [PubMed] [Google Scholar]
- Grewer C, Gameiro A, Rauen T. SLC1 glutamate transporters. Pflugers Arch. 2014;466:3–24. doi: 10.1007/s00424-013-1397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean Y, Grillet M, Augustin H, Ferveur JF, Featherstone DE. A glial amino-acid transporter controls synapse strength and courtship in Drosophila. Nat Neurosci. 2008;11:54–61. doi: 10.1038/nn2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygoruk A, Fei H, Daniels RW, Miller BR, Diantonio A, Krantz DE. A tyrosine-based motif localizes a Drosophila vesicular transporter to synaptic vesicles in vivo. J Biol Chem. 2010;285:6867–78. doi: 10.1074/jbc.M109.073064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot TS, Miller GW. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol Neurobiol. 2009;39:149–170. doi: 10.1007/s12035-009-8059-y. [DOI] [PubMed] [Google Scholar]
- Guzman MS, De Jaeger X, Raulic S, Souza IA, Li AX, Schmid S, Menon RS, Gainetdinov RR, Caron MG, Bartha R, Prado VF, Prado MA. Elimination of the vesicular acetylcholine transporter in the striatum reveals regulation of behaviour by cholinergic-glutamatergic co-transmission. PLoS Biol. 2011;9:e1001194. doi: 10.1371/journal.pbio.1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MK, Blakely RD. The functional impact of SLC6 transporter genetic variation. Annu Rev Pharmacol Toxicol. 2007;47:401–41. doi: 10.1146/annurev.pharmtox.47.120505.105242. [DOI] [PubMed] [Google Scholar]
- Hamilton PJ, Campbell NG, Sharma S, Erreger K, Herborg Hansen F, Saunders C, Belovich AN, Sahai MA, Cook EH, Gether U, McHaourab HS, Matthies HJ, Sutcliffe JS, Galli A. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol Psychiatry. 2013;18:1315–23. doi: 10.1038/mp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Hardaway JA, Hardie SL, Whitaker SM, Baas SR, Zhang B, Bermingham DP, Lichtenstein AJ, Blakely RD. Forward genetic analysis to identify determinants of dopamine signaling in Caenorhabditis elegans using swimming-induced paralysis. G3 (Bethesda) 2013;2:961–75. doi: 10.1534/g3.112.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V, Wodarz A. Initial neurogenesis in Drosophila. Wiley Interdiscip Rev Dev Biol. 2013;2:701–21. doi: 10.1002/wdev.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, Zigmond MJ. Reactive dopamine metabolites and neurotoxicity: implications for Parkinson’s disease. Adv Exp Med Biol. 1996;387:97–106. doi: 10.1007/978-1-4757-9480-9_13. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–75. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–38. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol. 2011 doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovemann BT, Ryseck RP, Walldorf U, Stortkuhl KF, Dietzel ID, Dessen E. The Drosophila ebony gene is closely related to microbial peptide synthetases and shows specific cuticle and nervous system expression. Gene. 1998;221:1–9. doi: 10.1016/s0378-1119(98)00440-5. [DOI] [PubMed] [Google Scholar]
- Hua Z, Leal-Ortiz S, Foss SM, Waites CL, Garner CC, Voglmaier SM, Edwards RH. v-SNARE composition distinguishes synaptic vesicle pools. Neuron. 2011;71:474–87. doi: 10.1016/j.neuron.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Huang Y, Chinnappan R, Bocchini C, Gustin MC, Stern M. The Drosophila inebriated-encoded neurotransmitter/osmolyte transporter: dual roles in the control of neuronal excitability and the osmotic stress response. Genetics. 2002;160:561–9. doi: 10.1093/genetics/160.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Stern M. In vivo properties of the Drosophila inebriated-encoded neurotransmitter transporter. J Neurosci. 2002;22:1698–708. doi: 10.1523/JNEUROSCI.22-05-01698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliadi KG, Avivi A, Iliadi NN, Knight D, Korol AB, Nevo E, Taylor P, Moran MF, Kamyshev NG, Boulianne GL. nemy encodes a cytochrome b561 that is required for Drosophila learning and memory. Proc Natl Acad Sci U S A. 2008;105:19986–91. doi: 10.1073/pnas.0810698105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar AA, Hossain MM, Bernstein AI, Miller GW, Richardson JR, Bennett JW. Fungal-derived semiochemical 1-octen-3-ol disrupts dopamine packaging and causes neurodegeneration. Proc Natl Acad Sci U S A. 2013;110:19561–6. doi: 10.1073/pnas.1318830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 1976;262:215–36. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen JC, Wan J, Palos TP, Howard BD, Baloh RW. Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology. 2005;65:529–34. doi: 10.1212/01.wnl.0000172638.58172.5a. [DOI] [PubMed] [Google Scholar]
- Jia XX, Gorczyca M, Budnik V. Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. J Neurobiol. 1993;24:1025–44. doi: 10.1002/neu.480240804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Omote H, Moriyama Y. Vesicular GABA transporter (VGAT) transports beta-alanine. J Neurochem. 2013;127:482–6. doi: 10.1111/jnc.12393. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci U S A. 2005;102:3495–500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahsai L, Zars T. Learning and memory in Drosophila: behavior, genetics, and neural systems. Int Rev Neurobiol. 2011;99:139–67. doi: 10.1016/B978-0-12-387003-2.00006-9. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Clemencon B, Simonin A, Leuenberger M, Lochner M, Weisstanner M, Hediger MA. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol Aspects Med. 2014;34:108–20. doi: 10.1016/j.mam.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Karsai G, Pollak E, Wacker M, Vomel M, Selcho M, Berta G, Nachman RJ, Isaac RE, Molnar L, Wegener C. Diverse in- and output polarities and high complexity of local synaptic and non-synaptic signaling within a chemically defined class of peptidergic Drosophila neurons. Front Neural Circuits. 2013;7:127. doi: 10.3389/fncir.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Takuwa K, Kuniyoshi H, Juni N, Nakajima T, Yamamoto D, Kimura Y. Cloning and characterization of a Drosophila melanogaster cDNA encoding a glutamate transporter. Biosci Biotechnol Biochem. 1999;63:2042–4. doi: 10.1271/bbb.63.2042. [DOI] [PubMed] [Google Scholar]
- Kerr TM, Muller CL, Miah M, Jetter CS, Pfeiffer R, Shah C, Baganz N, Anderson GM, Crawley JN, Sutcliffe JS, Blakely RD, Veenstra-Vanderweele J. Genetic background modulates phenotypes of serotonin transporter Ala56 knock-in mice. Mol Autism. 2013;4:35. doi: 10.1186/2040-2392-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Kitamoto T, Wang W, Salvaterra PM. Structure and organization of the Drosophila cholinergic locus. J Biol Chem. 1998;273:2706–2713. doi: 10.1074/jbc.273.5.2706. [DOI] [PubMed] [Google Scholar]
- Kitamoto T, Xie X, Wu CF, Salvaterra PM. Isolation and characterization of mutants for the vesicular acetylcholine transporter gene in Drosophila. J Neurobiol. 2000;42:161–171. [PubMed] [Google Scholar]
- Knapek S, Kahsai L, Winther AM, Tanimoto H, Nassel DR. Short neuropeptide F acts as a functional neuromodulator for olfactory memory in Kenyon cells of Drosophila mushroom bodies. J Neurosci. 2013;33:5340–5. doi: 10.1523/JNEUROSCI.2287-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–60. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koon AC, Ashley J, Barria R, DasGupta S, Brain R, Waddell S, Alkema MJ, Budnik V. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat Neurosci. 2011;14:190–9. doi: 10.1038/nn.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey JF, Pasca SP, Shcheglovitov A, Yazawa M, Schwemberger R, Rasmusson R, Dolmetsch RE. Timothy syndrome is associated with activity-dependent dendritic retraction in rodent and human neurons. Nat Neurosci. 2013;16:201–9. doi: 10.1038/nn.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–84. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen MB, Sonders MS, Mortensen OV, Larson GA, Zahniser NR, Amara SG. Dopamine transport by the serotonin transporter: a mechanistically distinct mode of substrate translocation. J Neurosci. 2011;31:6605–15. doi: 10.1523/JNEUROSCI.0576-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal HO, Chang HY, Terrell AN, Brooks ES, Pulido D, Simon AF, Krantz DE. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol Dis. 2010;40:102–112. doi: 10.1016/j.nbd.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal HO, Krantz DE. SLC18: Vesicular neurotransmitter transporters for monoamines and acetylcholine. Mol Aspects Med. 2013;34:360–72. doi: 10.1016/j.mam.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal HO, Terrell A, Lam HA, Djapri C, Jang J, Hadi R, Roberts L, Shahi V, Chou MT, Biedermann T, Huang B, Lawless GM, Maidment NT, Krantz DE. Drosophila modifier screens to identify novel neuropsychiatric drugs including aminergic agents for the possible treatment of Parkinson’s disease and depression. Mol Psychiatry. 2014;19:235–242. doi: 10.1038/mp.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SM, Kumar N, Neckameyer WS. GABAergic modulation of motor-driven behaviors in juvenile Drosophila and evidence for a nonbehavioral role for GABA transport. J Neurobiol. 2004;61:189–208. doi: 10.1002/neu.20061. [DOI] [PubMed] [Google Scholar]
- Leal SM, Neckameyer WS. Pharmacological evidence for GABAergic regulation of specific behaviors in Drosophila melanogaster. J Neurobiol. 2002;50:245–61. doi: 10.1002/neu.10030. [DOI] [PubMed] [Google Scholar]
- Lebestky T, Chang JS, Dankert H, Zelnik L, Kim YC, Han KA, Wolf FW, Perona P, Anderson DJ. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64:522–36. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Gaspar P, Nicolas D, Hornung JP. Transitory uptake of serotonin in the developing sensory pathways of the common marmoset. J Comp Neurol. 2006;499:677–89. doi: 10.1002/cne.21137. [DOI] [PubMed] [Google Scholar]
- Lee D, Su H, O’Dowd DK. GABA receptors containing Rdl subunits mediate fast inhibitory synaptic transmission in Drosophila neurons. J Neurosci. 2003;23:4625–34. doi: 10.1523/JNEUROSCI.23-11-04625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Rohila S, Han KA. The octopamine receptor OAMB mediates ovulation via Ca2+/calmodulin-dependent protein kinase II in the Drosophila oviduct epithelium. PLoS One. 2009;4:e4716. doi: 10.1371/journal.pone.0004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron. 2012;76:175–91. doi: 10.1016/j.neuron.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Li H, Chaney S, Forte M, Hirsch J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol. 2000;10:211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Lima F, Prado VF, Prado MA, Kushmerick C. Quantal release of acetylcholine in mice with reduced levels of the vesicular acetylcholine transporter. J Neurochem. 2010;113:943–51. doi: 10.1111/j.1471-4159.2010.06657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Placais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012a;488:512–6. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- Liu Q, Liu S, Kodama L, Driscoll MR, Wu MN. Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr Biol. 2012b;22:2114–23. doi: 10.1016/j.cub.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WW, Wilson RI. Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. Proc Natl Acad Sci U S A. 2013;110:10294–9. doi: 10.1073/pnas.1220560110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Peter D, Roghani A, Schuldiner S, Prive GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that supresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- Madsen KL, Thorsen TS, Rahbek-Clemmensen T, Eriksen J, Gether U. Protein interacting with C kinase 1 (PICK1) reduces reinsertion rates of interaction partners sorted to Rab11-dependent slow recycling pathway. J Biol Chem. 2012;287:12293–308. doi: 10.1074/jbc.M111.294702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makos MA, Han KA, Heien ML, Ewing AG. Using In Vivo Electrochemistry to Study the Physiological Effects of Cocaine and Other Stimulants on the Drosophila melanogaster Dopamine Transporter. ACS Chem Neurosci. 2010;1:74–83. doi: 10.1021/cn900017w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malutan T, McLean H, Caveney S, Donly C. A high-affinity octopamine transporter cloned from the central nervous system of cabbage looper Trichoplusia ni. Insect Biochem Mol Biol. 2002;32:343–57. doi: 10.1016/s0965-1748(01)00114-x. [DOI] [PubMed] [Google Scholar]
- Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr Biol. 2009;19:R700–13. doi: 10.1016/j.cub.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Mathews EA, Mullen GP, Hodgkin J, Duerr JS, Rand JB. Genetic interactions between UNC-17/VAChT and a novel transmembrane protein in Caenorhabditis elegans. Genetics. 2013;192:1315–25. doi: 10.1534/genetics.112.145771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ. Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. J Neurosci. 2009;29:3538–50. doi: 10.1523/JNEUROSCI.5564-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, Galli A, Blakely RD. Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci. 2008;28:7040–6. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbungu D, Ross LS, Gill SS. Cloning, functional expression, and pharmacology of a GABA transporter from Manduca sexta. Arch Biochem Biophys. 1995;318:489–97. doi: 10.1006/abbi.1995.1258. [DOI] [PubMed] [Google Scholar]
- McClung C, Hirsh J. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr Biol. 1998;8:109–12. doi: 10.1016/s0960-9822(98)70041-7. [DOI] [PubMed] [Google Scholar]
- McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD. Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci. 2007;27:14216–27. doi: 10.1523/JNEUROSCI.2992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean H, Verellen L, Caveney S, Donly C. Molecular cloning and functional characterization of a neuronal choline transporter from Trichoplusia ni. Insect Biochem Mol Biol. 2005;35:61–72. doi: 10.1016/j.ibmb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Melnattur KV, Lee CH. Visual circuit assembly in Drosophila. Dev Neurobiol. 2011;71:1286–96. doi: 10.1002/dneu.20894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon KP, Carrillo RA, Zinn K. Development and plasticity of the Drosophila larval neuromuscular junction. Wiley Interdiscip Rev Dev Biol. 2013;2:647–70. doi: 10.1002/wdev.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M, Linn CEJ, White K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu L, Ito K, Bacon JP, Strausfeld NJ. Optic glomeruli and their inputs in Drosophila share an organizational ground pattern with the antennal lobes. J Neurosci. 2012;32:6061–71. doi: 10.1523/JNEUROSCI.0221-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ. Further studies on the nature and causes of gene mutations. In: Jones DF. Proceedings of the 6th International Congress of Genetics; Wisconsin: Brooklyn Botanic Gardens; 1932. pp. 213–255. [Google Scholar]
- Mutch SA, Kensel-Hammes P, Gadd JC, Fujimoto BS, Allen RW, Schiro PG, Lorenz RM, Kuyper CL, Kuo JS, Bajjalieh SM, Chiu DT. Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci. 2011;31:1461–70. doi: 10.1523/JNEUROSCI.3805-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nall AH, Sehgal A. Small-molecule screen in adult Drosophila identifies VMAT as a regulator of sleep. J Neurosci. 2013;33:8534–40. doi: 10.1523/JNEUROSCI.0253-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naves LA, Van der Kloot W. Transmitter packaging at frog neuromuscular junctions exposed to anticholinesterases; the role of second-stage acetylcholine loading. J Neurophysiol. 1996;76:2614–25. doi: 10.1152/jn.1996.76.4.2614. [DOI] [PubMed] [Google Scholar]
- Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, Lin CF, Stevens C, Wang LS, Makarov V, Polak P, Yoon S, Maguire J, Crawford EL, Campbell NG, Geller ET, Valladares O, Schafer C, Liu H, Zhao T, Cai G, Lihm J, Dannenfelser R, Jabado O, Peralta Z, Nagaswamy U, Muzny D, Reid JG, Newsham I, Wu Y, Lewis L, Han Y, Voight BF, Lim E, Rossin E, Kirby A, Flannick J, Fromer M, Shakir K, Fennell T, Garimella K, Banks E, Poplin R, Gabriel S, DePristo M, Wimbish JR, Boone BE, Levy SE, Betancur C, Sunyaev S, Boerwinkle E, Buxbaum JD, Cook EH, Jr, Devlin B, Gibbs RA, Roeder K, Schellenberg GD, Sutcliffe JS, Daly MJ. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–5. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer WS, Cooper RL. GABA transporters in Drosophila melanogaster: molecular cloning, behavior, and physiology. Invert Neurosci. 1998;3:279–94. doi: 10.1007/BF02577688. [DOI] [PubMed] [Google Scholar]
- Oland LA, Gibson NJ, Tolbert LP. Localization of a GABA transporter to glial cells in the developing and adult olfactory pathway of the moth Manduca sexta. J Comp Neurol. 2010;518:815–38. doi: 10.1002/cne.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak WL. Mutations affecting the vision of Drosophila melanogaster. In: King RC, editor. Handbook of Genetics. Vol. 3. Plenum Publishing Corp; New York: 1975. [Google Scholar]
- Parikh V, St Peters M, Blakely RD, Sarter M. The presynaptic choline transporter imposes limits on sustained cortical acetylcholine release and attention. J Neurosci. 2013;33:2326–37. doi: 10.1523/JNEUROSCI.4993-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–61. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Park SK, George R, Cai Y, Chang HY, Krantz DE, Friggi-Grelin F, Birman S, Hirsh J. Cell type-specific limitation on in vivo serotonin storage following ectopic expression of the Drosophila serotonin transporter, dSERT. J Neurobiol. 2006;66:452–462. doi: 10.1002/neu.20222. [DOI] [PubMed] [Google Scholar]
- Park SS, Schulz EM, Lee D. Disruption of dopamine homeostasis underlies selective neurodegeneration mediated by alpha-synuclein. Eur J Neurosci. 2007;26:3104–12. doi: 10.1111/j.1460-9568.2007.05929.x. [DOI] [PubMed] [Google Scholar]
- Parsons SM. Transport mechanisms in acetylcholine and monoamine storage. Faseb J. 2000;14:2423–34. doi: 10.1096/fj.00-0203rev. [DOI] [PubMed] [Google Scholar]
- Pendleton RG, Rasheed A, Hillman R. Effects of adrenergic agents on locomotor behavior and reproductive development in Drosophila. Drug Development Research. 2000;50:142–146. [Google Scholar]
- Penmatsa A, Gouaux E. How LeuT shapes our understanding of the mechanisms of sodium-coupled neurotransmitter transporters. J Physiol. 2014;592:863–9. doi: 10.1113/jphysiol.2013.259051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo AB, Karam CS, Zhang Y, Ma CL, McCabe BD, Javitch JA. Amphetamine-induced behavior requires CaMKII-dependent dopamine transporter phosphorylation. Mol Psychiatry. 2014;19:279–81. doi: 10.1038/mp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo AB, Karam CS, Zhang Y, Yano H, Freyberg RJ, Karam DS, Freyberg Z, Yamamoto A, McCabe BD, Javitch JA. The membrane-raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Mol Psychiatry. 2013;18:824–33. doi: 10.1038/mp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poodry CA, Hall L, Suzuki DT. Developmental properties of Shibire: a pleiotropic mutation affecting larval and adult locomotion and development. Dev Biol. 1973;32:373–86. doi: 10.1016/0012-1606(73)90248-0. [DOI] [PubMed] [Google Scholar]
- Porzgen P, Park SK, Hirsh J, Sonders MS, Amara SG. The antidepressant-sensitive dopamine transporter in Drosophila: a primordial carrier for catecholamines. Mol Pharmacol. 2001;59:83–95. doi: 10.1124/mol.59.1.83. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Larsen KE, Krantz DE, Liu Y, Haycock JW, Setlik W, Gershon MD, Edwards RH, Sulzer D. Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J Neurosci. 2000;20:7297–7306. doi: 10.1523/JNEUROSCI.20-19-07297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado VF, Roy A, Kolisnyk B, Gros R, Prado MA. Regulation of cholinergic activity by the vesicular acetylcholine transporter. Biochem J. 2013;450:265–74. doi: 10.1042/BJ20121662. [DOI] [PubMed] [Google Scholar]
- Pramod AB, Foster J, Carvelli L, Henry LK. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW. The role of SNARE proteins in trafficking and function of neurotransmitter transporters. Handb Exp Pharmacol. 2006:181–96. doi: 10.1007/3-540-29784-7_9. [DOI] [PubMed] [Google Scholar]
- Reimer RJ. SLC17: a functionally diverse family of organic anion transporters. Mol Aspects Med. 2013;34:350–9. doi: 10.1016/j.mam.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro FM, Black SA, Prado VF, Rylett RJ, Ferguson SS, Prado MA. The “ins” and “outs” of the high-affinity choline transporter CHT1. J Neurochem. 2006;97:1–12. doi: 10.1111/j.1471-4159.2006.03695.x. [DOI] [PubMed] [Google Scholar]
- Richardt A, Rybak J, Störtkuhl KF, Meinertzhagen IA, Hovemann BT. Ebony protein in the Drosophila nervous system: optic neuropile expression in glial cells. J Comp Neurol. 2002;452:93–102. doi: 10.1002/cne.10360. [DOI] [PubMed] [Google Scholar]
- Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- Rival T, Soustelle L, Cattaert D, Strambi C, Iche M, Birman S. Physiological requirement for the glutamate transporter dEAAT1 at the adult Drosophila neuromuscular junction. J Neurobiol. 2006;66:1061–74. doi: 10.1002/neu.20270. [DOI] [PubMed] [Google Scholar]
- Rival T, Soustelle L, Strambi C, Besson MT, Iche M, Birman S. Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr Biol. 2004;14:599–605. doi: 10.1016/j.cub.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Rizki TM, Rizki RM. The cellular defense system of Drosophila melanogaster. In: King RC, Akai H, editors. Insect ultrastructure. Plenum; New York: 1984. pp. 579–604. [Google Scholar]
- Robertson SD, Matthies HJ, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol. 2009;39:73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues HA, de Fonseca MC, Camargo WL, Lima PM, Martinelli PM, Naves LA, Prado VF, Prado MA, Guatimosim C. Reduced expression of the vesicular acetylcholine transporter and neurotransmitter content affects synaptic vesicle distribution and shape in mouse neuromuscular junction. PLoS One. 2013;8:e78342. doi: 10.1371/journal.pone.0078342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez GJ, Roman DL, White KJ, Nichols DE, Barker EL. Distinct recognition of substrates by the human and Drosophila serotonin transporters. J Pharmacol Exp Ther. 2003;306:338–46. doi: 10.1124/jpet.103.048751. [DOI] [PubMed] [Google Scholar]
- Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–77. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- Roelofs J, Van Haastert PJ. Genes lost during evolution. Nature. 2001;411:1013–4. doi: 10.1038/35082627. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Broadie K. Electrophysiological analysis of synaptic transmission in central neurons of Drosophila larvae. J Neurophysiol. 2002;88:847–60. doi: 10.1152/jn.2002.88.2.847. [DOI] [PubMed] [Google Scholar]
- Roman G, Davis RL. Molecular biology and anatomy of Drosophila olfactory associative learning. Bioessays. 2001;23:571–81. doi: 10.1002/bies.1083. [DOI] [PubMed] [Google Scholar]
- Romero-Calderón R, Shome RM, Simon AF, Daniels RW, DiAntonio A, Krantz DE. A screen for neurotransmitter transporters expressed In the visual system of Drosophila melanogaster identifies three novel genes. Developmental Neurobiology. 2007;67:550–69. doi: 10.1002/dneu.20342. [DOI] [PubMed] [Google Scholar]
- Romero-Calderón R, Uhlenbrock G, Boryz J, Simon AF, Grygoruk A, Yee SK, Shyer A, Ackerson LC, Maidment NT, Meinertzhagen IA, Hovemann BT, Krantz DE. A glial variant of the vesicular monoamine transporter is required to store histamine in the Drosophila visual system. PLoS Genet. 2008;4(11):e1000245. doi: 10.1371/journal.pgen.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YS, Golic KG. Gene targeting by homologous recombination in Drosophila. Science. 2000;288:2013–8. doi: 10.1126/science.288.5473.2013. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Kramer R, Blakely RD, Murphy DL, Verrey F. The SLC6 transporters: perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Arch. 2014;466:25–42. doi: 10.1007/s00424-013-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager JJ, Torres GE. Proteins interacting with monoamine transporters: current state and future challenges. Biochemistry. 2011;50:7295–310. doi: 10.1021/bi200405c. [DOI] [PubMed] [Google Scholar]
- Sakrikar D, Mazei-Robison MS, Mergy MA, Richtand NW, Han Q, Hamilton PJ, Bowton E, Galli A, Veenstra-Vanderweele J, Gill M, Blakely RD. Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation. J Neurosci. 2012;32:5385–97. doi: 10.1523/JNEUROSCI.6033-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu SK, Ross LS, Gill SS. A cocaine insensitive chimeric insect serotonin transporter reveals domains critical for cocaine interaction. Eur J Biochem. 2002;269:3934–44. doi: 10.1046/j.1432-1033.2002.03084.x. [DOI] [PubMed] [Google Scholar]
- Sandoval GM, Duerr JS, Hodgkin J, Rand JB, Ruvkun G. A genetic interaction between the vesicular acetylcholine transporter VAChT/UNC-17 and synaptobrevin/SNB-1 in C. elegans. Nat Neurosci. 2006;9:599–601. doi: 10.1038/nn1685. [DOI] [PubMed] [Google Scholar]
- Sang TK, Chang HY, Lawless GM, Ratnaparkhi A, Mee L, Ackerson LC, Maidment NT, Krantz DE, Jackson GR. A Drosophila model of mutant human parkin-induced toxicity demonstrates selective loss of dopaminergic neurons and dependence on cellular dopamine. J Neurosci. 2007;27:981–992. doi: 10.1523/JNEUROSCI.4810-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MS, Park CK, Foss SM, Li H, Voglmaier SM. Sorting of the vesicular GABA transporter to functional vesicle pools by an atypical dileucine-like motif. J Neurosci. 2013;33:10634–46. doi: 10.1523/JNEUROSCI.0329-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–6. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer SW, Jong CJ, Ramila KC, Azuma J. Physiological roles of taurine in heart and muscle. J Biomed Sci. 2010;17(Suppl 1):S2. doi: 10.1186/1423-0127-17-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicker K, Uzelac Z, Gesmonde J, Bulling S, Stockner T, Freissmuth M, Boehm S, Rudnick G, Sitte HH, Sandtner W. Unifying concept of serotonin transporter-associated currents. J Biol Chem. 2013;287:438–45. doi: 10.1074/jbc.M111.304261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schioth HB, Roshanbin S, Hagglund MG, Fredriksson R. Evolutionary origin of amino acid transporter families SLC32, SLC36 and SLC38 and physiological, pathological and therapeutic aspects. Mol Aspects Med. 2014;34:571–85. doi: 10.1016/j.mam.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Seal B, Daniels GM, Wolfgang WJ, Forte MA, Amara SG. Identification and characterization of a cDNA encoding a neuronal glutamate transporter from Drosophila melanogaster. Receptors Channels. 1998;6:51–64. [PubMed] [Google Scholar]
- Seelig JD, Jayaraman V. Feature detection and orientation tuning in the Drosophila central complex. Nature. 2013;503:262–6. doi: 10.1038/nature12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–7. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007;51:333–55. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AF, Daniels R, Romero-Calderón R, Grygoruk A, Chang HY, Najibi R, Shamouelian D, Salazar E, Solomon M, Ackerson LC, Maidment NT, Diantonio A, Krantz DE. Drosophila vesicular monoamine transporter mutants can adapt to reduced or eliminated vesicular stores of dopamine and serotonin. Genetics. 2009;181:525–541. doi: 10.1534/genetics.108.094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehnge H, Huang X, Becker M, Whitley P, Conover D, Stern M. A neurotransmitter transporter encoded by the Drosophila inebriated gene. Proc Natl Acad Sci U S A. 1996;93:13262–7. doi: 10.1073/pnas.93.23.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H-J, Ming G-L, Fon E, Bellocchio E, Edwards RH, Poo M-M. Expression of a putative vesicular acetylcholine transporter facilitates quantal transmitter packaging. Neuron. 1997;18:815–826. doi: 10.1016/s0896-6273(00)80320-7. [DOI] [PubMed] [Google Scholar]
- Soustelle L, Besson MT, Rival T, Birman S. Terminal glial differentiation involves regulated expression of the excitatory amino acid transporters in the Drosophila embryonic CNS. Dev Biol. 2002;248:294–306. doi: 10.1006/dbio.2002.0742. [DOI] [PubMed] [Google Scholar]
- St Johnston D. Using mutants, knockdowns, and transgenesis to investigate gene function in Drosophila. Wiley Interdiscip Rev Dev Biol. 2013;2:587–613. doi: 10.1002/wdev.101. [DOI] [PubMed] [Google Scholar]
- Stacey SM, Muraro NI, Peco E, Labbe A, Thomas GB, Baines RA, van Meyel DJ. Drosophila glial glutamate transporter Eaat1 is regulated by fringe-mediated notch signaling and is essential for larval locomotion. J Neurosci. 2010;30:14446–57. doi: 10.1523/JNEUROSCI.1021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M, Ganetzky B. Identification and characterization of inebriated, a gene affecting neuronal excitability in Drosophila. J Neurogenet. 1992;8:157–72. doi: 10.3109/01677069209083445. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Sinakevitch I, Vilinsky I. The mushroom bodies of Drosophila melanogaster: an immunocytological and golgi study of Kenyon cell organization in the calyces and lobes. Microsc Res Tech. 2003;62:151–69. doi: 10.1002/jemt.10368. [DOI] [PubMed] [Google Scholar]
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol. 2002;12:633–8. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- Stuart AE, Borycz J, Meinertzhagen IA. The dynamics of signaling at the histaminergic photoreceptor synapse of arthropods. Prog Neurobiol. 2007;82:202–27. doi: 10.1016/j.pneurobio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Suh J, Jackson FR. Drosophila ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron. 2007;55:435–47. doi: 10.1016/j.neuron.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13:537–51. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–49. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes PA, Condron BG. Development and sensitivity to serotonin of Drosophila serotonergic varicosities in the central nervous system. Dev Biol. 2005;286:207–16. doi: 10.1016/j.ydbio.2005.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes PA, Norman HS, Condron BG. Variation in serotonergic and dopaminergic neuronal survival in the central nervous system of adult Drosophila. Cell Tissue Res. 2004;317:327–31. doi: 10.1007/s00441-004-0940-4. [DOI] [PubMed] [Google Scholar]
- Tabb JS, Ueda T. Phylogenetic studies on the synaptic vesicle glutamate transport system. J Neurosci. 1991;11:1822–8. doi: 10.1523/JNEUROSCI.11-06-01822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–46. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Talkowski ME, Kirov G, Bamne M, Georgieva L, Torres G, Mansour H, Chowdari KV, Milanova V, Wood J, McClain L, Prasad K, Shirts B, Zhang J, O’Donovan MC, Owen MJ, Devlin B, Nimgaonkar VL. A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum Mol Genet. 2008;17:747–58. doi: 10.1093/hmg/ddm347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimgan MS, Berg JS, Stuart AE. Comparative sequence analysis and tissue localization of members of the SLC6 family of transporters in adult Drosophila melanogaster. J Exp Biol. 2006;209:3383–404. doi: 10.1242/jeb.02328. [DOI] [PubMed] [Google Scholar]
- Tickoo S, Russell S. Drosophila melanogaster as a model system for drug discovery and pathway screening. Curr Opin Pharmacol. 2002;2:555–60. doi: 10.1016/s1471-4892(02)00206-0. [DOI] [PubMed] [Google Scholar]
- Torres G, Horowitz JM. Activating properties of cocaine and cocaethylene in a behavioral preparation of Drosophila melanogaster. Synapse. 1998;29:148–61. doi: 10.1002/(SICI)1098-2396(199806)29:2<148::AID-SYN6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Traiffort E, O’Regan S, Ruat M. The choline transporter-like family SLC44: properties and roles in human diseases. Mol Aspects Med. 2013;34:646–54. doi: 10.1016/j.mam.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Ueno T, Tomita J, Tanimoto H, Endo K, Ito K, Kume S, Kume K. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat Neurosci. 2012;15:1516–23. doi: 10.1038/nn.3238. [DOI] [PubMed] [Google Scholar]
- Umesh A, Gill SS. Immunocytochemical localization of a Manduca sexta gamma-aminobutyric acid transporter. J Comp Neurol. 2002;448:388–98. doi: 10.1002/cne.10271. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. Loading and recycling of synaptic vesicles in the Torpedo electric organ and the vertebrate neuromuscular junction. Prog Neurobiol. 2003;71:269–303. doi: 10.1016/j.pneurobio.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109:5469–74. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickrey TL, Condron B, Venton BJ. Detection of endogenous dopamine changes in Drosophila melanogaster using fast-scan cyclic voltammetry. Anal Chem. 2009;81:9306–13. doi: 10.1021/ac901638z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickrey TL, Xiao N, Venton BJ. Kinetics of the dopamine transporter in Drosophila larva. ACS Chem Neurosci. 2013;4:832–7. doi: 10.1021/cn400019q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina-Vilaseca A, Sorkin A. Lysine 63-linked polyubiquitination of the dopamine transporter requires WW3 and WW4 domains of Nedd4–2 and UBE2D ubiquitin-conjugating enzymes. J Biol Chem. 2010;285:7645–56. doi: 10.1074/jbc.M109.058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019–27. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ferdousy F, Lawal H, Huang Z, Daigle JG, Izevbaye I, Doherty O, Thomas J, Stathakis DG, O’Donnell JM. Catecholamines up integrates dopamine synthesis and synaptic trafficking. J Neurochem. 2011;119:1294–305. doi: 10.1111/j.1471-4159.2011.07517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25:6221–34. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI. Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci. 2013;36:217–41. doi: 10.1146/annurev-neuro-062111-150533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter S, Brunk I, Walther DJ, Holtje M, Jiang M, Peter JU, Takamori S, Jahn R, Birnbaumer L, Ahnert-Hilger G. Gαo2 regulates vesicular glutamate transporter activity by changing its chloride dependence. J Neurosci. 2005;25:4672–80. doi: 10.1523/JNEUROSCI.0549-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopp PJ, Williams BL, Selegue JE, Carroll SB. Drosophila pigmentation evolution: divergent genotypes underlying convergent phenotypes. Proc Natl Acad Sci U S A. 2003;100:1808–13. doi: 10.1073/pnas.0336368100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–63. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TR. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv Genet. 1987;24:127–222. [PubMed] [Google Scholar]
- Wu CF, Wong F. Frequency characteristics in the visual system of Drosophila: genetic dissection of electroretinogram components. J Gen Physiol. 1977;69:705–24. doi: 10.1085/jgp.69.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Prentice H. Role of taurine in the central nervous system. J Biomed Sci. 2010;17(Suppl 1):S1. doi: 10.1186/1423-0127-17-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MN, Koh K, Yue Z, Joiner WJ, Sehgal A. A genetic screen for sleep and circadian mutants reveals mechanisms underlying regulation of sleep in Drosophila. Sleep. 2008;31:465–72. doi: 10.1093/sleep/31.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, Vidensky S, Brennan J, Poulsen D, Won Park J, Li Jeon N, Robinson MB, Rothstein JD. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–94. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Hersh LB. The vesicular monoamine transporter 2 contains trafficking signals in both its N-glycosylation and C-terminal domains. J Neurochem. 2007;100:1387–96. doi: 10.1111/j.1471-4159.2006.04326.x. [DOI] [PubMed] [Google Scholar]
- Ye R, Blakely RD. Natural and engineered coding variation in antidepressant-sensitive serotonin transporters. Neuroscience. 2011;197:28–36. doi: 10.1016/j.neuroscience.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc Natl Acad Sci U S A. 1997;94:4131–6. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Naganuma F, Iida T, Nakamura T, Harada R, Mohsen AS, Kasajima A, Sasano H, Yanai K. Molecular mechanism of histamine clearance by primary human astrocytes. Glia. 2013;61:905–16. doi: 10.1002/glia.22484. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Terry DS, Shi L, Quick M, Weinstein H, Blanchard SC, Javitch JA. Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue. Nature. 2011;474:109–13. doi: 10.1038/nature09971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Danbolt NC. GABA and Glutamate Transporters in Brain. Front Endocrinol. 2013;4:165. doi: 10.3389/fendo.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]