Highlight
A leucine-rich repeat receptor-like kinase LP2 is transcriptionally controlled by zinc finger protein DST and serves as a negative regulator in drought response
Key words: Abiotic stress, leaf panicle 2, LRR, Oryza sativa, RLK, water channel.
Abstract
Drought is a recurring climatic hazard that reduces the crop yields. To avoid the negative effects of drought on crop production, extensive efforts have been devoted to investigating the complex mechanisms of gene expression and signal transduction during drought stress. Receptor-like kinases (RLKs) play important roles in perceiving extracellular stimuli and activating downstream signalling responses. The rice genome contains >1100 RLK genes, of which only two are reported to function in drought stress. A leucine-rich repeat (LRR)-RLK gene named Leaf Panicle 2 (LP2) was previously found to be strongly expressed in leaves and other photosynthetic tissues, but its function remains unclear. In the present study, it was shown that the expression of LP2 was down-regulated by drought and abscisic acid (ABA). Transgenic plants overexpressing LP2 accumulated less H2O2, had more open stomata in leaves, and showed hypersensitivity to drought stress. Further investigation revealed that transcription of LP2 was directly regulated by the zinc finger transcription factor DROUGHT AND SALT TOLERANCE (DST). In addition, LP2 was identified as a functional kinase localized to the plasma membrane and interacted with the drought-responsive aquaporin proteins OsPIP1; 1, OsPIP1; 3, and OsPIP2; 3. Thus, the findings provided evidence that the LRR-RLK LP2, transcriptionally regulated by the drought-related transcription factor DST, served as a negative regulator in drought response.
Introduction
Plants are sessile organisms and are frequently exposed to biotic and abiotic stresses during their life cycle. Among abiotic stresses, drought is one of the most common environmental factors that limit crop productivity, especially in Asia where at least 23 million ha of rice (20% of the total world rice area) are drought prone (Pandey et al., 2000). The economic costs of drought can be enormous. For example, the 2004 drought in Thailand was estimated to have affected 2 million ha of crop area, and had effects on >8 million people (Pandey et al., 2000). Drought affects the development and grain yield of rice at different growth stages, such as germination, vegetative growth, spikelet development and fertility, and grain filling (Wade et al., 1999), by suppressing leaf expansion, tillering and mid-day photosynthesis, and by reducing photosynthetic rates and leaf area due to early senescence (Nooden and Leopold, 1988; Kramer and Boyer, 1995). Considerable effort has been made to discover the key genes and signalling pathways involved in plant responses to drought stress. Detailed analysis will help in understanding the mechanism of drought signalling transduction and lay a foundation for engineering strategies to improve stress tolerance of crops around the world.
Perception and processing of extracellular signals through plasma membrane receptor-like protein kinases (RLKs) in plants lead to alterations in the concentrations of cellular ions and molecules that activate protein phosphorylation pathways. This in turn regulates expression of stress-responsive genes to generate physiological, biochemical, and other adaptive responses that reduce or eliminate the problem (Lee et al., 2011; Xing et al., 2011). RLKs belong to the serine/threonine protein kinase family. Through phosphorylation and dephosphorylation of the Ser/Thr residues, RLKs convert external signals to intracellular cytoplasmic signals (Stone and Walker, 1995; Morillo and Tax, 2006). RLKs comprise a major gene family in plants, with at least 610 members in Arabidopsis and ~1132 members in rice (Shiu et al., 2004; Morillo and Tax, 2006). Based on the N-terminal extracellular domains, RLKs fall into multiple subfamilies, and those containing a leucine-rich repeat domain (LRR-RLKs) constitute the largest subfamily in plants. In rice, ~309 LRR-RLK genes have been identified and classified into five subgroups based on phylogenetic analysis (Sun and Wang, 2011). LRR-RLKs are implicated in diverse signalling events that lead to such things as resistance to pathogens, plant development, and growth (Sun and Wang, 2011). Accumulating data suggest that LRR-RLKs also play an important role in regulating environmental stress responses. Five LRR-RLKs involved in stress response in plants have been identified at the molecular level. Srlk (Salt-induced Receptor-Like Kinase) encoding an LRR kinase in Medicago truncatula was reported to regulate the response of roots to salt stress (de Lorenzo et al., 2009). GHR1 (GUARD CELL HYDROGEN PEROXIDE-RESISTANT1) mediates abscisic acid (ABA) and hydrogen peroxide (H2O2)-regulated stomatal movement in Arabidopsis (Hua et al., 2012). Arabidopsis RLK7 is involved in the control of germination speed and tolerance to oxidation stress (Pitorre et al., 2010). RPK1 (receptor-like protein kinase 1) functions as a positive regulator of ABA signalling; transgenic Arabidopsis plants overexpressing RPK1 showed increased ABA sensitivity in root growth and stomatal closure, and displayed less transpirational water loss (Osakabe et al., 2010).
To date, only two LRR-RLK members in rice are reported to play a role in response to environmental stress. OsSIK1 is expressed mainly in stems and spikelets, and its expression is induced by salt, drought, or H2O2 treatment. Transgenic rice plants overexpressing OsSIK1 showed higher tolerance to salt and drought stresses, whereas knock-out mutants were sensitive to the same stresses (Ouyang et al., 2010). OsSIK1-overexpressing plants accumulated much less H2O2, and stomatal density was reduced by 8.4–17.8% in leaves (Ouyang et al., 2010). OsSIK2 is an S-domain RLK and is expressed mainly in rice leaves and leaf sheaths (Chen et al., 2013). OsSIK2 was induced by NaCl, drought, cold, dark, and ABA treatment, and transgenic plants overexpressing OsSIK2 exhibited enhanced tolerance to salt and drought stress compared with controls (Chen et al., 2013). Although the importance of RLK genes in abiotic stress has been well recognized in both monocots and dicots, the regulatory mechanisms governing their expression remain elusive.
Aquaporins, known as membrane intrinsic proteins (MIPs), are membrane channels that facilitate the transport of water and small neutral molecules (Maurel et al., 2008; Li et al., 2014). In plants, aquaporins regulate various physiological processes such as leaflet movement, CO2 assimilation, osmoregulation, uptake of mineral elements, and stomatal conductance (Gerbeau et al., 1999; Hanba et al., 2004; Siefritz et al., 2004; Ma et al., 2006). Aquaporins are also reported to be involved in various abiotic stresses, such as drought, salinity, and low temperature (Lian et al., 2004; Prak et al., 2008; Matsumoto et al., 2009; Mosa et al., 2012). There are 35 and 39 MIP members in the Arabidopsis and rice genomes, respectively (Wallace and Roberts, 2004; Sakurai et al., 2005). On the basis of sequence homology and localization, plant MIPs are classified into four subfamilies: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), small basic intrinsic proteins (SIPs), and nodulin-26 like intrinsic proteins (NIPs) (Li et al., 2014). The PIP subfamily can be further divided into two phylogenetic subgroups PIP1 and PIP2. PIP1 isoforms generally have very low or no water channel activity, whereas PIP2 subgroup members possess high water channel activity (Kammerloher et al., 1994; Weig et al., 1997). The activity of PIPs is regulated by post-translational modifications and protein interactions (Chaumont and Tyerman, 2014). In a phosphoproteomic study on rice plasma membranes, several aquaporins expressed in shoots were phosphorylated (Whiteman et al., 2008). However, knowledge of the protein kinases that phosphorylate aquaporins is still poorly understood.
In this study, we carried out a functional analysis on an LRR-RLK LP2 in rice. Expression of LP2 was down-regulated by various abiotic stresses. Transgenic plants overexpressing LP2 accumulated reduced levels of H2O2 and were sensitive to drought stress. Further analyses indicated that LP2 was transcriptionally regulated by a previously reported C2H2 zinc finger transcriptional factor DST (DROUGHT AND SALT TOLERANCE). It was also shown that LP2 interacted in vivo with three PIPs. It is reported herein that DST-regulated LRR-RLK LP2 participates in drought stress response.
Materials and methods
Plant materials and growth conditions
Rice (Oryza sativa) japonica cv. Nipponbare was used in this study. Drought treatment was applied mainly according to published methods (Ning et al., 2011) with some modifications. Seeds of wild-type and LP2-overexpressing transgenic plants were soaked in water for 2 d, and left for another 5 d. Seven-day-old seedlings were planted in small pots containing the same amount of soil and grown in a phytotron (PAR 384 μmol m–2 s–1, 25 ºC day/20 ºC darkness, 12h photoperiod). After 2 weeks, water was removed from the tray to begin drought treatment. Plant phenotypes were observed after 6, 7, and 8 d. Nine days after treatment, the plants were moved back to the watered trays for recovery. Survival rates of the transgenic lines and wild-type plants were recorded 4 d after rehydration. The drought stress experiment was performed at least three times.
Generation of LP2- and DST-overexpressing transgenic rice plants
For construction of the LP2 overexpression vector, the full-length cDNA of LP2 was amplified and inserted into the pCUbi1390 vector under control of the Zea mays ubiquitin promoter. For construction of the DST overexpression vector, the full-length cDNA of DST was amplified and inserted into pCUbi1390 with a 3×FLAG-tag at the C-terminus. The constructs were introduced into Nipponbare by Agrobacterium tumefaciens-mediated transformation as described previously (Hiei et al., 1994). Transgenic rice lines and their progeny were grown in paddy fields in Beijing (39°54’N, summer season, temperate climate) or Hainan (18°16’N, winter season, subtropical climate) under local growing conditions. Seeds of T2 homozygous overexpression lines were subjected to molecular and phenotypic analyses. The primer sequences used for the construction of this vector are listed in Supplementary Table S1 available at JXB online.
Quantitative measurement of H2O2
H2O2 contents were determined with a Hydrogen Peroxide assay kit (Beyotime, S0038) according to the manufacturer’s protocol.
Imaging of rice stomata and measurement of stomatal density
Imaging of rice stomata was conducted as described previously (Huang et al., 2009) with some modifications. Leaves of 4-week-old plants were detached and immediately put between cardboard sheets to avoid curling, and frozen with liquid nitrogen. Stomatal images were obtained using an FEI Quanta 200 environmental scanning electron microscope (ESEM; FEI Corporation, The Netherlands).
Subcellular localization of LP2–GFP proteins
The coding sequence of LP2 was amplified and cloned into the N-terminus of green fluorescent protein (GFP) under control of the Cauliflower mosaic virus (CaMV) 35S promoter in the transient expression vector pA7-GFP, generating recombinant pA7-LP2-GFP. The recombinant vector was then transformed into rice protoplasts according to protocols described previously (Chen et al., 2006), or into onion (Allium spp.) epidermal cells via shotgun bombardment (PDS-1000/He; Bio-Rad). The GFP signal was visualized using a confocal laser-scanning microscope (LSM 700; Carl Zeiss).
RNA preparation and qPCR analysis
Total rice RNA was extracted with an RNA Prep Pure Kit (Zymo Research) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 2 μg of total RNA using a SuperScript II Kit (TaKaRa). Primer pairs used for qPCR are listed in Supplementary Table S1 at JXB online. qPCR analysis was conducted using an ABI7500 fast qPCR system with the SYBR Premix Ex Taq (TaKaRa; RR041A). The procedure was as follows: initial polymerase activation for 30 s at 95 °C followed by 40 cycles of 95 °C for 5 s and 60 °C for 34 s. For each sample, qPCR was performed with three technical replicates on three biological replicates. The 2–ΔΔCT method was used to analyse relative transcript levels in gene expression (Livak and Schmittgen, 2001). Primers used for ChIP-PCR are listed in Supplementary Table S1. For detecting the expression level of LP2 in the dst mutant, 4-week-old plants were used for RNA isolation.
Yeast one-hybrid assay
The assay was carried out as described previously (Gao et al., 2013). To generate AD-DST, the full-length DST coding sequence was amplified by reverese transcription–PCR (RT–PCR) from the Nipponbare cultivar and ligated into the pB42AD vector (Clontech) digested with EcoRI. To generate LP2p::LacZ reporter genes, various fragments of the LP2 promoter were amplified from Nipponbare genomic DNA and inserted into the corresponding sites in the reporter plasmid pLacZi. Plasmids were co-transformed into yeast strain EGY48. Transformants were grown on SD/Trp-/Ura plates for 48h and then transferred onto X- gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) plates for blue colour development.
ChIP-PCR assay
Chromatin immunoprecipitation (ChIP) assays were performed as described previously (Zhang et al., 2012) with some modifications. Approximately 2g of LP2-FLAG-overexpressing transgenic callus was ground to fine powder with liquid nitrogen for further analysis. Monoclonal mouse anti-Flag antibody (Sigma, St. Louis, MO, USA) was used in a ChIP assay. Chromatin precipitated with a pre-immune serum was used as the negative control, whereas the chromatin before precipitation was used as an input control. Primers used for ChIP-PCR are listed in Supplementary Table S1 at JXB online.
Firefly luciferase complementation imaging assay
The assay was carried out as described previously (Hua et al., 2012). LP2 and RAR1 sequences were fused upstream of N-Luc in the pCAMBIA-NLuc vector, and OsPIP1; 1, OsPIP1; 3, OsPIP2; 3, as well as AvrB were fused downstream of C-Luc in the pCAMBIACLuc vector.
Results
Identification and sequence analysis of the LP2 gene
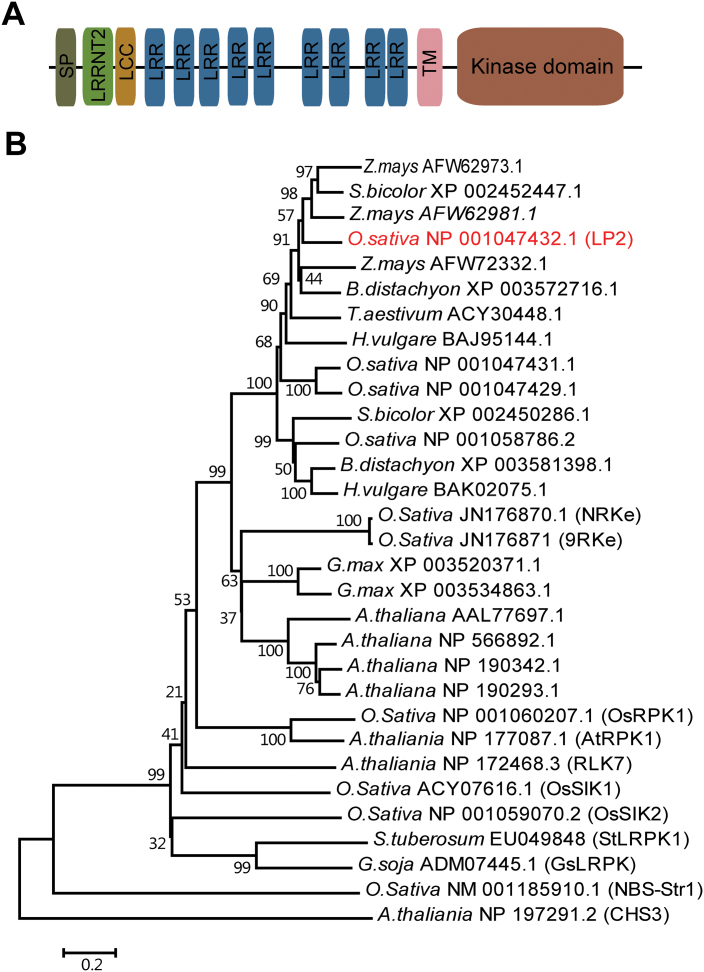
The expression level of an LRR-RLK was highly reduced in an analysis of microarray data from the drought-tolerant dst mutant (Huang et al., 2009). The gene, named Leaf Panicle 2 (LP2; Os02g40240), was strongly expressed in leaves and other photosynthetic tissues (Thilmony et al., 2009). To characterize further the function of LP2 in drought response, the protein sequence was analysed. The protein contains a putative N-terminal signal peptide (SP), a putative extracellular domain containing 11 LRR domains (one LRRNT_2 domain, an LCC domain, and nine LRR domains), a transmembrane domain (TM), and an S_TKc intracellular kinase domain identified by the SMART program (Fig. 1A). BLAST searches of available genome sequences revealed that a homologue sharing the highest sequence homology with LP2 (47.76% amino acid identity) was from Brachypodium distachyon. Close homologues of LP2 were also identified in monocots such as Zea mays, Sorghum bicolor, Triticum aestivum, Hordeum vulgare, and Oryza sativa (Fig. 1B); however, the functions of these genes were largely unknown. There are several LRR-RLKs in O. sativa, Arabidopsis thaliana, and Glycine soja that function in abiotic stress (Wu et al., 2009; Ouyang et al., 2010; Pitorre et al., 2010; Yang et al., 2010; Zhang et al., 2011; Ray et al., 2012; Chen et al., 2013; Shi et al., 2014; Yang et al., 2014). Among them, OsSIK1 and OsSIK2 were reported to improve drought and salt stress tolerance in rice plants (Ouyang et al., 2010; Chen et al., 2013), but OsSIK1 and OsSIK2 share only 30.56% and 18.04% amino acid identities with LP2, respectively.
Fig. 1.
Protein domain structure and phylogenetic analysis of LP2. (A) Schematic depiction of the major domains in LP2. (B) Phylogenetic analysis of LP2 and other LRR receptor-like kinases constructed by the Neighbor–Joining method. The LP2 protein is shown in red.
LP2 is preferentially expressed in vegetative tissues and LP2 is a functional kinase localized to the plasma membrane
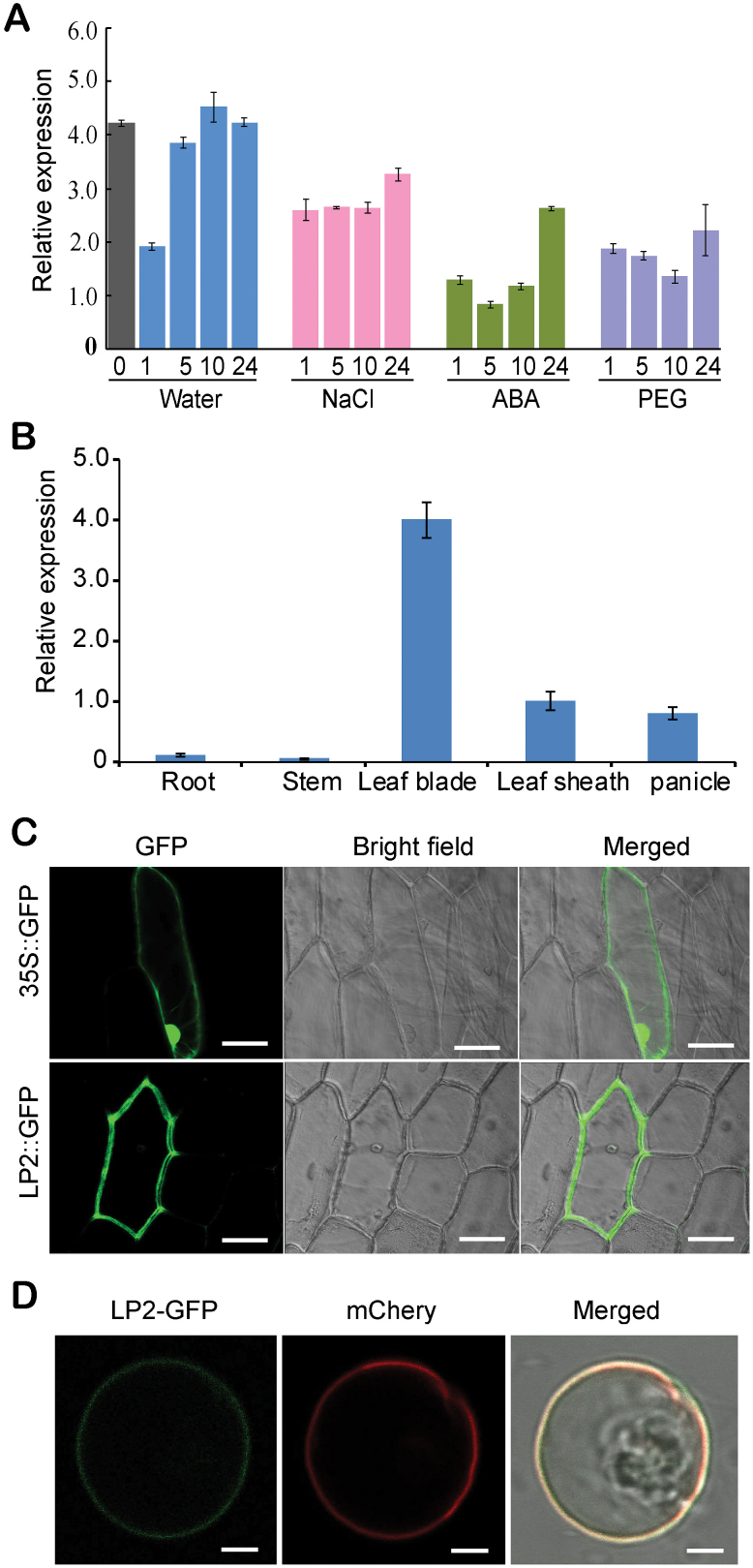
To confirm whether the expression of LP2 is regulated by abiotic stress, quantitative RT–PCR (qRT–PCR) was used to investigate the transcriptional profile of LP2 under various stress conditions. LP2 was down-regulated after 1h of water treatment, but then steadily increased to the original level after 5h (Fig. 2A). However, expression was highly inhibited by polyethylene glycol (PEG) and ABA treatments after 24h (Fig. 2A). A previous study showed that LP2 was highly expressed in leaves and other photosynthetic tissues (Thilmony et al., 2009). The tissue expression profiles of LP2 were investigated by qRT–PCR analyses. LP2 was preferentially expressed in leaf blades and sheaths, moderately expressed in young panicles, but was not detectable in other tissues, such as roots and stems (Fig. 2B). Transient expression of an LP2–GFP fusion protein in onion epidermal cells and rice protoplasts indicated that LP2–GFP is localized in the plasma membrane (Fig. 2C, D), suggesting a potential role for LP2 in the plasma membrane.
Fig. 2.
Transcript analysis and subcellular localization of LP2. (A) Determination of LP2 transcript levels by qRT–PCR in response to water, NaCl, ABA, and PEG. (B) Tissue-specific expression pattern of LP2 (means ±SD, n=3). (C) Subcellular localization of the fused LP2–GFP in onion epidermal cells. Bars=100 μm. (D) Subcellular localization of the fused LP2–GFP in rice protoplasts. Bar=5 μm.
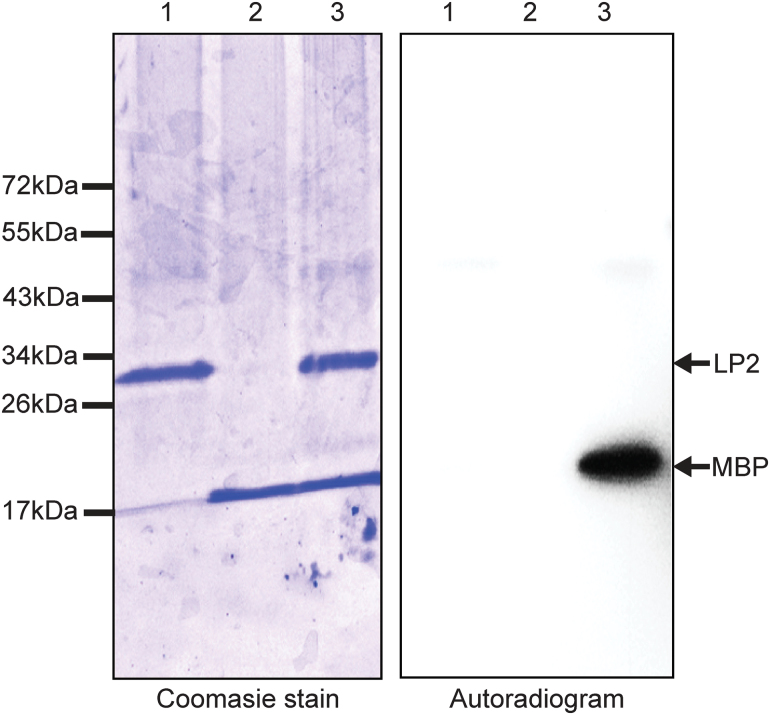
To determine if LP2 has kinase activity, the intracellular kinase domain (residues 682–978) of LP2 fused to a 6× His tag was expressed in Escherichia coli strain BL21. An in vitro kinase assay was then performed with myelin basic protein (MBP) as substrate. The kinase domain showed no autophosphorylation activity, although it phosphorylated the artificial substrate MBP (Fig. 3).
Fig. 3.
In vitro kinase assays with the LP2 kinase domain using MBP as substrate. Protein was visualized by Coomassie Blue staining (left panel), and protein phosphorylation was detected by autoradiography ([32P], right panel). The experiment was repeated twice with similar results.
Up-regulation of LP2 expression leads to drought sensitivity of transgenic plants
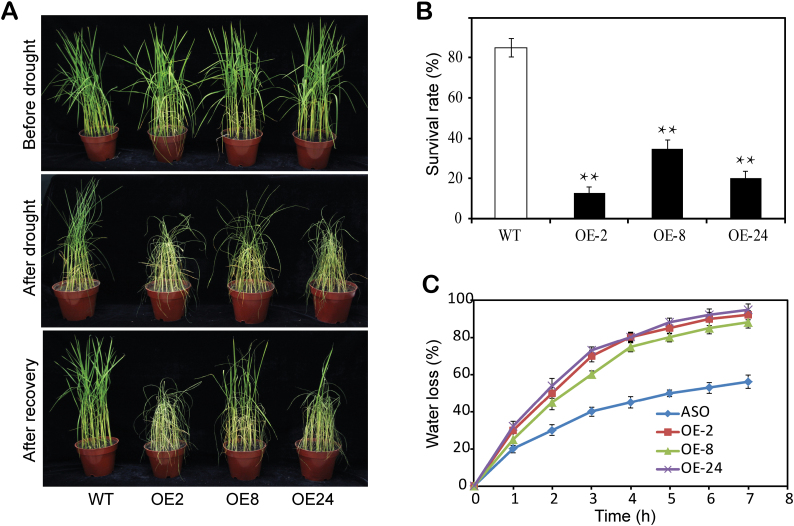
Because the expression level of LP2 was inhibited by PEG and ABA treatment, the effect of LP2 overexpression on plant drought sensitivity was next tested. The full-length open reading frame of LP2 under control of the maize ubiquitin promoter was transformed into japonica rice cultivar Nipponbare. Fifteen independent transgenic lines were identified and hygromycin-resistant T2 plants were then screened and confirmed by qRT–PCR analysis (Supplementary Fig. S1 at JXB online). Three independent transgenic lines (OE-2, OE-8, and OE-24) with a high level of expression of LP2 were chosen for further investigation. Four-week-old plants were subjected to drought stress (Fig. 4A, upper panel). After 8 d of drought treatment, the leaves of the control showed only slight rolling and wilting, but leaves of the three transgenic lines exhibited severe drought-induced rolling and wilting (Fig. 4A, middle panel). After re-watering for 2 d, growth of control plants was almost identical to that of the non-stressed control, whereas growth of the transgenic lines remained severely inhibited (Fig. 4A, bottom panel). Furthermore, the survival rates of OE-2, OE-8, and OE-24 plants were markedly lower than those of the controls. After rehydration, survival rates were only 13, 35, and 20% in OE-2, OE-8, and OE-24 lines, respectively, compared with 85% for the control (Fig. 4B). The results indicated that overexpression of LP2 reduced drought tolerance in rice. The rate of water loss from detached leaves of the wild-type and LP2 overexpression lines was then measured. The overexpression lines lost water much faster than wild-type plants (Fig. 4C). Thus LP2 was shown to be a negative regulator of the drought signalling pathway.
Fig. 4.
Drought tolerance testing of LP2-overexpressing rice. (A) Phenotypes of wild-type and transgenic plants before drought treatment (top panel), after withholding water for 1 week (middle panel), and after recovery for 3 d (bottom panel). (B) Survival of transgenic and wild-type plants. Data are means ±SD from three independent biological replicates. Asterisks indicate statistically significant differences compared with the control (Student’s t-test: **P<0.01). (C) Water loss rate from transgenic and wild-type plants. Water loss was expressed as a percentage relative to the total water content. Error bars are based on three replicates. For each repeat, 20 fully expanded leaves from ~4-week-old plants were used.
LP2 overexpression results in decreased stomatal closure and increased stomatal density
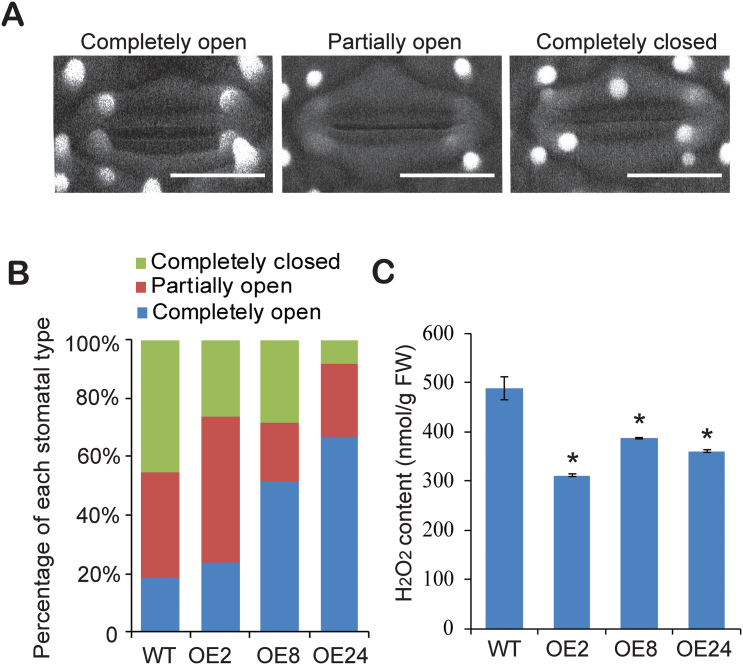
Plant response to drought stress is closely associated with stomatal movement (Hetherington and Woodward, 2003). It was therefore investigated whether the altered phenotypes of LP2 overexpression plants under drought stress were correlated with differences in stomatal aperture by environmental scanning electron microscopy; 45.5% of stomata were completely closed in wild-type plants under drought stress, whereas only 26, 28.6, and 8.3% were completely closed in LP2-overexpressing lines OE-2, OE-8, and OE-24, respectively (Fig. 5A, B). In addition, 36.4% of stomata were partially open in the wild-type plants, but 50.0, 20.0, and 25.0% of stomata were partially open in the LP2-overexpressing lines OE-2, OE-8, and OE-24, respectively (Fig. 5B). Furthermore, 18.2% of stomata were completely open in the wild-type plants, compared with 24.3, 51.4, and 66.7% in the respective LP2-overexpressing lines (Fig. 5B). These results suggested that increased drought sensitivity in LP2-overexpressing plants was largely due to enhanced stomatal opening. Since H2O2 induces stomatal closure (McAinsh et al., 1996; Hamilton et al., 2000; Apel and Hirt, 2004; Mittler et al., 2004; Bright et al., 2006), H2O2 contents in leaves of LP2 overexpression lines were determined and lower accumulation relative to the control was found (Fig. 5C). These results suggested that the increased stomatal opening in LP2 overexpression plants under drought stress may be due to decreased reactive oxygen (ROS) production.
Fig. 5.
Down-regulation of H2O2 in LP2-overexpressing plants inhibits stomatal closure. (A) Scanning electron microscopy images of three levels of stomatal opening. Scale bar=10 μm. (B) Percentages of three levels of stomatal opening in wild-type and LP2-overexpressing plants (n=63 stomata for the wild type; n=55 stomata for OE-2; n=72 stomata for OE-8; n=42 stomata for OE-24). (C) Quantitative measurements of H2O2 in seedling leaves of wild-type and LP2-overexpressing plants. Data are means ±SD from three independent biological replicates. Asterisks indicate statistically significant differences compared with the control (Student’s t-test: *P<0.05).
LP2 expression is directly regulated by DST
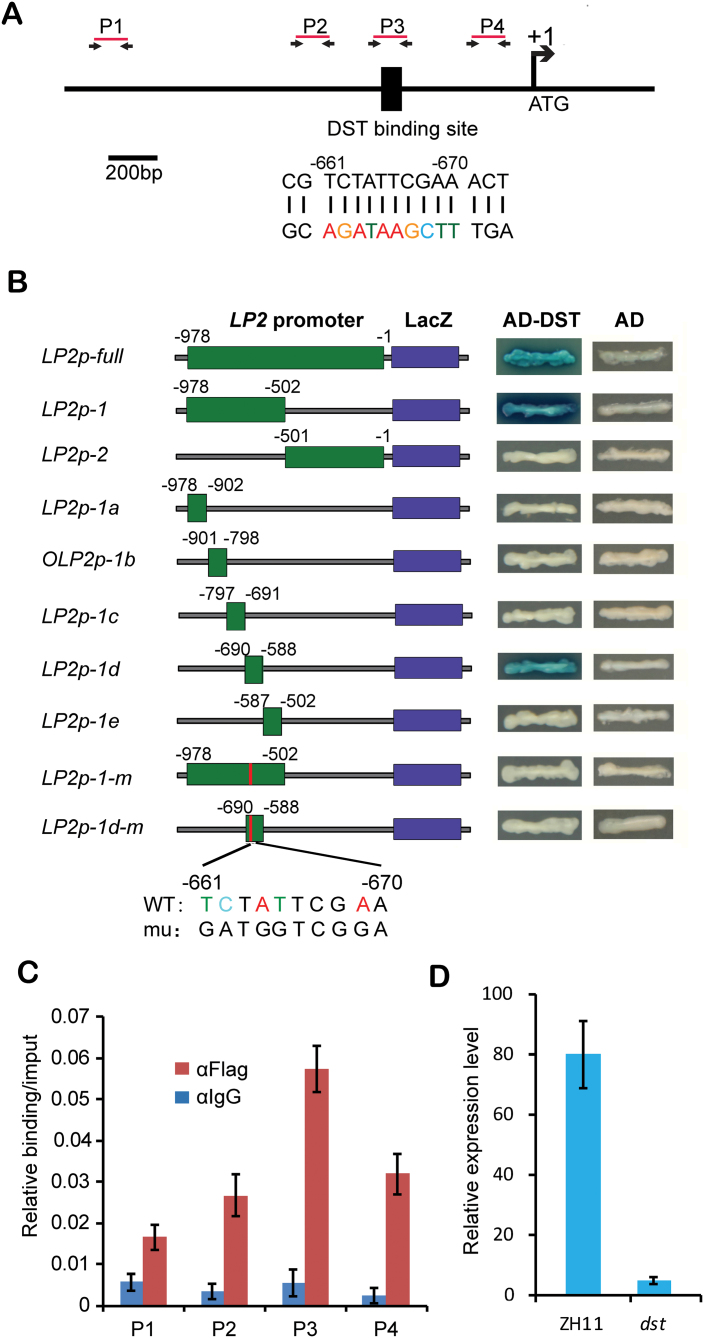
Analysis of the promoter sequence of LP2 revealed a DST-binding element (DBE) at position –670 to –661 (Huang et al., 2009; Fig. 6A). DST is a zinc finger transcription factor that negatively regulates stomatal closure by direct modulation of genes related to H2O2 homeostasis (Huang et al., 2009). To test the possibility that DST directly binds to the DBE in the LP2 promoter, a yeast one-hybrid assay was performed. The results showed that DST protein binds directly to the promoter sequence of LP2 (–2126 to –1) (Fig. 6B). To determine whether the predicted DBE is functional, several truncation derivatives of the –2126 to –1 promoter sequence were subjected to transactivity assays. As shown in Fig. 6B, only the fragment including the DBE interacted with DST in yeast. When the DBE was deleted or mutated, there was no binding (Fig. 6B). A chromatin immunoprecipitation-quantitative real-time PCR (ChIP-qPCR) assay was further applied to investigate the interaction between DST and the LP2 promoter. The results indicated a significant enrichment of DST binding to the DBE of the LP2 promoter, in contrast to its negligible binding to other positions (Fig. 6C). The expression level of LP2 in the dst mutant was also assayed and, as shown in Fig. 6D, expression of LP2 was greatly decreased in the mutant. These results indicated that DST bound to the DBE at position –670 to –661 of the LP2 promoter to regulate LP2 expression directly.
Fig. 6.
LP2 is transcriptionally regulated by DST via direct binding to the promoter. (A) Schematic diagram of the promoter regions of LP2. A black line represents the promoter region of LP2; the black box on the line represents the putative DST-binding site. Upper numbers indicate relative distances from the ATG initiation codon shown as +1 (scale bars=200bp). (B) Yeast one-hybrid assays showing that DST activates the LacZ reporter gene driven by the LP2 promoter containing the putative DST-binding motif, but not LacZ reporter genes driven by the LP2 promoter without the binding motif, or with mutations in the binding motif. Red bars in the construct of LP2p-1-m and LP2p-1d-m indicate the positions of mutations. AD, activation domain. (C) ChIP-qPCR assays showing that LP2 promoter fragments containing the putative DST-binding site are specifically enriched. Four pairs of primers were used for the ChIP-qPCR experiment (means ±SD, n=3). Immunoprecipitation with a pre-immune (Pre.) serum was used as the negative control. (D) Comparison of transcript abundance of LP2 in the wild type (ZH11) and dst mutant by qRT–PCR.
LP2 physically interacts with plasma membrane aquaporins
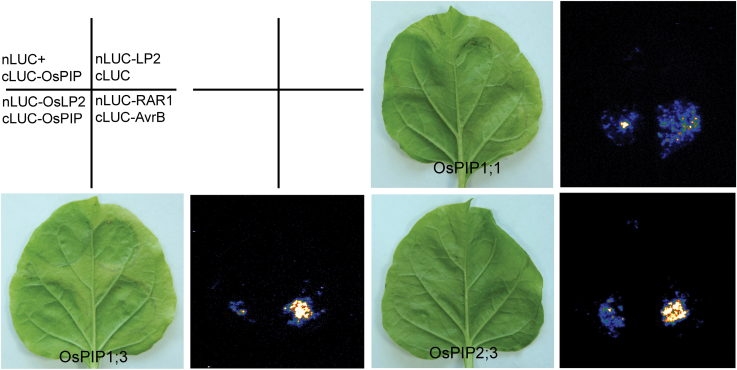
In rice, several aquaporins including OsPIP1; 1, OsPIP1; 3, and OsPIP2; 3 were shown to play crucial roles in response to drought stress by transgenic analyses (Lian et al., 2004; Yu et al., 2006). Recently, SIRK1, a member of the LRR-RLK family in Arabidopsis, was shown to interact with, and activate, multiple aquaporins via phosphorylation (Wu et al., 2013). To investigate if LP2 interacted with these aquaporins, Nluc-LP2 and Cluc-PIP vectors were constructed for firefly luciferase complementation imaging assays (Chen et al., 2008). Co-expression of NLuc-LP2 and cLUC-OsPIP1; 1 resulted in strong LUC complementation, similar to NLuc-LP2/cLUC-OsPIP1; 3, NLuc-LP2/cLUC-OsPIP2; 3, and the positive control, whereas co-expression of NLuc/cLUC-OsPIP or NLuc-LP2/Cluc led to negligible LUC activity (Fig. 7). To evaluate if LP2 can specifically phosphorylate theses aquaporins in vitro, attempts were made to express OsPIP1; 1, OsPIP1; 3, and OsPIP2; 3 in E. coli; these failed however. New measures need to be taken to address this problem.
Fig. 7.
LP2 physically interacts with plasma membrane aquaporins. Interaction of LP2 with three aquaporins as indicated by firefly luciferase complementation imaging assays in Nicotianana benthamiana leaves. nLUC-RAR1 and cLUC-AvrB were used as positive controls. nLUC and cLUC-OsPIP as well as nLUC-LP2 and cLUC were used as negative controls.
Discussion
Unlike animals, higher plants, which are sessile and cannot escape their surroundings, must adapt to environmental change by various molecular responses. For example, when water deficit occurs in the soil, plants respond by activating drought resistance pathways that induce or change the expression level of drought-responsive genes that produce transcription factors, protein kinases, and osmoprotectant-synthesizing enzymes (Hadiarto and Tran, 2011). RLKs are signalling proteins that perceive environmental signals by extracellular domains and transduce them into the cell via a transmembrane domain and an intracellular kinase domain (Morillo and Tax, 2006). RLKs convey signals to target proteins in the cytoplasm by catalytic processes of protein kinase activity. In plants, RLKs comprise a large gene family and regulate various plant processes, including growth and development as well as abiotic stresses (Osakabe et al., 2013). RLK7 (RECEPTOR-LIKE KINASE 7) encoding an LRR-RLK is implicated in control of germination speed and tolerance to oxidative stress (Pitorre et al., 2010). Arabidopsis RPK1 (RECEPTOR-LIKE KINASE 1) participates in early ABA signalling as well as abiotic stress responses; overproduction of RPK1 leads to enhanced drought tolerance accompanied by enhanced expression of stress- and H2O2-responsive genes (Osakabe et al., 2005, 2010). OsSIK1 and OsSIK2 overexpression results in enhanced drought tolerance in rice (Ouyang et al., 2010; Chen et al., 2013). Although the role of the RLK genes in regulating the environmental stress response is well recognized in plants, less is known about the regulatory mechanism of these genes. In the present study, it was shown that the LRR-RLK gene LP2 was transcriptionally regulated by the C2H2 zinc finger transcription factor DST, via direct binding to its promoter. Expression of LP2 was reduced after salt, ABA, and PEG treatments. Overexpression of LP2 in rice reduced the H2O2 content and stomatal closure, resulting in drought sensitivity of transgenic plants. Furthermore, it was found that LP2 interacted in vivo with OsPIPs. These findings established a new branch in the pathway of DST-mediated drought sensing pathway in plants.
Expression of LP2 was previously found to be extremely down-regulated in the dst mutant (Huang et al., 2009). DST is a zinc finger transcription factor that regulates drought and salt tolerance by controlling H2O2-induced stomatal closure. This suggested the possibility that LP2 played a role in abiotic stress response and acted downstream of DST. In the present study, analysis of the LP2 promoter revealed a DBE in the upstream region of –670 to –661, and a yeast one-hybrid assay showed that only the fragment including the DBE can interact with DST in yeast, suggesting that LP2 transcription is likely to be directly regulated by DST. In addition, LP2 was negatively regulated by drought stress, consistent with the induction expression pattern of DST (Fig. 2A; Huang et al., 2009). Overproduction of LP2 in transgenic plants led to drought sensitivity and a faster rate of water loss, corresponding to lower stomatal closure and H2O2 content (Figs 4, 5). H2O2 is as an important signalling molecule that plays a key role in induction of leaf stomatal closure (McAinsh et al., 1996; Hamilton et al., 2000; Apel and Hirt, 2004; Mittler et al., 2004; Bright et al., 2006; Tan et al., 2014). Thus, LP2 functions as a negative regulator of drought tolerance. The present studies added a new member to the DST-mediated drought signalling pathway.
So far, only two LRR-RLK family genes, OsSIK1 and OsSIK2, affecting drought response have been reported in rice (Ouyang et al., 2010; Chen et al., 2013). This study reports a third gene, LP2, also involved in drought response. This finding adds further support to the central role of LRR-RLK proteins in regulating drought response in a staple crop plant. However, there are several important differences in regard to LP2 compared with OsSIK1 and OsSIK2. First, although the three proteins all belong to the LRR-RLK gene family, LP2 does not share significant primary sequence homology with OsSIK1 and OsSIK2 (amino acid identities are only 30.27% and 18.04%, respectively). Phylogenetic analysis revealed that the three proteins are in different subgroups (Fig. 1B). Secondly, LP2 is mainly expressed in leaves and other green tissues, and its expression is reduced by drought, ABA, and PEG treatments (Fig. 2), whereas OsSIK1 is mainly expressed in the stem and spikelet, and OsSIK2 is expressed in the leaf and leaf sheath, and their expression levels are induced by NaCl and drought treatments. Thirdly, although transgenic plants overexpressing each gene accumulate reduced levels of H2O2 in leaves, they show different phenotypes; LP2-overexpressing plants are sensitive to drought stress (Fig. 4), whereas OsSIK1- and OsSIK2-overexpressing plants show higher tolerance to drought stress than control plants. These results indicate that LP2 and the other two OsSIK genes may play different roles in drought response.
Discovery of aquaporins in plants led to a paradigm shift in the understanding of water relations. A large number of plant aquaporins were identified and explained according to their importance in regulating water flow through membranes and in maintaining cellular homeostasis at all developmental stages and under different environmental conditions (Hachez et al., 2006; Chaumont and Tyerman, 2014). However, water is not the sole molecule that diffuses through aquaporins. Accumulating evidence indicates that aquaporins represent an important membrane-selective pathway for diffusion of several neutral solutes, including glycerol, urea, ammonia, carbon dioxide, H2O2, and the metalloids boric acid, silicic acid, and arsenite (Tyerman et al., 2002; Maurel et al., 2008; Gomes et al., 2009; Hachez and Chaumont, 2010; Ma, 2010; Miwa and Fujiwara, 2010; Bienert and Chaumont, 2014; Chaumont and Tyerman, 2014; Kaldenhoff et al., 2014). These solutes are essential for plant growth and development, thus suggesting a role for aquaporins in these processes. Transgenic analyses showed that several aquaporins also have confirmed roles in response to abiotic stress, including drought, salt, and low temperature stresses (Siefritz et al., 2002; Lian et al., 2004; Yu et al., 2006; Prak et al., 2008; Matsumoto et al., 2009; Liu et al., 2013; Li et al., 2014). PIPs represent one subfamily of aquaporins and comprise 11 members in rice. Transgenic analyses revealed that multiple rice PIPs, including OsPIP1; 1, OsPIP1; 3, and OsPIP2; 3, play important roles in drought response (Lian et al., 2004; Yu et al., 2006). In addition, PIP activities are regulated by post-translational modifications and protein interactions (Chaumont and Tyerman, 2014). By using a LUC assay in the present study, it was shown that LP2 interacted with these three PIPs in tobacco cells (Fig. 7). This indicated that these aquaporins might be phosphorylation targets of LP2. More recently, SIRK1, a member of the LRR-RLK family in Arabidopsis, was identified to undergo rapid transient phosphorylation after resupply of sucrose to sucrose-starved seedlings, and shown to interact directly with aquaporins to activate them via phosphorylation (Wu et al., 2013). It was believed that LP2 regulated the activity of OsPIP1; 1, OsPIP1; 3, and OsPIP2; 3 in a similar way, but the hypothesis could not be confirmed in an in vitro kinase assay, because of failure in expressing the three PIP proteins. The biological meaning of LP2–OsPIP interactions remains to be further investigated.
It is well known that the phytohormone ABA is an important regulator of plant response to abiotic stress such as drought by mediating stomatal closure. The main molecular mechanism was proposed as follows: the ABA produced binds to ABA PYR (pyrabactin resistance) receptors and inhibits the activity of PP2C protein phosphatases, including ABI1, which acts as the suppressor of downstream key protein kinases, such as SnRK2 kinases; in turn, ABA activates the S-type anion channel SLAC1 via SnRK2 kinases (Schmidt et al., 1995; Park et al., 2009; Hubbard et al., 2010). However, some stress response pathways are independent of ABA (Zhu, 2002; Yamaguchi-Shinozaki and Shinozaki, 2006). It was reported that the DST-mediated signalling pathway was ABA independent (Huang et al., 2009). Thus, in the present study LP2, through direct transcriptional regulation by DST, participated in drought stress signalling most probably in an ABA-independent way. In addition, unlike the above ABA–PYR–PP2C–SnRK2–SLAC1 pathway, which promotes stomatal closure, the DST–LP2 pathway enhances stomatal opening by reduction of H2O2 accumulation. Therefore, under normal conditions, it is possible that DST promotes transcription of LP2, leading to the stomatal opening and in turn promoting plant growth. When plants are exposed to abiotic stresses, the active ABA content level is elevated and positive regulatory pathways are activated, resulting in stomatal closure. At the same time, expression of LP2 is significantly inhibited by stress conditions (Fig. 2A) in a similar manner to its upstream regulator DST (Huang et al., 2009). Therefore, the two pathways may act co-ordinately to maintain plant growth and development.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Expression levels of the LP2 gene in WT and transgenic lines.
Table S1. Primers used in this study.
Acknowledgements
This work was supported by the National Natural Science Foundation (31371601), and the 863 National High-tech R&D Program of China (2012AA100101). We thank Dr Hongxuan Lin for providing the dst mutant, and Dr Robert McIntosh (University of Sydney), Dr Chunming Wang (Nanjing Agricultural University), and Dr Tao Jiang (Toronto University) for critical reading of the manuscript.
References
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Chaumont F. 2014. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochimica et Biophysica Acta 1840, 1596–1604. [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. 2006. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. The Plant Journal 45, 113–122. [DOI] [PubMed] [Google Scholar]
- Chaumont F, Tyerman SD. 2014. Aquaporins: highly regulated channels controlling plant water relations. Plant Physiology 164, 1600–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zou Y, Shang Y, Lin H, Wang Y, Cai R, Tang X, Zhou JM. 2008. Firefly luciferase complementation imaging assay for protein–protein interactions in plants. Plant Physiology 146, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LJ, Wuriyanghan H, Zhang YQ, et al. 2013. An S-domain receptor-like kinase, OsSIK2, confers abiotic stress tolerance and delays dark-induced leaf senescence in rice. Plant Physiology 163, 1752–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Tao L, Zeng L, Vega-Sanchez ME, Umemura K, Wang GL. 2006. A highly efficient transient protoplast system for analyzing defence gene expression and protein–protein interactions in rice. Molecular Plant Pathology 7, 417–427. [DOI] [PubMed] [Google Scholar]
- de Lorenzo L, Merchan F, Laporte P, Thompson R, Clarke J, Sousa C, Crespi M. 2009. A novel plant leucine-rich repeat receptor kinase regulates the response of Medicago truncatula roots to salt stress. The Plant Cell 21, 668–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Zheng XM, Fei G, et al. 2013. Ehd4 ecodes a novel and Oryza-genus-specific regulator of photoperiodic flowering in rice. PLoS Genetics 9, e1003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbeau P, Guclu J, Ripoche P, Maurel C. 1999. Aquaporin Nt-TIPa can account for the high permeability of tobacco cell vacuolar membrane to small neutral solutes. The Plant Journal 18, 577–587. [DOI] [PubMed] [Google Scholar]
- Gomes D, Agasse A, Thiebaud P, Delrot S, Geros H, Chaumont F. 2009. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochimica et Biophysica Acta 1788, 1213–1228. [DOI] [PubMed] [Google Scholar]
- Hachez C, Chaumont F. 2010. Aquaporins: a family of highly regulated multifunctional channels. Advances in Experimental Medicine and Biology 679, 1–17. [DOI] [PubMed] [Google Scholar]
- Hachez C, Zelazny E, Chaumont F. 2006. Modulating the expression of aquaporin genes in planta: a key to understand their physiological functions? Biochimica et Biophysica Acta 1758, 1142–1156. [DOI] [PubMed] [Google Scholar]
- Hadiarto T, Tran L. 2011. Progress studies of drought-responsive genes in rice. Plant Cell Reports 30, 297–310. [DOI] [PubMed] [Google Scholar]
- Hamilton DW, Hills A, Kohler B, Blatt MR. 2000. Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proceedings of the National Academy of Sciences, USA 97, 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M. 2004. Overexpression of the barley aquaporin HvPIP2; 1 increases internal CO2 conductance and CO2 assimillation in the leaves of transgenic rice plants. Plant and Cell Physiology 45, 521–529. [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI. 2003. The role of stomata in sensing and driving environmental change. Nature 424, 901–908. [DOI] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T. 1994. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. The Plant Journal 6, 271–282. [DOI] [PubMed] [Google Scholar]
- Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z, Guo Y, Chen Z, Gong Z. 2012. A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. The Plant Cell 24, 2546–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XY, Chao DY, Gao JP, Zhu MZ, Shi M, Lin HX. 2009. A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes and Development 23, 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. 2010. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes and Development 24, 1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Kai L, Uehlein N. 2014. Aquaporins and membrane diffusion of CO2 in living organisms. Biochimica et Biophysica Acta 1840, 1592–1595. [DOI] [PubMed] [Google Scholar]
- Kammerloher W, Fischer U, Piechottka GP, Schaffner AR. 1994. Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. The Plant Journal 6, 187–199. [DOI] [PubMed] [Google Scholar]
- Kramer PJ, Boyer JS. 1995. Water relations of plants and soils . San Diego: Academic Press. [Google Scholar]
- Lee SK, Kim BG, Kwon TR, et al. 2011. Overexpression of the mitogen-activated protein kinase gene OsMAPK33 enhances sensitivity to salt stress in rice (Oryza sativa L.). Journal of Bioscience 36, 139–151. [DOI] [PubMed] [Google Scholar]
- Li GW, Santoni V, Maurel C. 2014. Plant aquaporins: roles in plant physiology. Biochimica et Biophysica Acta 1840, 1574–1582. [DOI] [PubMed] [Google Scholar]
- Lian HL, Yu X, Ye Q, Ding X, Kitagawa Y, Kwak SS, Su WA, Tang ZC. 2004. The role of aquaporin RWC3 in drought avoidance in rice. Plant and Cell Physiology 45, 481–489. [DOI] [PubMed] [Google Scholar]
- Liu C, Fukumoto T, Matsumoto T, et al. 2013. Aquaporin OsPIP1; 1 promotes rice salt resistance and seed germination. Plant Physiology and Biochemistry 63, 151–158. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Ma JF. 2010. Silicon transporters in higher plants. Advances in Experimental Medicine and Biology 679, 99–109. [DOI] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. 2006. A silicon transporter in rice. Nature 440, 688–691. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Lian HL, Su WA, Tanaka D, Liu C, Iwasaki I, Kitagawa Y. 2009. Role of the aquaporin PIP1 subfamily in the chilling tolerance of rice. Plant and Cell Physiology 50, 216–229. [DOI] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. 2008. Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology 59, 595–624. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Clayton H, Mansfield TA, Hetherington AM. 1996. Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiology 111, 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Science 9, 490–498. [DOI] [PubMed] [Google Scholar]
- Miwa K, Fujiwara T. 2010. Boron transport in plants: co-ordinated regulation of transporters. Annals of Botany 105, 1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillo SA, Tax FE. 2006. Functional analysis of receptor-like kinases in monocots and dicots. Current Opinion in Plant Biology 9, 460–469. [DOI] [PubMed] [Google Scholar]
- Mosa KA, Kumar K, Chhikara S, Mcdermott J, Liu Z, Musante C, White JC, Dhankher OP. 2012. Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Research 21, 1265–1277. [DOI] [PubMed] [Google Scholar]
- Ning Y, Jantasuriyarat C, Zhao Q, et al. 2011. The SINA E3 ligase OsDIS1 negatively regulates drought response in rice. Plant Physiology 157, 242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooden LD, Leopold AC. 1988. Senescence and aging in plants . San Diego: Academic Press. [Google Scholar]
- Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran L. 2013. Sensing the environment: key roles of membrane-localized kinases in plant perception and response to abiotic stress. Journal of Experimental Botany 64, 445–458. [DOI] [PubMed] [Google Scholar]
- Osakabe Y, Maruyama K, Seki M, Satou M, Shinozaki K, Yamaguchi-Shinozaki K. 2005. Leucine-rich repeat receptor-like kinase1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis. The Plant Cell 17, 1105–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y, Mizuno S, Tanaka H, Maruyama K, Osakabe K, Todaka D, Fujita Y, Kobayashi M, Shinozaki K, Yamaguchi-Shinozaki K. 2010. Overproduction of the membrane-bound receptor-like protein kinase1, RPK1, enhances abiotic stress tolerance in Arabidopsis. Journal of Biological Chemistry 285, 9190–9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang SQ, Liu YF, Liu P, Lei G, He SJ, Ma B, Zhang WK, Zhang JS, Chen SY. 2010. Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. The Plant Journal 62, 316–329. [DOI] [PubMed] [Google Scholar]
- Pandey S, Behura DD., Villano R, Naik D. 2000. Economic cost of drought and farmers’ coping mechanisms: a study of rainfed rice systems in Eastern India . IRRI Discussion Paper Series 39. Philippines: International Rice Research Institute. [Google Scholar]
- Park SY, Fung P, Nishimura N, et al. 2009. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324, 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitorre D, Llauro C, Jobet E, Guilleminot J, Brizard JP, Delseny M, Lasserre E. 2010. RLK7, a leucine-rich repeat receptor-like kinase, is required for proper germination speed and tolerance to oxidative stress in Arabidopsis thaliana. Planta 232, 1339–1353. [DOI] [PubMed] [Google Scholar]
- Prak S, Hem S, Boudet J, Viennois G, Sommerer N, Rossignol M, Maurel C, Santoni V. 2008. Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins: role in subcellular trafficking of AtPIP2; 1 in response to salt stress. Molecular and Cellular Proteomics 7, 1019–1030. [DOI] [PubMed] [Google Scholar]
- Ray S, Kapoor S, Tyagi AK. 2012. Analysis of transcriptional and upstream regulatory sequence activity of two environmental stress-inducible genes, NBS-Str1 and BLEC-Str8, of rice. Transgenic Research 21, 351–366. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M. 2005. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant and Cell Physiology 46, 1568–1577. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Schelle I, Liao YJ, Schroeder JI. 1995. Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proceedings of the National Academy of Sciences, USA 92, 9535–9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CC, Feng CC, Yang MM, Li JL, Li XX, Zhao BC, Huang ZJ, Ge RC. 2014. Overexpression of the receptor-like protein kinase genes AtRPK1 and OsRPK1 reduces the salt tolerance of Arabidopsis thaliana. Plant Science 217–218, 63–70. [DOI] [PubMed] [Google Scholar]
- Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH. 2004. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. The Plant Cell 16, 1220–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefritz F, Otto B, Bienert GP, van der Krol A, Kaldenhoff R. 2004. The plasma membrane aquaporin NtAQP1 is a key component of the leaf unfolding mechanism in tobacco. The Plant Journal 37, 147–155. [DOI] [PubMed] [Google Scholar]
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R. 2002. PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. The Plant Cell 14, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Walker JC. 1995. Plant protein kinase families and signal transduction. Plant Physiology 108, 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Wang GL. 2011. Genome-wide identification, characterization and phylogenetic analysis of the rice LRR-kinases. PLoS One 6, e16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JJ, Tan ZH, Wu FQ, et al. 2014. A novel chloroplast-localized pentatricopeptide repeat protein involved in splicing affects chloroplast development and abiotic stress response in rice. Molecular Plant 7, 1329–1349. [DOI] [PubMed] [Google Scholar]
- Thilmony R, Guttman M, Thomson JG, Blechl AE. 2009. The LP2 leucine-rich repeat receptor kinase gene promoter directs organ-specific, light-responsive expression in transgenic rice. Plant Biotechnology Journal 7, 867–882. [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Niemietz CM, Bramley H, 2002. Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant, Cell and Environment 25, 173–194. [DOI] [PubMed] [Google Scholar]
- Wade LJ, McLaren CG, Quintana L, et al. 1999. Genotype by environment interactions across diverse rainfed lowland rice environments. Field Crops Research 64, 35–50. [Google Scholar]
- Wallace IS, Roberts DM. 2004. Homology modeling of representative subfamilies of Arabidopsis major intrinsic proteins. Classification based on the aromatic/arginine selectivity filter. Plant Physiology 135, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weig A, Deswarte C, Chrispeels MJ. 1997. The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiology 114, 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman SA, Nuhse TS, Ashford DA, Sanders D, Maathuis FJ. 2008. A proteomic and phosphoproteomic analysis of Oryza sativa plasma membrane and vacuolar membrane. The Plant Journal 56, 146–156. [DOI] [PubMed] [Google Scholar]
- Wu T, Tian Z, Liu J, Xie C. 2009. A novel leucine-rich repeat receptor-like kinase gene in potato, StLRPK1, is involved in response to diverse stresses. Molecular Biology Reports 36, 2365–2374. [DOI] [PubMed] [Google Scholar]
- Wu XN, Rodriguez CS, Pertl-Obermeyer H, Obermeyer G, Schulze WX. 2013. Sucrose-induced receptor kinase SIRK1 regulates a plasma membrane aquaporin in Arabidopsis. Molecular and Cellular Proteomics 12, 2856–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing HT, Guo P, Xia XL, Yin WL. 2011. PdERECTA, a leucine-rich repeat receptor-like kinase of poplar, confers enhanced water use efficiency in Arabidopsis. Planta 234, 229–241. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. 2006. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annual Review of Plant Biology 57, 781–803. [DOI] [PubMed] [Google Scholar]
- Yang H, Shi Y, Liu J, Guo L, Zhang X, Yang S. 2010. A mutant CHS3 protein with TIR–NB–LRR–LIM domains modulates growth, cell death and freezing tolerance in a temperature-dependent manner in Arabidopsis. The Plant Journal 63, 283–296. [DOI] [PubMed] [Google Scholar]
- Yang L, Wu K, Gao P, Liu X, Li G, Wu Z. 2014. GsLRPK, a novel cold-activated leucine-rich repeat receptor-like protein kinase from Glycine soja, is a positive regulator to cold stress tolerance. Plant Science 215–216, 19–28. [DOI] [PubMed] [Google Scholar]
- Yu X, Peng YH, Zhang MH, Shao YJ, Su WA, Tang ZC. 2006. Water relations and an expression analysis of plasma membrane intrinsic proteins in sensitive and tolerant rice during chilling and recovery. Cell Research 16, 599–608. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Cao YL, Zhao J, Li XH, Xiao JH, Wang SP. 2011. A pair of orthologs of a leucine-rich repeat receptor kinase-like disease resistance gene family regulates rice response to raised temperature. BMC Plant Biology 11, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZX, Chen J, Lin SS, Li Z, Cheng RH, Fang CX, Chen HF, Lin WX. 2012. Proteomic and phosphoproteomic determination of ABA’s effects on grain-filling of Oryza sativa L. inferior spikelets. Plant Science 185, 259–273. [DOI] [PubMed] [Google Scholar]
- Zhu JK. 2002. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology 53, 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.