Abstract
The molecular mechanisms underlying oogenesis and maternally controlled embryogenesis in fish are not fully understood, especially in marine species. Our aim was to study the egg and embryo transcriptome during oogenesis and early embryogenesis in Atlantic cod. Follicles from oogenesis stages (pre-, early-, and late-vitellogenic), ovulated eggs, and two embryonic stages (blastula, gastrula) were collected from broodstock fish and fertilized eggs. Gene expression profiles were measured in a 44 K oligo microarray consisting of 23,000 cod genes. Hundreds of differentially expressed genes (DEGs) were identified in the follicle stages investigated, implicating a continuous accumulation and degradation of polyadenylated transcripts throughout oogenesis. Very few DEGs were identified from ovulated egg to blastula, showing a more stable maternal RNA pool in early embryonic stages. The highest induction of expression was observed between blastula and gastrula, signifying the onset of zygotic transcription. During early vitellogenesis, several of the most upregulated genes are linked to nervous system signaling, suggesting increasing requirements for ovarian synaptic signaling to stimulate the rapid growth of oocytes. Highly upregulated genes during late vitellogenesis are linked to protein processing, fat metabolism, osmoregulation, and arrested meiosis. One of the genes with the highest upregulation in the ovulated egg is involved in oxidative phosphorylation, reflecting increased energy requirements during fertilization and the first rapid cell divisions of early embryogenesis. In conclusion, this study provides a large-scale presentation of the Atlantic cod's maternally controlled transcriptome in ovarian follicles through oogenesis, ovulated eggs, and early embryos. Mol. Reprod. Dev. 81: 619–635, 2014. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
Atlantic cod (Gadus morhua L.) is an important species both within fisheries and aquaculture. Cod is iteroparous with synchronous oocyte development, and females spawn up to 19 batches with up to 300,000 small pelagic eggs each over several weeks during the spawning season (February–May) (Kjesbu, 1989). Viability of eggs and embryos is unpredictable, and mortality as well as malformations in early-life stages are high (Brown et al., 2003; van der Meeren and Ivannikov, 2006; Avery et al., 2009; Fjelldal et al., 2009; Taranger et al., 2010). In this context, increased knowledge of cod egg and early embryo development will significantly aid both wild-stock management and aquaculture of cod.
The development of eggs (oogenesis) in cod (reviewed by Kjesbu and Kryvi, 1989) (Fig. 1) starts with oogonia (the precursors for oocytes), which are characterized by their small size and the presence of only one nucleus. Oogenesis initiates as oogonia transition to oocytes, and at the same time, follicle cells start to surround the newly formed oocytes. Primary oocyte growth is characterized by the formation of peripheral nucleoli, a circumnuclear ring, and an extracellular egg envelope. Cortical alveoli appear at the periphery as the circumnuclear ring breaks down. Formation of yolk granules at the periphery of the cytoplasm marks the onset of true vitellogenesis. The yolk content in oocytes increases markedly, and the cortical alveoli increase in size and number. At maturation, the irregular nucleus migrates to the animal pole, the oocyte hydrates and increases in size, and is eventually ovulated into the ovarian lumen. At ovulation, the egg contains all the components required to initiate and drive early embryogenesis. Importantly, the presence of mRNAs synthesized and/or deposited in the oocyte during oogenesis is crucial for the synthesis of proteins needed for the first developmental events to take place, since zygotic gene transcription is not activated until several cell divisions have completed (1982a and 1982b).
Figure 1.
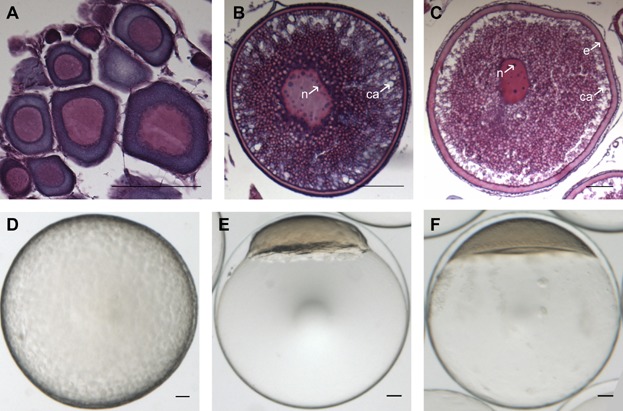
Overview of the developmental stages of Atlantic cod follicles, eggs, and embryos assessed with the microarray. Histological sections of pre-, early-, and late-vitellogenic follicles (A, B, and C, respectively) and photos of an unfertilized egg (D) and embryo at blastula stage (23.5 hr post-fertilization (hpf)) (E) and gastrula stage (58 hpf) (F). e, egg envelope; ca, cortical alveoli; n, nucleolus. Scale bar, 100 µm. [Color figure can be viewed in the online issue which is available at wileyonlinelibrary.com]
Following fertilization, non-yolk cytoplasm accumulates at the animal pole and forms the blastodisc. Numerous, rapid blastomere cleavages then follow. When 9–10 cleavage cycles have completed, the blastodisc consists of ∼500 cells clustered together like a ball (blastula), and the embryo enters the midblastula transition (Kane and Kimmel, 1993). This midblastula transition is characterized by cell cycle lengthening and loss of cell synchrony, and often coincides with the maternal to zygotic transition (MZT), when a gradual shift from degradation of maternal RNAs to activation of zygotic transcription occurs (reviewed by Tadros and Lipshitz, 2009). From the time of gastrulation onwards, the embryo relies on zygotically expressed transcripts to control further development.
Recent efforts have been made with large-scale methods to gain more insight into the molecular mechanisms that control egg development. In the model species zebrafish (Danio rerio Hamilton), a range of methods have been applied to study the transcriptome of gonads and isolated follicles, inlcluding microarrays (Li et al., 2004; Santos et al., 2007; Sreenivasan et al., 2008), mass sequencing of expressed sequence tags (ESTs) (Zeng and Gong, 2002), and serial analysis of gene expression (SAGE) (Knoll-Gellida et al., 2006). Likewise, increasing knowledge about oogenesis is emerging in salmonids like rainbow trout (Oncorhynchus mykiss Walbaum) and coho salmon (Oncorhynchus kisutch Walbaum), as changes in the ovarian transcriptome during different stages of oogenesis have been studied with microarrays and suppression subtractive hybridization (SSH) (von Schalburg et al., 2005, 2008; Bobe et al., 2006; MacKenzie et al., 2006; Luckenbach et al., 2008). Such studies have identified numerous participants that may be essential for oogenesis, greatly enhancing our knowledge of molecular mechanisms underlying this complex process in fish (Cerdà et al., 2008a). Nevertheless, large-scale studies on transcriptome dynamics during oogenesis are limited for marine species. In Senegalese sole (Solea senegalensis Kaup), the ovarian transcriptome at different maturational stages has been studied by microarray (Cerdà et al., 2008b; Tingaud-Sequeira et al., 2009). Goetz et al. (2006) analyzed 1,361 ESTs from the cod ovary at final maturation and ovulation, and Breton et al. (2012) identified genes differentially expressed between mid- and late-vitellogenic ovaries by a subtractive hybridization-based approach. In addition, the expression of 50 genes was recently measured in ovaries at six different stages by quantitative reverse-transcriptase PCR (qPCR) (Breton and Berlinsky, 2013). To our knowledge, no study has so far revealed (on a larger scale) which genes are developmentally regulated through the course of oogenesis in Atlantic cod.
A few reports have followed the fish transcriptome throughout embryogenesis using microarrays, in zebrafish (Mathavan et al., 2005), gilthead sea bream (Sparus auratus L.) (Sarropoulou et al., 2005), and mummichog (Fundulus heteroclitus) (Bozinovic et al., 2011). Environmental-based changes in the mummichog embryonic transcriptome have been described (Tingaud-Sequeira et al., 2013). The first large-scale analyses of the Atlantic cod transcriptome during embryogenesis were recently performed by Drivenes et al. (2012), Kleppe et al. (2012), and Lanes et al. (2012), respectively using microarray (7,000 genes), cDNA sequencing (257 genes), and qPCR (25 genes). Additional studies with a larger gene-diversity coverage would be beneficial to reveal the whole picture of cod embryogenesis. In order to understand the the egg and embryo development in this species, it would be beneficial to combine information about embryogenesis with information about stages during ovarian maturation when maternal RNAs become stored in the egg. Little information exists about the dynamics of maternal RNA storage, usage, and subsequent degradation.
The Atlantic cod genome is now sequenced, and more transcripts are available (Star et al., 2011). The aim of this study was therefore to identify genes and pathways associated with specific stages in Atlantic cod oogenesis and early embryo development. Consequently, expression profiles of more than 23,000 genes were measured using a 44 K oligo microarray in pre-vitellogenic, early-, and late-vitellogenic follicles, ovulated eggs, and embryos at blastula and gastrula stages. Genes and pathways differentially up- and downregulated between specific stages of development were also identified. To our knowledge, this is the first study to characterize the cod transcriptome throughout oogenesis and early embryogenesis at such a large scale. Increased knowledge around pathway activity will contribute to the overall goal, which is to understand mechanisms that contribute to viability of cod eggs and embryos.
RESULTS
Transcriptome Dynamics
A range of genes were significantly upregulated from pre- to early-vitellogenic follicles (349), from early- to late-vitellogenic follicles (376), from late- vitellogenic follicles to ovulated eggs (149), from ovulated eggs to blastula embryos (30), and from blastula to gastrula embryos (657). The corresponding numbers for downregulated genes were 555, 532, 655, 10, and 396, respectively. In general, more transcripts of genes were downregulated than induced throughout oogenesis, with the largest difference detected from late-vitellogenic follicles to ovulated eggs, during which 506 more genes were downregulated then upregulated (Fig. 2). The smallest difference was observed from ovulated eggs to blastula, where 20 more genes were upregulated than downregulated (Fig. 2). From blastula to gastrula, many more genes were upregulated than downregulated (Fig. 2). The complete gene lists can be found in Supplemental material (Additional Files 1a–j).
Figure 2.
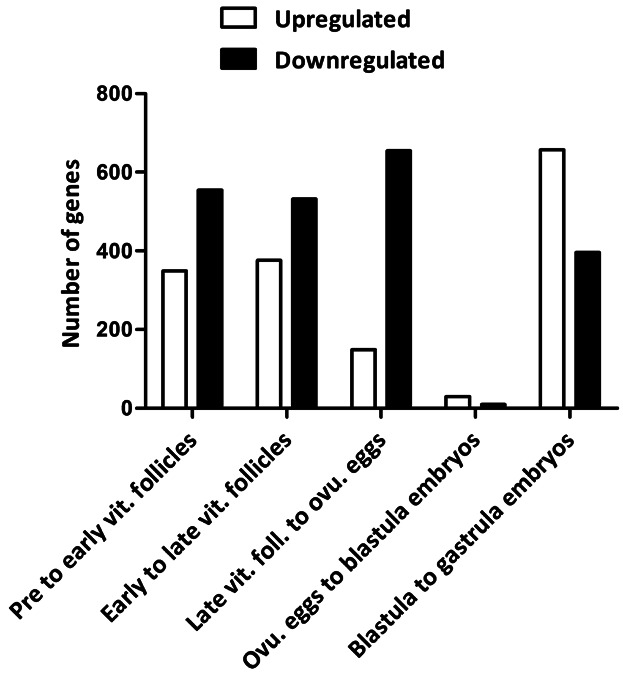
Number of up- and downregulated genes between specific stages of oogenesis and embryogenesis in Atlantic cod. Number of up- (white bars) and downregulated genes (black bars) (y-axis) are shown for each stage comparison (x-axis). vit., vitellogenic; foll., follicles; ovu., ovulated.
Regulated Genes and Associated Pathways
Differentially expressed genes (DEGs) detected in this study were linked to KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways in order to identify their systems-level function. An overview of the pathways represented by the highest numbers (≥10) of DEGs can be seen in Supplemental material (Additional File 2). “Housekeeping” pathways tend to encompass a large proportion of the DEGs, whereas smaller pathways that might be mainly up- or downregulated will be masked due to their lower contribution of DEGs. As we consider pathways that are clearly activated or silenced, and therefore represented by mostly up- or downregulated genes, respectively, we focused on the ratio between the number of upregulated and downregulated genes within each specific pathway. In addition, we applied a cutoff for this ratio in order to limit the long list of pathways annotated to the DEGs: up/down ≥5 for upregulated pathways, and down/up ≥5 for downregulated pathways. We added “1” to all “numbers of genes” within a pathway to avoid arithmetic errors that would otherwise result from division by pathways with zero genes. Figure 3 shows an overview of the up- and downregulated pathways. As few genes were differentially expressed from ovulated eggs to blastula embryos, this group was not included. The most up- and downregulated (based on fold change (FC)) genes from one stage to the next are shown in Figure 4, and are also presented with associated KEGG pathways in the following sections.
Figure 3.
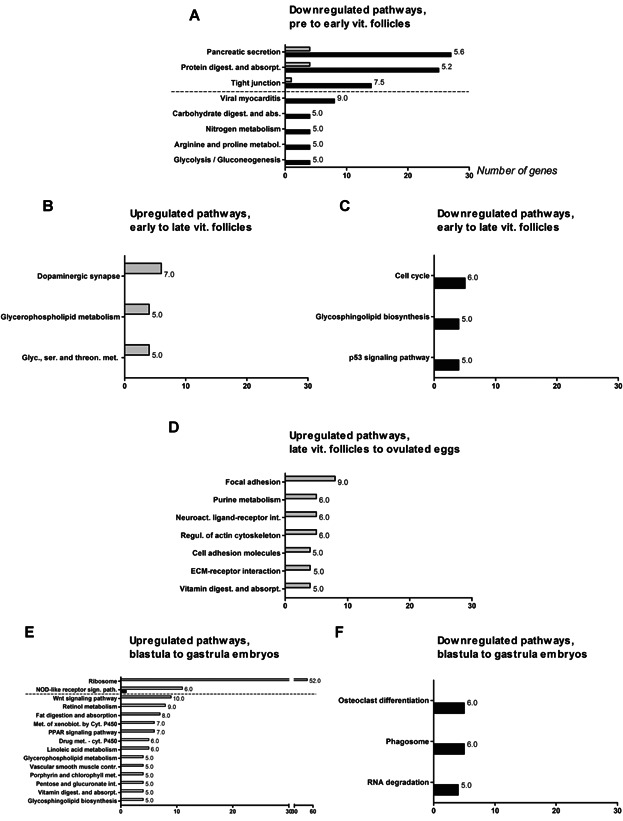
Up- and downregulated KEGG pathways between specific stages of oogenesis and embryogenesis in Atlantic cod. Downregulated pathways from pre- to early-vitellogenic follicles (A); up- and downregulated pathways from early- to late-vitellogenic follicles (B and C, respectively); upregulated pathways from late-vitellogenic follicles to ovulated eggs (D); up- and downregulated pathways from blastula to gastrula embryos (E and F, respectively). The name of each KEGG pathway (y-axis) and the number of genes (x-axis) are shown. The dotted line separates pathways represented by 10 or more genes from pathways represented by less than 10 genes. The number at the top of each bar is the ratio (see the section “Regulated genes and associated pathways”) between up- and downregulated genes for that pathway. Gray and black bars represent up- and downregulated pathways, respectively. vit., vitellogenic.
Figure 4.
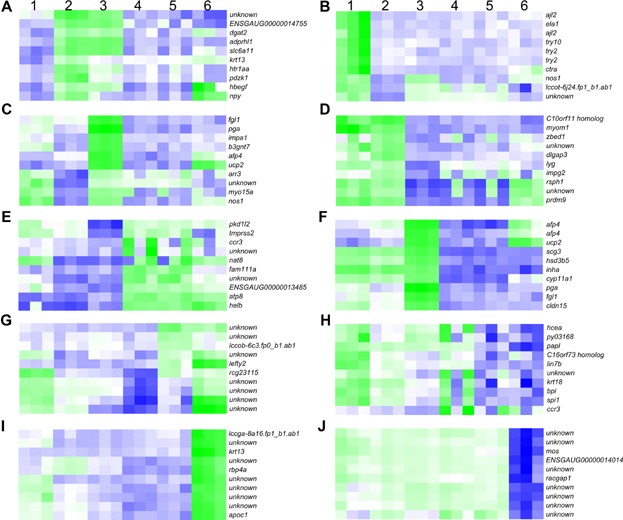
Heat maps of the expression of the most-regulated (based on fold change) genes from one developmental stage to the next in Atlantic cod, ranging from pre-vitellogenic follicles to gastrulating embryos. The most-up-/downregulated genes are shown for periods between: (A, B) pre- to early-vitellogenic follicles; (C, D) early- to late-vitellogenic follicles; (E, F) late-vitellogenic follicles to ovulated eggs; (G, H) ovulated eggs to blastula embryos; (I, J) blastula to gastrula embryos. 1, pre-vitellogenic follicles; 2, early-vitellogenic follicles; 3, late-vitellogenic follicles; 4, unfertilized eggs; 5, blastula; 6, gastrula. Green and blue colors represent up- and downregulation of genes, respectively. In cases of predicted genes, the Ensembl gene IDs (when possible) or GenBank EST ID's are shown. The predicted genes include: ENSGAUG00000014755, similar to protein tyrosine phosphatase, receptor type, Q isoform 1 precursor; lccot-6j24.fp1_b1.ab1, similar to F57G4.9; ENSGAUG00000013485, hypothetical protein LOC100005938; lccob-6c3.fp0_b1.ab1, similar to predicted protein; lccga-8a16.fp1_b1.ab1, hypothetical protein; and ENSGAUG00000014014, hypothetical protein LOC559540.
From Pre- to Early-Vitellogenic Follicles
The following genes are most-upregulated from pre- to early-vitellogenic follicles (Fig. 4A): sodium- and chloride-dependent GABA transporter 3 (slc6a11, FC 117.2), neuropeptide y (npy, FC 42.8), Na(+)/H(+) exchange regulatory cofactor NHE-RF3 (pdzk1, FC 39.2), protein ADP-ribosylarginine hydrolase-like protein 1 (adprhl1, FC 36.1), diacylglycerol O-acyltransferase 2 (dgat2, FC 31.0), 5-hydroxytryptamine receptor 1a-alpha (htr1aa, FC 28.2), proheparin-binding EGF-like growth factor (hbegf, FC 26.6), keratin, type 1 cytoskeletal 13 (krt13, FC 24.9), a predicted gene (similar to protein tyrosine phosphatase, receptor type, Q isoform 1 precursor, FC 35.8), and an unknown gene (FC 24.1). Nervous system signaling pathways are associated with three of these genes (npy, slc6a11, and htr1aa).
A high number of the downregulated genes (555) (Fig. 2) are linked to the pathways pancreatic secretion and protein digestion and absorption (27 and 25 genes, respectively) (Fig. 3A). A number of other pathways (tight junction, viral myocarditis, carbohydrate digestion and absorption, nitrogen metabolism, arginine and proline metabolism, and glycolysis/gluconeogenesis) are also downregulated at this stage, among which tight junction is represented by more than 10 genes. Among the most downregulated genes from pre- to early-vitellogenic follicles are two lactose-binding lectin l-2 (ajl2, FC 2665.5 and 767.1) and two trypsin-2 (try2, FC 503.9 and 416.5) genes. In addition, elastase-1 (ela1, FC 1558.4), chymotrypsin a (ctra, FC 1422.2), trypsin 10 (try10, FC 820.3), nitric oxide synthase (nos1, FC 473.8), a predicted gene (similar to f57g4.9, FC 446.4), and an unknown gene (FC 518.0) are within this group of genes (Fig. 4B). Three of the genes (try2, try10, ela1) are annotated with protein digestion and absorption.
From Early- to Late-Vitellogenic Follicles
Three pathways are upregulated from early- to late-vitellogenic follicles: dopaminergic synapse, glycerophospholipid metabolism and glycine, serine and threonine metabolism (Fig. 3B). The following genes show the most marked upregulation (Fig. 4C): pepsin a (pga, FC 955.5), UDP-GlcNAc:betaGal beta-1.3-N-acetylglucosaminyltransferase 7 (b3gnt7, FC 539.9), inositol monophosphatase 1 (impa1, FC 475.0), mitochondrial uncoupling protein 2 (ucp2, FC 430.8), nitric oxide synthase, brain (nos1, FC 298.8), arrestin-C (arr3, FC 192.6), myosin XVA (myo15a, FC 139.1), type-4 ice-structuring protein (afp4, FC 137.7), fibrinogen-like protein 1 (fgl1, FC 103.2), and an unknown gene (FC 107.0). pga, ucp2, arr3 and impa1 are annotated with protein digestion and absorption, peroxisome proliferation-activated receptor (PPAR) signaling, meiotic arrest, and osmoregulation, respectively. myo15a and fgl1 are linked to immune-related pathways.
Cell cycle, glycosphingolipid biosynthesis, and the p53-signaling pathway are downregulated from early- to late-vitellogenic follicles (Fig. 3C). The following genes show the most marked downregulation from early- to late-vitellogenic follicles (Fig. 4D): radial spoke head 1 homolog (rsph1, FC 420.1), leucine-rich repeat-containing protein C10orf11 homolog (c10orf11homolog, FC 411.9), histone-lysine N-methyltransferase PRDM9 (prdm9, FC 185.8), myomesin-1 (myom1, FC 158.7), zinc finger BED domain-containing protein 1 (zbed1, FC 127.6), lyzosome g (lyg, FC 91.0), interphotoreceptor matrix proteoglycan 2 (impg2, FC 72.4), disks large-associated protein 3 (dlgap3, FC 64.3), and two unknown genes (FC 158.0 and 75.9). Nervous system signaling pathways are associated with two of the genes (prdm9 and dlgap3).
From Late-Vitellogenic Follicles to Ovulated (Unfertilized) Eggs
Upregulated pathways from late-vitellogenic follicles to ovulated eggs include: focal adhesion, purine metabolism, neuroactive ligand-receptor interaction, regulation of actin cytoskeleton, cell adhesion molecules, ECM–receptor interaction, and vitamin digestion and absorption (Fig. 3D). The following genes show the most marked upregulation (Fig. 4E): polycystic kidney disease protein 1-like 2 (pkd1l2, FC 109.2), DNA helicase B (helb, FC 84.7), transmembrane protease serine 2 (tmprss2, FC 63.2), CC chemokine type 3 (ccr3, FC 58.3), ATP synthase F0 subunit 8 (atp8, FC 52.3), protein FAM111A (fam111a, FC 51.1), probable N-acetyltransferase 8 (nat8, FC 36.7), a predicted gene (hypothetical protein LOC100005938, FC 68.7), and two unknown genes (FC 67.7 and 49.0). atp8 is annotated with the KEGG pathway oxidative phosphorylation.
Two afp4 genes (FC 5924.8 and 1420.3) are represented among the most-downregulated genes from late-vitellogenic follicles to ovulated eggs. Also within this group of genes are secretogranin-3 (scg3, FC 3060.3), pga (FC 3008.0), cholesterol side-chain cleavage enzyme, mitochondrial (cyp11a1, FC 1164.3), mitochondrial uncoupling protein 2 (ucp2, FC 1101.1), inhibin alpha chain (inha, FC 941.0), fibrinogen-like protein 1 (fgl1, FC 746.6), claudin-15 (cldn15, FC 695.3), and 3 beta-hydroxysteroid dehydrogenase type 5 (hsd3b5, FC 685.9) (Fig. 4F). Two of these genes (cyp11a1 and hsd3b5) are linked to the KEGG pathway steroid biosynthesis. In addition, pga, ucp2, cldn15, and fgl1 are annotated with protein digestion and absorption, PPAR signaling, cell adhesion molecules, and immune related pathways, respectively.
From Ovulated (Unfertilized) Eggs to Blastula Embryos
Few genes are differentially expressed from ovulated eggs to blastula embryos (Fig. 2). The following genes show the most marked upregulation (Fig. 4G): rCG23115 (FC 114.4), left-right determination factor 2 (lefty2, FC 10.4), a predicted gene (similar to predicted protein, FC 10.5), and 7 unknown genes (FC 80.1, 19.4, 17.9, 17.2, 16.8, 14.6, and 10.5). lefty2 is associated with the transforming growth factor (TGF)-beta signaling pathway (left-right determination).
The only genes downregulated from ovulated eggs to blastula embryos are high choriolytic enzyme 1 (hcea, FC 39.1), circumsporozoite protein (py03168, FC 14.3), ccr3 (FC 12.0), bacterial permeability-increasing protein (bpi, FC 10.1), transcription factor PU.1 (spi1, FC 9.3), uncharacterized protein C16orf73 homolog (c16orf73 homolog, FC 8.3), keratin, type 1 cytoskeletal 18 (krt18, FC 8.2), iron/zinc purple acid phosphatase-like protein (papl, FC 6.0), protein lin-7 homolog B (lin7b, FC 6.0), and an unknown gene (FC 11.5) (Fig. 4H). hcea, spi1, and bpi are annotated with protein digestion and absorption, osteoclast differentiation, and toll-like receptor signaling, respectively. Furthermore, papl is linked to a number of pathways including folate biosynthesis, two-component system, and aminobenzoate degradation.
From Blastula to Gastrula Embryos
The pathway ribosome is represented by the highest number (51) of upregulated genes from blastula to gastrula embryos. The nucleotide-binding oligomerization domain (NOD)-like receptor signaling pathway is also represented by more than 10 upregulated genes. Less dominating, although upregulated, pathways include wnt signaling pathway, retinol metabolism, fat digestion and absorption, metabolism of xenobiotics by cytochrome P450, linoleic acid metabolism, glycerophospholipid metabolism, vascular smooth muscle contraction, porphyrin and chlorophyll metabolism, pentose and glucuronate interaction, vitamin digestion and absorption, and glycosphingolipid biosynthesis (Fig. 3E). During this period of development, the following genes show the most marked upregulation: retinol-binding protein 4-A (rbp4a, FC 10582.0), apolipoprotein C-1 (apoc1, FC 9999.3), keratin, type 1 cytoskeletal 13 (krt13, FC 8161.2), a predicted gene (hypothetical protein, FC 3080.3), and 6 unknown genes (FC 5391.3, 4607.9, 3975.3, 3593.1, 3569.3, and 3194.6) (Fig. 4I). No KEGG pathways could be annotated to this group of genes.
Three pathways are downregulated, including osteoclast differentiation, phagosome, and RNA degradation (Fig. 3F). The genes showing the most marked downregulation include serine/threonine-protein kinase mos (mos, FC 183.3), rac GTPase-activating protein 1 (racgap1, FC 149.4), a predicted gene (hypothetical protein LOC559540, FC 185.9), and 7 unknown genes (FC 258.9, 234.9, 172.5, 168.1, 163.8, 160.1, and 150.1) (Fig. 4J). One gene, mos, is linked to the MAPK-signaling pathway, regulation of actin cytoskeleton, oocyte meiosis, and progesterone-mediated oocyte maturation.
Markers for Stage-Specific Processes
Previous studies (reviewed by Lubzens et al., 2010) have identified a number of genes that mark specific stages/processes within oogenesis and embryogenesis in different fish species, and such genes were also found within our list (Additional File 1) of significantly regulated genes in cod follicles, eggs, and embryos. growth and differention factor 9 (gdf9) was highly expressed in all maternal stages, and significantly decreased at the gastrula stage (Fig. 5A). The gene encoding the Steroidogenic acute regulatory protein (Star) was significantly downregulated from late-vitellogenic follicles to ovulated eggs, with peak expression during late-vitellogenic follicles. In addition, the related genes cyp11a1 and cytochrome P450 aromatase a (cyp19a1) showed almost identical expression profiles to star (Fig. 5B). Several genes encoding Insulin-like growth factor (Igf)-binding proteins were found to be differentially expressed, either upregulated from early- to late-vitellogenic follicles and/or downregulated from late-vitellogenic follicles to ovulated eggs (Fig. 5B). A number of zona pellucida genes (zona pellucida sperm-binding proteins 2-4 (zp2, zp3 and zp4) and zona pellucida-like domain-containing protein 1 (zpld1)) were found to be differentially expressed, where most were expressed through oogenesis and significantly downregulated in ovulated eggs (Fig. 5B). anti-müllarian hormone (amh) was downregulated from late-vitellogenic follicles to ovulated eggs (Fig. 5B). A vitellogenin (vtg) was significantly downregulated from pre-vitellogenic to early-vitellogenic follicles, and significantly upregulated from early- to late-vitellogenic oocytes (Fig. 5B). Other vitellogenins were also observed, although no significant changes in expression were measured (data not shown). The genes encoding Syntabulin (Sybu) and Zygote arrest protein 1 (Zar1) were highly expressed throughout oogenesis, followed by a sharp downregulation at the gastrula stage (Fig. 5C).
Figure 5.
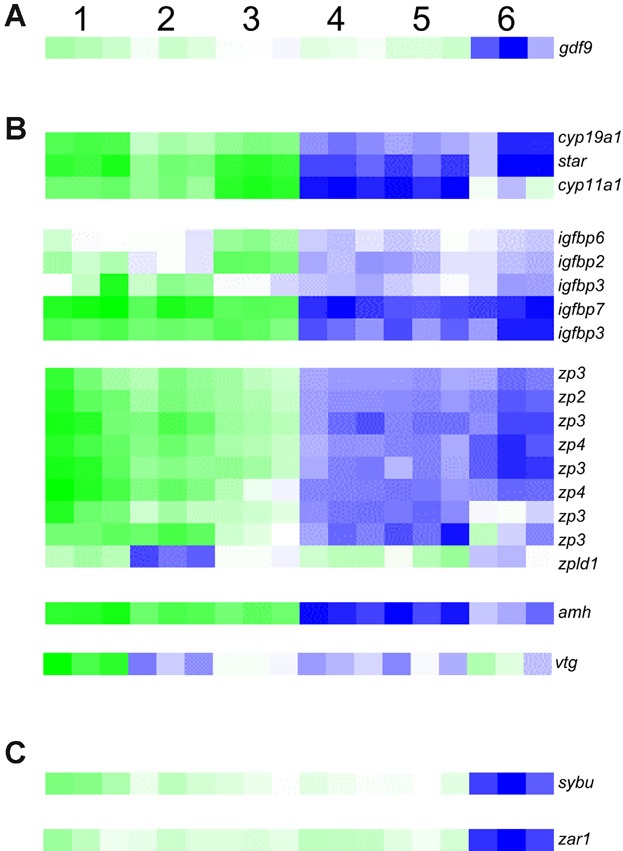
Heat maps for the expression of selected genes that previously have been shown to be involved in specific processes related oogenesis and the MZT. Genes involved in primary oocyte growth (A), vitellogenesis (B), and the MZT (C) are shown. 1, pre-vitellogenic follicles; 2, early-vitellogenic follicles; 3, late-vitellogenic follicles; 4, unfertilized eggs; 5, blastula; 6, gastrula. Green and blue colors represent up- and downregulation of genes, respectively. [Color figure can be viewed in the online issue which is available at wileyonlinelibrary.com]
DISCUSSION
A dominant pattern in the cod transcriptome during oogenesis is the continuous accumulation and degradation of transcripts. Interestingly, the latest stage of oogenesis shows a marked increase in degradation of transcripts. These results are in line with observations in mouse (Mus musculus L.), where a controlled degradation of maternal RNAs has been observed at later stages of maturation (Su et al., 2007). More recent findings in mouse have shown that the stability of maternal RNAs is essential for normal fertility (Medvedev et al., 2011). It is well established that maternal RNAs are stored in oocytes during oogenesis (reviewed by Stitzel and Seydoux, 2007), and such mRNAs are subsequently translated in an inter-dependent manner, ensuring the correct timing for specific protein synthesis (Vasudevan et al., 2006); less is known about the timing of maternal RNA incorporation or production. Our observation of a steady upregulation of genes throughout the maturing follicular stages implies that mRNAs are actively accumulated starting at pre-vitellogenic stages, and continues gradually throughout oogenesis in cod.
Another complex feature of the cod early-development transcriptomes is the remarkable stability from the mature egg to the blastula stage. Few transcripts are up- or downregulated during this time, showing a high homogeneity of maternal RNAs during early development of the cod egg and embryo. This is in contrast to the findings of Ferg et al. (2007) in zebrafish, where they characterized a pool of fast-degrading transcripts by the 64-cell stage. This comparison suggests a later post-ovulatory degradation of maternal transcripts in cod versus zebrafish. The few induced genes observed over the same period of embryogenesis in the current study may be explained by early zygotic activity before blastula stages (Mathavan et al., 2005; Vesterlund et al., 2011; Drivenes et al., 2012), which would indicate possible functions for these genes in the activation of the zygotic transcriptome or specific processes in the early embryo. Nevertheless, the early-induced genes could also be explained by an underrepresentation of stored maternal RNAs due to their short poly(A) tail, as observed in zebrafish (Aanes et al., 2011). In general, the highest number of upregulated genes is observed between blastula and gastrula embryos. This can be explained by the onset of zygotic transcription and the initiation of organogenesis in cod embryos (Drivenes et al., 2012; Kleppe et al., 2012).
A dominant feature from pre- to early-vitellogenic follicles is the downregulation of genes involved in protein degradation pathways (Fig. 3A). During this period, oocytes are arrested in prophase I and are awaiting activation by the maturation signal (Kondo et al., 1997). In reproductive-arrested (diaphase) Drosophila melanogaster oocytes, protein degradation pathways are also activated by highly expressed trypsins (Baker and Russell, 2009). Interestingly, trypsins were also identified in the current study. One could speculate that protein degradation pathways contribute to the arrested growth of oocytes. More than 10 genes involved in tight junction are also downregulated from pre-to early-vitellogenic follicles. Tight-junction protein complexes serve as barriers that regulate paracellular transport across epithelia (Van Itallie and Anderson, 2004). Several tight-junction proteins, such as claudins, are regulated during oogenesis in zebrafish (Clelland and Kelly, 2010). Likewise, several of the claudins were downregulated from pre- to mid-late-vitellogenic follicles in cod, which supports the hypothesis that changes in the abundance of tight junction proteins could be responsible for modifications in the follicular layer at this stage. The role of tight-junction proteins in oogenesis is further supported by their responsiveness to sex steroids (Clelland and Kelly, 2010).
One of the most upregulated genes from pre- to early-vitellogenic follicles is htr1aa, a member of the Htr1a family of G-protein coupled receptors for the neurotransmitter serotonin, which can act both pre- and post-synaptically (Piñeyro and Blier, 1999). Another highly upregulated gene involved in the serotonergic synapse is slc6a11, a member of the family of Na+/Cl− transporters which contributes to neurotransmitter uptake (Grossmann and Nelson, 2002). The neurotransmitter npy is also highly upregulated in cod follicles during this stage. Npy has previously been identified in ovaries of mammals (Keator et al., 2010). Furthermore, this gene has previously been associated with ovarian expression in snakeskin gourami (Trichogaster pectoralis Regan) (Boonanuntanasarn et al., 2012) and cod (Kehoe and Volkoff, 2007), but has not been shown to be upregulated at a certain stage of oogenesis. Results from cow (Bos primigenius), however, show that npy upregulation coincides with the onset of follicular development (Hulshof et al., 1994). npy is an important regulator of energy homeostasis in fish and mammals (Wu et al., 2012), so upregulation of local npy expression as well as other players in nervous system signaling may indicate activation of processes important for energy uptake and rapid oocyte growth during early vitellogenesis. A similar process may occur during late-vitellogenesis as well, since the dopaminergic synapse pathway is upregulated at that stage.
Among genes previously implicated in the regulation of pre-vitellogenic growth are different growth factors like gdf9 (Lubzens et al., 2010), a TGFβ factor essential for female fertility in mouse (Yan et al., 2001). In zebrafish and European sea bass (Dicentrachus labrax L.), gdf9 expression is elevated in the ovary during primary ovarian growth, with subsequent reduction during secondary growth (Liu and Ge, 2007; Halm et al., 2008), indicating an important role for this gene in primary ovarian growth in fish. gdf9 seems to also be of maternal importance in cod as its expression is high throughout oogenesis; its major downregulation took place during gastrulation, which may reflect a possible function related to follicular-somatic-cell survival, as observed in mammals (Paulini and Melo, 2011).
At late vitellogenesis, glycerophospholipid metabolism is upregulated while glycospingolipid biosynthesis is downregulated. This implies that there is less de novo synthesis of lipids and more degradation for storage. Furthermore, the involvement of the highly upregulated ucp2 in PPAR signaling implies an active uptake of fats during late-vitellogenic stages (Oku and Umino, 2008). It is well known that lipids accumulate in the fish oocyte during vitellogenesis, forming nutritional and energetic reserves for the subsequent embryo and larvae. Furthermore, Breton et al. (2012) recently reported that maturation and ovulatory competency during cod vitellogenesis are associated with genes involved in lipid metabolism. In coho salmon, ovary accumulation of lipids during vitellogenesis is associated with increased ovarian expression of star (Campbell et al., 2006), which encodes a protein that regulates the transport of cholesterol across the mitochondrial membrane, a rate-limiting step in steroid synthesis (Stocco, 1998). Similarly, we observed that ucp2 and star expression increases from early- to late-vitellogenic oocytes in cod. The associated cyp11a1 and cyp19a1, which are essential for estradiol-17β synthesis (reviewed by Uno et al., 2012), show similar expression profiles to star, partly in agreement with the findings of Breton et al. (2012). Increased expression of star, cyp19a1, and cyp11a1 increases the steroid synthesis capacity, including the production of estradiol-17β, which is increased in parallel with follicle-stimulating hormone (FSH) in coho salmon plasma during cortical alveoli synthesis. One could thus speculate that the transition from primary to secondary growth is stimulated by FSH in cod; plasma levels of FSH were not measured in this study. In coho salmon, accumulation of lipids is also associated with increased plasma Insulin-like growth factor 1 (Igf-1) and increased ovarian mRNA levels of igf-1 and igf-2 (Campbell et al., 2006). Conversely, we observed that igf-1 and its receptor igf-1r are expressed throughout cod oogenesis, with no significant changes in mRNA levels. Nevertheless, expression of genes encoding Igf-binding proteins peaks during the late-vitellogenic stage, which reflects the possible involvement of the Igf system in lipid accumulation in cod.
pga is associated with the protein digestion pathway, and is strictly upregulated during late vitellogenesis. Whereas Pga is one of the main proteolytic enzymes of the digestive system (Dee et al., 2009), it has never been associated with oocytes before. Little is known about which enzymes process vitellogenin and yolk proteins during oogenesis in fish; perhaps Pga participates in this process. We also found an arrestin (arr3) upregulated at late vitellogenesis. Arrestins have previously been shown to be involved in the G-protein signaling pathway that relieves meiotic arrest in Xenopus laevis and mouse (Wang and Liu, 2003; Lowther et al., 2011). The occurence of this arrestin pathway and the decrease in cell-cycle pathways in cod late-vitellogenic oocytes together suggest that final maturation is approaching and signals associated with late-meiotic steps might be activated. Osmoregulation in both goby (Gillichthys mirabilis Cooper) and eel (Anguilla anguilla L.) has been linked to high experession of impa1 (Evans and Somero, 2008; Kalujnaia et al., 2010). Interestingly, we observed induction of impa1 in follicles at late vitellogenesis; it may therefore be possible that this upregulation is linked to the preparation of the egg for the marine environment, where it may soon be fertilized.
A number of cell-structure-related pathways are upregulated in ovulated eggs, including focal adhesion, regulation of actin cytoskeleton, cell adhesion molecules, and extracellular-matrix–receptor interaction. During egg maturation, the oocyte undergoes large reorganizations as it divides and expels the first polar body (reviewed by Lubzens et al., 2010). Further reorganization occurs during fertilization and subsequent cell divisions; the changes detected in these pathways might be in preparation for the above-mentioned processes. Structural components of the egg envelope, the Zona pellucida proteins, accumulate in the growing oocytes (Arukwe and Goksøyr, 2003). In agreement with previous findings in cod (Drivenes et al., 2012), the high expression levels of such genes in follicle stages, with subsequent downregulation by the ovulated-egg stage, reflect their maternal role.
One of the most upregulated genes in the ovulated egg is atp8, which is involved in oxidative phosphorylation. atp8 is one of the 13 protein-coding mitochondrial genes in cod (Johansen and Bakke, 1996). Mitochondrial genes were recently shown to encode a significant proportion of the high-abundance transcripts in cod eggs and embryos at the 1–2 cell and blastula stages (Kleppe et al., 2012), reflecting a high energy demand in eggs and embryos at the time of fertilization and during the first rapid cell cleavages of embryogenesis. Another gene associated with the accumulation of energy store for the egg is amh (Luckenbach et al., 2008), and this seems to be true for cod as well since we observed a high expression of it in all follicle stages investigated, with a significant downregulation in ovulated eggs.
Two of the most downregulated genes from late-vitellogenic follicles to ovulated eggs, cyp11a1 and hsd3b5, are involved in steroid-hormone biosynthesis. Steroid hormones are produced by theca and granulosa cells in the follicle; during ovulation, the egg is released from these surrounding cells. This may explain the sharp decrease in steroidogenic transcripts at the ovulated-egg stage. Termination of vitellogenesis and the initiation of maturation (resumption of meiosis) are also associated with a switch in the ovarian steroidogenic pathway, wherein the production of estradiol-17β is replaced by the production of maturation-inducing steroid (MIS) (Nagahama and Yamashita, 2008). The downregulation of steroidogenic genes cyp11a1 and hsd3b5 during this period may therefore reflect maturation-related changes in steroidogenesis.
lefty2 is one of the highest-upregulated genes from ovulated eggs to blastula embryos. Lefty2 is a TGFβ factor that plays an important role in left-right patterning in the embryo (Meno et al., 1996). A significant upregulation of lefty2 during cleavage stages may reflect early preparation for right-left asymmetry in cod. The expression of this gene is detected at the blastomere stage in zebrafish embryos (Thisse et al., 2001), which might indicate that lefty2 is one of the early zygotic genes.
A number of different pathways are linked to a few genes that are downregulated from ovulated eggs to the blastula-stage embryo. The gene with the highest fold change, hcea, is annotated with the KEGG pathway protein digestion and absorption, and its encoded protein is an astacin metalloproteinase that functions as a hatching enzyme (digestion of the egg envelope) in teleost embryos (Yasumasu et al., 1989). Maternally deposited variants of hatching enzymes in cod were also observed by Drivenes et al. (2012), although the information about potential additional functions of hatching enzymes at this stage is limited. One possible role for Hcea could be similar to that of Alveolin, which is an astacin metalloprotease similar to Hcea that acts as a “trigger” for the hardening of the egg envelope upon fertilization in medaka (Oryzias latipes Temminck and Schlegel) (Shibata et al., 2000). Although the hcea identified in this study is most similar to a gene encoding a hatching enzyme, it is possibile that this factor instead contributes to egg envelope hardening in cod.
The high number of upregulated genes involved in the ribosome pathway from blastula to gastrula embryos supports the suggestion that the zygotic transcription is initiated during this period of development. Ranking second to the ribosome pathway is the NOD-like receptor signaling pathway, which was represented by more than 10 upregulated genes. NOD-like receptors sense intracellular pathogens and activate innate immune responses in humans (reviewed by Chen et al., 2009), and a number of orthologs are found in teleosts (Laing et al., 2008). Upregulation of genes linked to NOD-like receptor signaling during gastrulation may indicate early activation of some of the players in the cod innate immune system. Other known, developmental pathways are also upregulated (Fig. 3E), further strengthening the importance in the transition between blastula and gastrula stages. A few pathways are downregulated, including osteclast differentiation, phagosome, and RNA degradation. These processes could be related to the degradation of maternal RNA and associated proteins.
Some maternal RNAs stored in the oocyte may be essential for the activation of zygotic transcription or even for embryonic events after the MZT (Putiri and Pelegri, 2008). zar1 is a maternal-effect gene necessary for post-MZT embryogenesis in the mouse (Wu et al., 2003), and is also suggested to play a role in the timing of MZT in cod (Drivenes et al., 2012). In the current study, zar1 was expressed in all follicle stages investigated, and significantly downregulated during the MZT, supporting the previous suggestion by Drivenes et al. (2012). Another maternal-effect gene is sybu, a regulator of dorsal axis formation in zebrafish. During oogenesis, RNAs encoded by sybu are transported to the vegetal pole of the oocyte, and subsequently translocated from there in a microtubule-dependent manner during embryogenesis. It is therefore suggested that Sybu is involved in vegetal-pole localization and microtubule-dependent transport of dorsal determinants, thereby linking oocyte animal-vegetal polarity with embryonic dorso-ventral polarity (Nojima et al., 2010). Interestingly, sybu is significantly downregulated during the MZT in cod, and it is highly expressed through all follicle and egg stages investigated as well as in early embryos (blastula). Nojima et al. (2010) suggested that Sybu localizes dorsal determinants to the vegetal pole during oogenesis and/or in early stage zygotes, which makes sense in relation to the expression pattern observed in the current study; we therefore suggest that Sybu may likely play a similar regulatory role in cod.
Apart from the biological results, our results may differ from previous studies of the cod transcriptome in follicles and early embryos due to different methods applied, sample types, and number of genes assayed per sample. For example, we measured gene expression in isolated follicle cells with a certain size range, whereas other studies used heterogenous ovary tissue (Goetz et al., 2006; Breton et al., 2012; Breton and Berlinsky 2013), which in addition to follicle cells of different size ranges contains connective cells. Hence, using whole-ovary samples from cod may introduce heterogeneity in data since a mix of stage-specific processes during oogenesis are being monitored. In previous studies involving the cod embryonic transcriptome, Drivenes et al. (2012) monitored 7,000 genes whereas more than 23,000 genes were included in the current study, which provides greater diversity. Another aspect to consider between these two microarray studies is the samples themselves. While Drivenes et al. (2012) used two to three pools of embryos from the same egg batch for each embryonic stage, we applied three pools of embryos from three different females for each embryonic stage. Consequently, the fold changes reported herein are a more robust indicator of regulation shared among different genetic backgrounds.
CONCLUSIONS
This study provides a large-scale presentation of the Atlantic cod maternally controlled transcriptome from pre-vitellogenic follicles through selected stages of oogenesis, unfertilized eggs, and early embryos until onset of zygotic transcription. We show that more than 3,000 genes are differentially expressed during this period of egg and embryo development. Several hundred genes are up- and downregulated throughout oogenesis, reflecting a continuous accumulation as well as degradation of polyadenylated maternal transcripts in the developing follicle. Furthermore, stages between the mature egg and blastula show high homogeneity as very few genes are differentially expressed. A high level of degradation as well as induction of gene expression from blastula to gastrula indicates that most maternal transcripts are degraded after blastula stages, while a wave of major zygotic transcription is initiated during the same period.
Several highly-upregulated genes during early vitellogenesis are associated with nervous system signaling; this may be related to increased synaptic signaling in the ovary that help initiate the rapid growth of oocytes. The highly-upregulated genes of late vitellogenesis are linked to protein processing and fat metabolism, as well as to osmoregulation and arrested meiosis, which, implicate the importance of controlling oogenesis and the timing of acclimation to the marine environment. Oxidative phosphorylation is linked to one of the genes with highest upregulation from late vitellogenesis to the ovulated egg, which coincides with fertilization and the main activity of the early embryo, namely to divide in a rapid and energy-consuming manner. These basic profiles of expression and pathway regulation will be beneficial for further studies of oogenesis and early embryo development in cod and other teleosts.
MATERIALS AND METHODS
Animals and Collection of Eggs
All samplings in this study were performed according to the guidelines approved by the Norwegian Animal Research Authority (NARA). Atlantic cod were hatched and reared at the Institute of Marine Research, Austevoll Research station (60.1°N). Fish of different ages were kept in netpens under ambient temperature and light conditions, and fed daily using a commercially available dry feed.
On February 17, 2011 (early in the spawning season), both maturing and spawning females were sacrified by a blow to the head, and follicles were collected and immediately put in RNA later. Connective tissue was removed, leaving isolated follicles for stage determination (see the section “Categorizing of follicles”) and subsequent analysis. To collect immature ovaries containing only pre-vitellogenic oocytes/follicles, 1-year-old females were also sacrified by a blow to the head, and gonad biopsies were sampled and put in RNA-later until further analysis. Immature female gonads were too tightly organized to isolate oocytes/follicles from the connective tissue; therefore, the samples referred to as pre-vitellogenic follicles in this study also contain connective tissue. Ovulated (unfertilized) eggs and embryos at blastula (23.5 hr post fertilization (hpf)) and gastrula (58 hpf) stages were collected in 2009 according to Kleppe et al. (2013). For an overview of all samples and subsequent analyses in this study, see Table 1.
Table 1.
Overview of the Samples of this Study
| Sample type | Amount (mg) | Analysis | Number of samples (n) |
|---|---|---|---|
| Immature ovary tissue, pooled from several females | 20 | Microarray and qPCR | 3 |
| Early vitellogenic follicles, pooled from several females | 20 | Microarray and qPCR | 3 |
| Late vitellogenic follicles, pooled from several females | 20 | Microarray and qPCR | 3 |
| Ovulated eggs, pooled from one femalea | 20 | Microarray | 3 |
| Blastula embryos, pooled from one femalea | 20 | Microarray and qPCR | 3 |
| Gastrula embryos, pooled from one femalea | 20 | Microarray and qPCR | 3 |
Samples from Kleppe et al. (2013). Each sample is a pool of eggs/embryos from one female. Three samples at the same developmental stage represent sibling pools from three different females.
Type and amount of each sample, downstream analysis and number of samples in each analysis are shown.
Categorizing of Follicles
Follicles in RNA later were manually sorted by size (diameter), and were staged based on criteria defined by Sivertsen (1935) and Kjesbu et al. (1991, 1996). Follicles of specific stages of oogenesis can be seen in Figure 1: immature/pre-vitellogenic (diameter < 250 µm (Fig. 1A)), early-vitellogenic (∼350 µm in diameter (Fig. 1B)), and late-vitellogenic (∼700 µm in diameter (Fig. 1C)) follicles were analyzed. Ovulated eggs and the embryonic developmental stages included in this study can be seen in Figure 1D–F.
RNA Extraction for Microarray and qPCR Analyses
Total RNA was extracted from 20 mg of follicles/eggs/embryos by applying RNeasy® Mini Kit (QIAGEN, Oslo, Norway), and genomic DNA was removed by using Turbo DNA-free™ Kit (Ambion, Austin, TX), according to the manufacturer's instructions. A NanoDrop® NP-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) was used to measure the quantity and quality of the RNA samples. The quality of the RNA samples was also checked using a 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA); and no samples with RIN values below 8.5 were accepted for further analysis.
Microarray Design and Assay, cDNA Synthesis and Microarray Hybridization
A custom, Agilent 44 k oligo design was constructed based on the Atlantic cod gene set described by Star et al. (2011). In addition, we supplemented this with approximately 6000 ESTs from oocyte, blastula, and gastrula libraries (Kleppe et al., 2012). The resulting design contained a transcriptome coverage with 23,857 unique genes, of which 19,973 received annotation through SwissProt and UniRef. For each developmental stage (pre-, early-, and late-vitellogenic follicles, unfertilized eggs, and blastula and gastrula embryos), three pooled samples (see Table 1) were included in the microarray analysis. A one-color microarray-based gene expression analysis (Agilent Technologies) protocol was applied, according to the manufacturer's guidelines. For each array, 200 ng of total RNA were used for cDNA synthesis. Labeling efficiency and amount of labeled cRNA were measured using a NanoDrop® NP-1000 spectrophotometer (NanoDrop Technologies). Slides were scanned using an Agilent B Scanner (G2505B).
Microarray Data Analyses: Importing Raw Data, Rank Product, and KEGG Annotation
The array raw data were read and processed by the Feature Extraction software (Agilent Technologies) before importing into J-express (Dysvik and Jonassen, 2001) for analysis. The data was quantile normalized (Bolstad et al., 2003), and missing values replacements were predicted by LS impute Adaptive (Bo et al., 2004). All data were log(2)-transformed and collapsed on contigs before downstream analysis.
Mitochondrial genes represent a significant fraction of the transcripts in cod eggs. As the microarray used in this study had been enriched with ∼6,000 probes from a previous study of the cod egg and early-embryo transcriptomes (Kleppe et al., 2012), many probes found in our array were of mitochondrial origin. The Ensembl annotation for cod does not contain mitochondrial genes, which means that the sequences from which the microarray probes were designed were not checked against mitochondrial DNA. This created an error when looking for over- and under-represented processes during oogensis and early embryogenesis. Consequently, these untranslated region (UTR)-sequences and UTR-contigs were mapped to both the genome and to the mitochondrial DNA. The sequences that had a BLAT hit above 70% identity to the mitochondrial DNA, in a gene or within 2,000-bp downstream of a gene, were annotated as a mitochondrial gene. The microarray probes were annotated based on the gene annotation of the sequences or contigs that they originated from. Subsequently, all probes representing the same mitochondrial gene were merged, thus obtaining a median gene-expression profile. This median profile was used when identifying processes that demarcated a specific stage of development.
To identify genes that were differentially expressed through egg development and early embryogenesis, mRNA levels in pre-, early-, and late-vitellogenic follicles, ovulated eggs, and blastula and gastrula embryos were compared from stage to stage applying the statistical method Rank product (Breitling et al., 2004). Only genes with a q-value below 0.05 were considered significantly up- or downregulated from one stage to the next. The Ensembl genes, UTR-sequences, and UTR-contigs that the microarray probes originated from were annotated with KEGG pathways, using the best-hit from BLAST against 12 of the species that are annotated in KEGG Pathways. All pathways containing genes of interest were visualized by mapping the up- and downregulated genes in different colors on the pathway map (Kanehisa et al., 2012). KEGG annotation BLAST scores were manually checked for each gene of interest. We provide MIAME-compliant description of the microarray study, available in the arrayexpress database (http://www.ebi.ac.uk/arrayexpress), with accession number E-MTAB-2170.
Verification of the Microarray by qPCR
Gene expression profiles of selected genes were measured by qPCR for most of the samples that were included in the microarray (three pooled samples from each of the following developmental stages (Table 1)): pre-, early-, and late-vitellogenic follicles, and blastula and gastrula embryos. As it is challenging to find stable reference genes for cod egg- and embryo stages (Mittelholzer et al., 2007; Olsvik et al., 2008; Kleppe et al., 2012; Lanes et al., 2012), an external reference gene was applied for the qPCR analysis. One picogram of rabbit hemoglobin alpha (hba) mRNA (SIGMA; Norway) was spiked into each RNA sample before cDNA synthesis (Kleppe et al., 2012). cDNA was produced using Superscript VILO cDNA synthesis (Invitrogen, Carlsbad, Germany). All primers used for amplification, and detection of genes were designed applying the software Primer Express 3.0 (Applied Biosystems, Foster city, CA) (see Table 2) together with hba-primers (Kleppe et al., 2013).
Table 2.
The Primers Used for qPCR
| Gene | Gene ID | Forward sequence | Reverse sequence |
|---|---|---|---|
| hba | NM_001082389.2 | 5′TCCCCACCACCAAGACCTACT | TGGGCTTTGATCTGCTCAGA |
| ajl2 | ENSGMOG00000020535 | 5′TGAAGAAGGCTCCTGGATGTG | CATTCCAACCACCTTTCATCACT |
| rsph1 | ENSGMOG00000019274 | 5′CCTGGAACAACGGCAAGATAG | CCCGGTCCCGTTGGAT |
| afp4 | ENSGMOG00000002484 | 5′GCTGATGGCGACTGTTGAAG | GGTCCTCCCGTCCTCGAT |
| sybu | ENSGMOG00000006474 | 5′GGCTCTACTCGCGACATGGT | TGCAATCCCATAATGCAACAG |
| ipo7 | ENSGMOG00000001681 | 5′GGAAAATAGCAGTCCGCTCACT | CCTCCTCTGGAGCTCAGCAA |
| pga | ENSGMOG00000001326 | 5′GCCTCTGCCTACGTGTCTCAGT | CGTAGAACTCCCTGATGAAGACATC |
| krt13 | ENSGMOG00000011430 | 5′ACACGAGGAGGAGCTACTGGTT | ACTCCCTCGTAGTGACTCCTAATCTC |
Gene short names, ID (Ensembl gene ID or GenBank expressed sequence tag ID (hba)) and corresponding sequences are shown.
qPCR was performed on an SDS 7900HT Fast Real-Time PCR system (Applied Biosystems, Oslo, Norway) system using Fast SYBR® green PCR Master Mix (Applied Biosystems). Thermal cycling conditions were: 95°C for 20 sec, and 40 cycles of 95°C for 1 sec followed by 60°C for 20 sec. PCR efficiencies were verified to be approximately equal between target and reference genes (standard-curve method using 250, 125, 62.5, 31.25, and 15.63 ng RNA). Melting-curve analysis revealed that each primer pair only produced one product. One hundred twenty-five micrograms of RNA were used to produce cDNA for downstream analysis of the relative transcript abundance. No-template controls were run for each gene per PCR plate. The relative gene expression level was calculated using the Comparative Ct method (Applied Biosystems). All data were normalized to rabbit hba mRNA. For each gene, the data were calibrated to the sample with the lowest value for ΔCt.
For the gene expression measurement with qPCR, a one-way ANOVA with the post test Tukey's multiple comparison test was applied on log-converted values to reveal significant differences between groups. Statistical tests were performed using GraphPad Prism 5.04 (GraphPad Software Inc., La Jolla, CA). A P-value of ≤0.05 indicated a significant difference. The gene expression profiles measured by qPCR can be seen in Figure 6.
Figure 6.
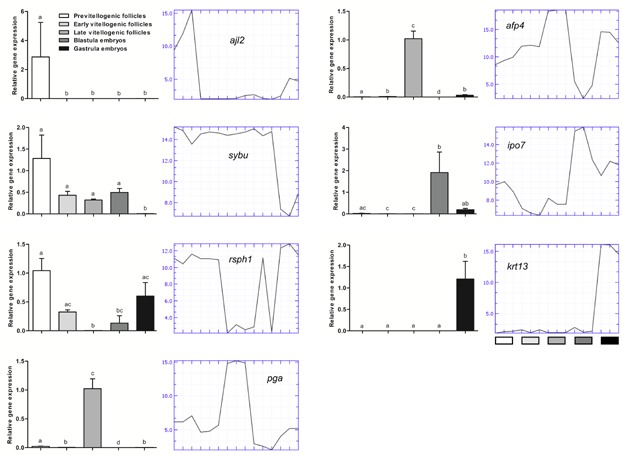
Verification of the microarray by qPCR. Gene expression of the selected genes ajl2, sybu, rsph1, pga, afp4, ipo7, and krt13 relative to hba, in follicles (pre-, early-, and late-vitellogenic) and embryos (blastula and gastrula). Graphs to the left represent gene expression profiles measured by qPCR, while graphs to the right represent gene expression profiles measured by microarray for the same stages using the same RNA samples (three pooled samples from each developmental stage). All qPCR data are shown as mean ± standard error of the mean from three animals (n = 3). [Color figure can be viewed in the online issue which is available at wileyonlinelibrary.com]
Acknowledgments
The authors would like to thank the Norwegian Microarray Consortium for providing guidance with analyses and publication of microarray data, Prof. Jon Vidar Helvik and Dr. Eva Andersson for supervision, Dr. Ørjan Karlsen for participating in samplings, Stig Mæhle for performing microarray hybridizations and Anne Torsvik for performing histological sections. This study was funded by the EU-project LIFECYCLE (FP7222719, http://www.lifecycle.gu.se/, 19.12.2013).
Glossary
- ESTs
expressed sequence tags
- FC
fold change
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MZT
maternal-to-zygotic transition
- NOD
nucleotide-binding oligomerization domain
- PPAR
peroxisome proliferator-activated receptor
- qPCR
quantitative reverse-transcriptase PCR
- TGF
transforming growth factor.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web-site.
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
REFERENCES
- Aanes H, Winata CL, Lin CH, Chen JP, Srinivasan KG, Lee SG, Lim AY, Hajan HS, Collas P, Bourque G, Gong Z, Korzh V, Aleström P, Mathavan S. Zebrafish mRNA sequencing deciphers novelties in transcriptome dynamics during maternal to zygotic transition. Genome Res. 2011;21:1328–1338. doi: 10.1101/gr.116012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arukwe A, Goksøyr A. Eggshell and egg yolk proteins in fish: hepatic proteins for the next generation: Oogenetic, population, and evolutionary implications of endocrine disruption. Comp Hepatol. 2003;6:1–24. doi: 10.1186/1476-5926-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery TS, Killen SS, Hollinger TR. The relationship of embryonic development, mortality, hatching success, and larval quality to normal or abnormal early embryonic cleavage in Atlantic cod, Gadus morhua. Aquaculture. 2009;289:265–273. [Google Scholar]
- Baker DA, Russell S. Gene expression during Drosophila melanogaster egg development before and after reproductive diapause. BMC Genomics. 2009;10:242. doi: 10.1186/1471-2164-10-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo TH, Dysvik J, Jonassen I. LSimpute: Accurate estimation of missing values in microarray data with least squares methods. Nucleic Acids Res. 2004;32:e34. doi: 10.1093/nar/gnh026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobe J, Montfort J, Nguyen T, Fostier A. Identification of new participants in the rainbow trout (Oncorhynchus mykiss) oocyte maturation and ovulation processes using cDNA microarray. Reprod Biol Endocrinol. 2006;4:39. doi: 10.1186/1477-7827-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Boonanuntanasarn S, Jangprai A, Yoshizaki G. Characterization of neuropeptide Y in snakeskin gourami and the change in its expression due to feeding status and melanocortin 4 receptor expression. Gen Comp Endocrinol. 2012;179:184–195. doi: 10.1016/j.ygcen.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Bozinovic G, Sit TL, Hinton DE, Oleksiak MF. Gene expression throughout a vertebrate's embryogenesis. BMC Genomics. 2011;12:10. doi: 10.1186/1471-2164-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling B, Armengaud P, Amtmann A, Herzyk P. Rank products: A simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004;573:83–92. doi: 10.1016/j.febslet.2004.07.055. [DOI] [PubMed] [Google Scholar]
- Breton TS, Anderson JL, Goetz FW, Berlinsky DL. Identification of ovarian gene expression patterns during vitellogenesis in Atlantic cod (Gadus morhua. Gen Comp Endocrinol. 2012;179:296–304. doi: 10.1016/j.ygcen.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Breton TS, Berlinsky DL. Characterizing ovarian gene expression during oocyte growth in Atlantic cod (Gadus morhua. Comp Biochem Physiol Part D Genomics Proteomics. 2013;9:1–10. doi: 10.1016/j.cbd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Brown JA, Minkoff G, Puvanendran V. Larviculture of Atlantic cod (Gadus morhua): Progress, protocols and problems. Aquaculture. 2003;227:357–372. [Google Scholar]
- Campbell B, Dickey J, Beckman B, Young G, Pierce A, Fukada H, Swanson P. Previtellogenic oocyte growth in salmon: Relationships among body growth, plasma insulin-like growth factor-1, estradiol-17b, follicle-stimulating hormone and expression of ovarian genes for insulin-like growth factors, steroidogenic-acute regulatory protein and receptors for gonadotropins, growth hormone, and somatolactin. Biol Reprod. 2006;75:34–44. doi: 10.1095/biolreprod.105.049494. [DOI] [PubMed] [Google Scholar]
- Cerdà J, Bobe J, Babin PJ, Admon A, Lubzens E. Functional genomics and proteomic approaches for the study of gamete formation and viability in farmed finfish. Rev Fish Sci. 2008a;16:56–72. [Google Scholar]
- Cerdà J, Mercadé J, Lozano JJ, Manchado M, Tingaud-Sequeira A, Astola A, Infante C, Halm S, Viñas J, Castellana B, Asensio E, Cañavate P, Martinez-Rodriguez G, Piferrer F, Planas JV, Prat F, Yúfera M, Durany O, Subirada F, Rosell E, Maes T. Genomic resources for a commercial flatfish, the Senegalese sole (Solea senegalesis): EST sequencing, oligo microarray design, and development of the Soleamold bioinformatic platform. BMC Genomics. 2008b;9:508. doi: 10.1186/1471-2164-9-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Shaw MH, Kim Y-G, Nuñez G. NOD-Like receptors: Role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- Clelland ES, Kelly SP. Tight junction proteins in zebrafish ovarian follicles: Stage specific mRNA abundance and response to 17β-estradiol, human chorionic gonadotropin, and maturation inducing hormone. Gen Comp Endocrinol. 2010;168:388–400. doi: 10.1016/j.ygcen.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Dee DR, Filonowicz S, Horimoto Y, Yada RY. Recombinant prosegment peptide acts as a folding catalyst and inhibitor of native pepsin. Biochim Biophys Acta. 2009;1794:1795–1801. doi: 10.1016/j.bbapap.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Drivenes Ø, Taranger GL, Edvardsen RB. Gene expression profiling of Atlantic cod (Gadus morhua) embryogenesis using microarray. Mar Biotechnol (NY) 2012;14:167–176. doi: 10.1007/s10126-011-9399-y. [DOI] [PubMed] [Google Scholar]
- Dysvik B, Jonassen I. J-Express: Exploring gene expression data using Java. Bioinformatics. 2001;17:369–370. doi: 10.1093/bioinformatics/17.4.369. [DOI] [PubMed] [Google Scholar]
- Evans TG, Somero GN. A microarray-based transcriptomic time-course of hyper- and hypo-osmotic stress signaling events in the euryhaline fish Gillichthys mirabilis: Osmosensors to effectors. J Exp Biol. 2008;211:3636–3649. doi: 10.1242/jeb.022160. [DOI] [PubMed] [Google Scholar]
- Ferg M, Sanges R, Gehrig J, Kiss J, Bauer M, Lovas A, Szabo M, Yang L, Straehle U, Pankratz MJ, Olasz F, Stupka E, Müller F. The TATA-binding protein regulates maternal mRNA degradation and differential zygotic transcription in zebrafish. EMBO J. 2007;26:3945–3956. doi: 10.1038/sj.emboj.7601821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjelldal PG, van der Meeren T, Jørstad K, Hansen TJ. A radiological study on vertebral deformities in cultured and wild Atlantic cod (Gadus morhua, L.) Aquaculture. 2009;289:6–12. [Google Scholar]
- Goetz FW, McCauley L, Goetz GW, Norberg B. Using global genome approaches to address problems in cod mariculture. ICES J Mar Sci. 2006;63:393–399. [Google Scholar]
- Grossmann TR, Nelson N. Differential effect of pH on sodium binding by the various GABA transporters expressed in Xenopus oocytes. FEBS Lett. 2002;527:125–132. doi: 10.1016/s0014-5793(02)03194-0. [DOI] [PubMed] [Google Scholar]
- Halm S, Ibañez AJ, Tyler CR, Prat F. Molecular characterization of growth differentiation factor 9 (gdf9) and bone morphogenic protein 15 (bmp15) and their patterns of gene expression during the ovarian reproductive cycle in the European sea bass. Mol Cell Endocrinol. 2008;291:95–103. doi: 10.1016/j.mce.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Hulshof SC, Dijkstra G, Van der Beek EM, Bevers MM, Figueiredo JR, Beckers JF, Van den Hurk R. Immunohistochemical localization of vasoactive intestinal peptide and neuropeptide Y in the bovine ovary. Biol Reprod. 1994;50:553–560. doi: 10.1095/biolreprod50.3.553. [DOI] [PubMed] [Google Scholar]
- Johansen S, Bakke I. The complete mitochondrial DNA sequence of Atlantic cod (Gadus morhua): Relevance to taxonomic studies among codfishes. Mol Mar Biol Biotechnol. 1996;5:203–214. [PubMed] [Google Scholar]
- Kalujnaia S, McVee J, Kasciukovic T, Stewart AJ, Cramb G. A role for inositol monophosphatase 1 (IMPA1) in salinity adaption in the euryhaline eel (Anguilla Anguilla. FASEB J. 2010;25:3981–3991. doi: 10.1096/fj.10-161000. [DOI] [PubMed] [Google Scholar]
- Kane DA, Kimmel CB. The zebrafish midblastula transition. Development. 1993;119:447–456. doi: 10.1242/dev.119.2.447. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular datasets. Nucleic Acids Res. 2012;40:D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keator CS, Custer EE, Hoagland TA, Schreiber DT, Mah K, Lawson AM, Slayden OD, McCracken JA. Evidence for a potential role of neuropeptide Y in ovine corpus luteum function. Domest Anim Endocrinol. 2010;38:103–114. doi: 10.1016/j.domaniend.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Kehoe AS, Volkoff H. Cloning and characterization of neuropeptide Y (NPY) and cocaine and amphetamine regulated transcript (CART) in Atlantic cod (Gadus morhua. Comp Biochem Physiol A Physiol. 2007;146:451–461. doi: 10.1016/j.cbpa.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Kjesbu OS. The spawning activity of cod, Gadus morhua L. J Fish Biol. 1989;34:195–206. [Google Scholar]
- Kjesbu OS, Klungsøyr J, Kryvi H, Witthames PR, Greer Walker M. Fecundity, atresia, and egg size of captive Atlantic cod (Gadus morhua) in relation to proximate body composition. Can J Fish Aquat Sci. 1991;48:2333–2343. [Google Scholar]
- Kjesbu OS, Kryvi H. Oogenesis in cod, Gadus morhua L., studied by light and electron microscopy. J Fish Biol. 1989;34:735–746. [Google Scholar]
- Kjesbu OS, Kryvi H, Norberg B. Oocyte size and structure in relation to blood plasma steroid hormones in individually monitored, spawning Atlantic cod. J Fish Biol. 1996;49:1197–1215. [Google Scholar]
- Kleppe L, Edvardsen RB, Kuhl H, Malde K, Furmanek T, Drivenes Ø, Reinhardt R, Taranger GL, Wargelius A. Maternal 3'UTRs: From egg to onset of zygotic transcription in Atlantic cod. BMC Genomics. 2012;13:443. doi: 10.1186/1471-2164-13-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppe L, Karlsen Ø, Edvardsen RB, Norberg B, Andersson E, Taranger GL, Wargelius A. Cortisol treatment of prespawning female cod affects cytogenesis related factors in eggs and embryos. Gen Comp Endocrinol. 2013;189:84–895. doi: 10.1016/j.ygcen.2013.04.028. [DOI] [PubMed] [Google Scholar]
- Knoll-Gellida A, André M, Gattegno T, Forgue J, Admon A, Babin PJ. Molecular phenotype of zebrafish ovarian follicle by serial analysis of gene expression and proteomic profiling, and comparison with the transcriptomes of other animals. BMC Genomics. 2006;7:46. doi: 10.1186/1471-2164-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Yanagawa T, Yoshida N, Yamashita M. Introduction of cyclin B induces activation of the maturation-promoting factor and breakdown of germinal vesicle in growing zebrafish oocytes unresponsive to the maturation-inducing hormone. Dev Biol. 1997;190:142–152. doi: 10.1006/dbio.1997.8673. [DOI] [PubMed] [Google Scholar]
- Laing KJ, Purcell MK, Winton JR, Hansen JD. A genomic view of the NOD-like receptor family in teleost fish: Identification of a novel NLR subfamily in zebrafish. BMC Evol Biol. 2008;8:42. doi: 10.1186/1471-2148-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanes CF, Fernandes JM, Kiron V, Babiak I. Profiling of key apoptopic, stress, and immune-related transcripts during embryonic and postembryonic development of Atlantic cod (Gadus morhua L.) Theriogenology. 2012;78:1583.e2–1596.e2. doi: 10.1016/j.theriogenology.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Chia JM, Bartfai R, Christoffels A, Yue GH, Ding K, Ho MY, Hill JA, Stupka E, Orban L. Comparative analysis of the testis and ovary transcriptomes in zebrafish by combining experimental and computational tools. Comp Funct Genomics. 2004;5:403–418. doi: 10.1002/cfg.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Ge W. Growth differentiation factor 9 and its spatiotemporal expression and regulation in the zebrafish ovary. Biol Reprod. 2007;76:294–302. doi: 10.1095/biolreprod.106.054668. [DOI] [PubMed] [Google Scholar]
- Lowther KM, Nikolaev VO, Mehlmann LM. Endocytosis in the mouse oocyte and its contribution to cAMP signaling during meiotic arrest. Reproduction. 2011;141:737–747. doi: 10.1530/REP-10-0461. [DOI] [PubMed] [Google Scholar]
- Lubzens E, Young G, Bobe J, Cerdà J. Oogenesis in teleosts: How fish eggs are formed. Gen Comp Endocrinol. 2010;165:367–389. doi: 10.1016/j.ygcen.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Luckenbach JA, Iliev DB, Goetz FW, Swanson P. Identification of differentially expressed ovarian genes during primary and early secondary oocyte growth in coho salmon, Oncorhynchus kisutch. Reprod Biol Endocrinol. 2008;6:2. doi: 10.1186/1477-7827-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie S, Montserrat N, Mas M, Acerete L, Tort L, Krasnov A, Goetz FW, Planas JV. Bacterial lipopolysaccharide induces apoptosis in the trout ovary. Reprod Biol Endocrinol. 2006;4:46. doi: 10.1186/1477-7827-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathavan S, Lee SGP, Mak A, Miller LD, Murthy KRK, Govindarajan KR, Tong Y, Wu YL, Lam SH, Yang H, Ruan YJ, Korzh V, Gong ZY, Liu ET, Lufkin T. Transcriptome analysis of zebrafish embryogenesis using microarrays. PLOS Genet. 2005;1:260–276. doi: 10.1371/journal.pgen.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev S, Pan H, Schultz RM. Absence of MSY2 in mouse oocytes perturbs oocyte growth and maturation, RNA stability, and the transcriptome. Biol Reprod. 2011;85:575–583. doi: 10.1095/biolreprod.111.091710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meno C, Saijoh Y, Fujii H, Ikeda M, Yokoyama T, Yokoyama M, Toyoda Y, Hamada H. Left-right asymmetric expression of the TGF beta-family member lefty in mouse embryos. Nature. 1996;381:151–155. doi: 10.1038/381151a0. [DOI] [PubMed] [Google Scholar]
- Mittelholzer C, Andersson E, Consten D, Hirai T, Nagahama Y, Norberg B. 20β-hydroxysteroid dehydrogenase and cyp19a1 are differentially expressed during maturation in Atlantic cod (Gadus morhua. J Mol Endocrinol. 2007;39:319–328. doi: 10.1677/JME-07-0070. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Yamashita M. Regulation of oocyte maturation in fish. Dev Growth Differ. 2008;50:S195–S219. doi: 10.1111/j.1440-169X.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: Ι. Characterization and timing of cellular changes at the midblastula stage. Cell. 1982a;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell. 1982b;30(3):687–696. doi: 10.1016/0092-8674(82)90273-2. [DOI] [PubMed] [Google Scholar]
- Nojima H, Rothhämel S, Shimizu T, Kim C-H, Yonemura S, Marlow FL, Hibi M. Syntabulin, a motor protein linker, controls dorsal determination. Development. 2010;137:923–933. doi: 10.1242/dev.046425. [DOI] [PubMed] [Google Scholar]
- Oku H, Umino T. Molecular characterization of peroxisome proliferator-activated receptors (PPARs) and their gene expression in the differentiating adipocytes of red sea bream Pagrus major. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:268–277. doi: 10.1016/j.cbpb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Olsvik PA, Søfteland L, Lie KK. Selection of reference genes for qRT-PCR examination of wild populations of Atlantic cod Gadus morhua. BMC Res Notes. 2008;1:47. doi: 10.1186/1756-0500-1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulini F, Melo EO. The role of oocyte-secreted factors GDF9 and BMP15 in follicular development and oogenesis. Reprod Domest Anim. 2011;46:354–361. doi: 10.1111/j.1439-0531.2010.01739.x. [DOI] [PubMed] [Google Scholar]
- Piñeyro G, Blier P. Autoregulation of serotonin neurons: Role in antidepressant drug action. Pharmacol Rev. 1999;51:533–591. [PubMed] [Google Scholar]
- Putiri E, Pelegri F. Passing the torch at the oocyte to embryo transition. Trends Dev Biol. 2008;3:45–60. [Google Scholar]
- Santos EM, Workman VL, Paull GC, Filby AL, Van Look KJ, Kille P, Tyler CR. Molecular basis of sex and reproductive status in breeding zebrafish. Physiol Genomics. 2007;30:111–122. doi: 10.1152/physiolgenomics.00284.2006. [DOI] [PubMed] [Google Scholar]
- Sarropoulou E, Kotoulas G, Power DM, Geisler R. Gene expression profiling of gilthead sea bream during early development and detection of stress-related genes by the application of cDNA microarray technology. Physiol Genomics. 2005;23:182–191. doi: 10.1152/physiolgenomics.00139.2005. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Iwamatsu T, Oba Y, Kobayashi D, Tanaka M, Nagahama Y, Suzuki N, Yoshikuni M. Identification and cDNA cloning of Alveolin, an extracellular metalloproteinase, which induces chorion hardening of medaka (Oryzias latipes) eggs upon fertilization. J Biol Chem. 2000;275:8349–8354. doi: 10.1074/jbc.275.12.8349. [DOI] [PubMed] [Google Scholar]
- Sivertsen E. (Spawning of cod: with special focus on the yearly cycle of the status of the organs and generations.) Fiskeridirektoratets Skrifter Serie Havundersøkelser IV; 1935. Torskens gytning: med særlig henblikk på den årlige cyclus i generasjonsorganenes tilstand; pp. 1–29. [Google Scholar]
- Sreenivasan R, Cai M, Bartfai R, Wang X, Christoffels A, Orban L. Transcriptomic analyses reveal novel genes with sexually dimorphic expression in the zebrafish gonad and brain. PLoS ONE. 2008;3:e1791. doi: 10.1371/journal.pone.0001791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star B, Nederbragt AJ, Jentoft S, Grimholt U, Malmstrøm M, Gregers TF, Rounge TB, Paulsen J, Solbakken MH, Sharma A, Wetten OF, Lanzén A, Winer R, Knight J, Vogel JH, Aken B, Andersen O, Lagesen K, Tooming-Klunderud A, Edvardsen RB, Tina KG, Espelund M, Nepal C, Previti C, Karlsen BO, Moum T, Skage M, Berg PR, Gjøen T, Kuhl H, Thorsen J, Malde K, Reinhardt R, Du L, Johansen SD, Searle S, Lien S, Nilsen F, Jonassen I, Omholt SW, Stenseth NC, Jakobsen KS. The genome sequence of Atlantic cod reveals a unique immune system. Nature. 2011;477:207–210. doi: 10.1038/nature10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzel M, Seydoux G. Regulation of the oocyte-to zygote transition. Science. 2007;316:407–408. doi: 10.1126/science.1138236. [DOI] [PubMed] [Google Scholar]
- Stocco DM. A review of the characteristics of the protein required for the acute regulation of steroid hormone biosynthesis; the case for the steroidogenic acute regulatory (StAR) protein. Proc Soc Exp Biol Med. 1998;217:123–129. doi: 10.3181/00379727-217-44214. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol. 2007;302:104–117. doi: 10.1016/j.ydbio.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros W, Lipshitz HD. The maternal-to-zygotic transition: A play in two acts. Development. 2009;136:3033–3042. doi: 10.1242/dev.033183. [DOI] [PubMed] [Google Scholar]
- Taranger GL, Carrillo M, Schulz RW, Fontaine P, Zanuy S, Felip A, Weltzien F-A, Dufour S, Karlsen Ø, Norberg B, Andersson E, Hansen T. Control of puberty in farmed fish. Gen Comp Endocrinol. 2010;165:483–515. doi: 10.1016/j.ygcen.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Thisse B, Pflumio S, Fürthauer M, Loppin B, Heyer V, Degrave A, Woehl R, Lux A, Steffan T, Charbonnier XQ, Thisse C. Expression of the zebrafish genome during embryogenesis. ZFIN online publication; 2001. Supported by the grant number RR 15402-01 from the NIH. [Google Scholar]
- Tingaud-Sequeira A, Chauvigné F, Lozano J, Agulleiro MJ, Asensio E, Cerdà J. New insights into molecular pathways associated with flatfish ovarian development and atresia revealed by transcriptional analysis. BMC Genomics. 2009;10:434. doi: 10.1186/1471-2164-10-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingaud-Sequeira A, Lozano JJ, Zapater C, Otero D, Kube M, Reinhardt R, Cerdà J. A rapid transcriptome response is associated with desiccation resistance in aerially-exposed killifish embryos. PLoS ONE. 2013;8:e64410. doi: 10.1371/journal.pone.0064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno T, Ishizuka M, Itakura T. Cytochrome P450 (CYP) in fish. Environ Toxicol Pharmacol. 2012;34:1–113. doi: 10.1016/j.etap.2012.02.004. [DOI] [PubMed] [Google Scholar]
- van der Meeren T, Ivannikov V. Seasonal shift in spawning of Atlantic cod (Gadus morhua L.) by photoperiod manipulation: Egg quality in relation to temperature and intensive larval rearing. Aquac Res. 2006;37:898–913. [Google Scholar]
- Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda, M.D.) 2004;19:331–338. doi: 10.1152/physiol.00027.2004. [DOI] [PubMed] [Google Scholar]
- Vasudevan S, Seli E, Steitz JA. Metazoan oocyte and early embryo development program: A progression through translation regulatory cascades. Genes Dev. 2006;20:138–146. doi: 10.1101/gad.1398906. [DOI] [PubMed] [Google Scholar]
- Vesterlund L, Jiao H, Unneberg P, Hovatta O, Kere J. The zebrafish transcriptome during early development. BMC Dev Biol. 2011;11:30. doi: 10.1186/1471-213X-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schalburg KR, Rise ML, Brown GD, Davidson WS, Koop BF. A comprehensive survey of the genes involved in maturation and development of the rainbow trout ovary. Biol Reprod. 2005;72:687–699. doi: 10.1095/biolreprod.104.034967. [DOI] [PubMed] [Google Scholar]
- von Schalburg KR, Cooper GA, Yazawa R, Davidson WS, Koop BF. Microarray analysis reveals differences in expression of cell surface and extracellular matrix components during development of the trout ovary and testis. Comp Biochem Physiol Part D Genomics Proteomics. 2008;3:78–90. doi: 10.1016/j.cbd.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu XJ. A G protein-coupled receptor kinase induces Xenopus oocyte maturation. J Biol Chem. 2003;278:15809–15814. doi: 10.1074/jbc.M300320200. [DOI] [PubMed] [Google Scholar]
- Wu X, Viveiros MM, Eppig JJ, Bai Y, Fitzpatrick SL, Matzuk MM. Zygote arrest 1 (Zar1) is a novel maternal-effect gene critical for the oocyte-to-embryo transition. Nat Genet. 2003;33:187–191. doi: 10.1038/ng1079. [DOI] [PubMed] [Google Scholar]
- Wu S, Li B, Lin H, Li W. Stimulatory effects of neuropeptide Y on the growth of orange-spotted grouper (Epinephelus coioides. Gen Comp Endocrinol. 2012;179:159–166. doi: 10.1016/j.ygcen.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- Yasumasu S, Iuchi I, Yamagami K. Purification and partial characterization of high choriolytic enzyme (HCE), a component of the hatching enzyme of the teleost, Oryzias latipes. J Biochem. 1989;105:204–211. doi: 10.1093/oxfordjournals.jbchem.a122640. [DOI] [PubMed] [Google Scholar]
- Zeng S, Gong Z. Expressed sequence tag analysis of expression profiles of zebrafish testis and ovary. Gene. 2002;294:45–53. doi: 10.1016/s0378-1119(02)00791-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary
Supplementary


