Abstract
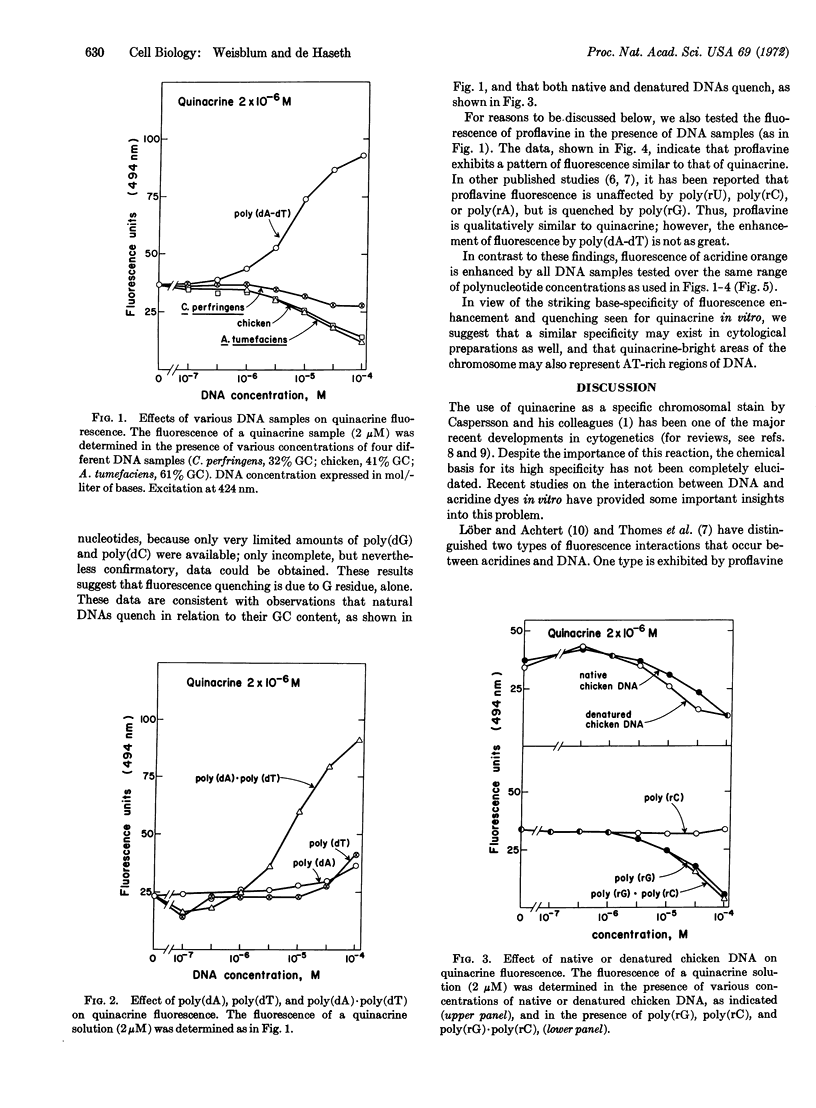
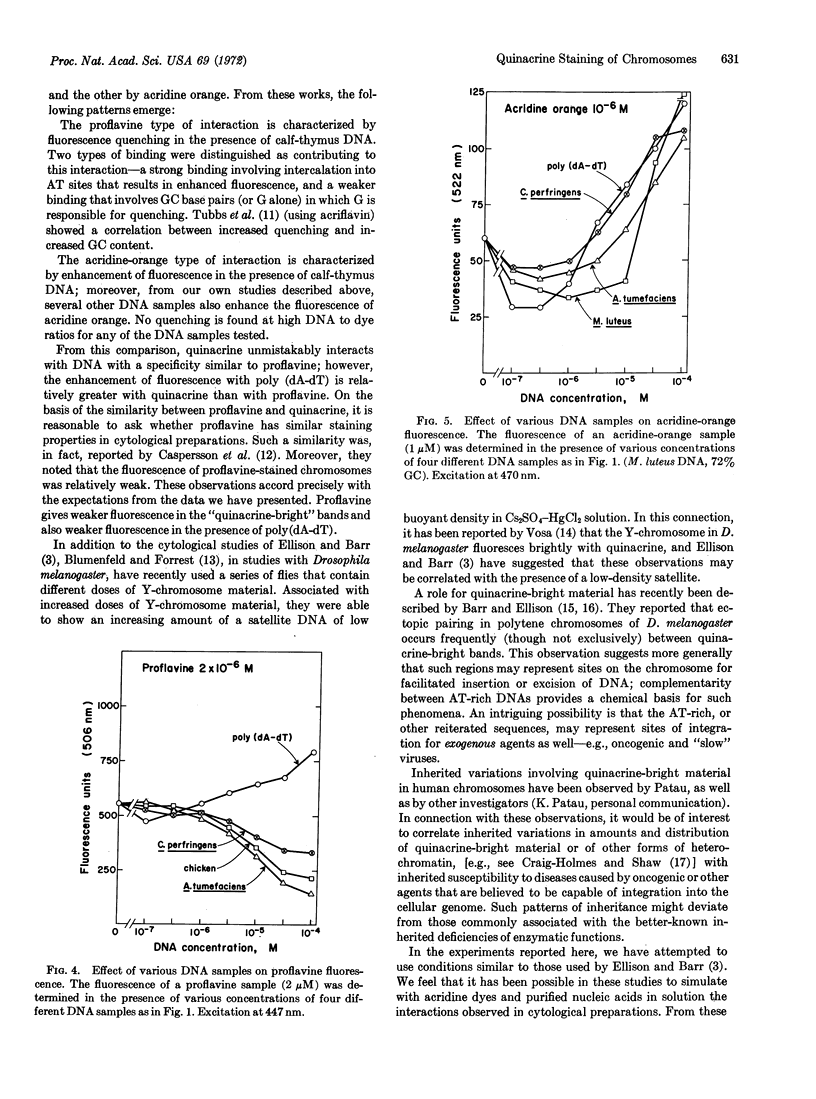
Fluorescence of quinacrine in the presence of different polynucleotides was studied to attempt to identify the specific nucleotides responsible for the fluorescence of stained chromosome preparations. A marked enhancement of fluorescence was seen in the presence of bihelical polynucleotides, such as poly(dA-dT), poly(dA)·poly(dT), and poly(rA)·poly(rU), but not in the presence of single-stranded polynucleotides, such as poly(dA), poly(dT), poly(rA), or poly(rU) alone. The higher was the GC content of natural DNAs, the more they quenched. Quenching was also seen with poly(dG) or poly(rG) alone, but not with poly(dC) or poly(rC) alone. Native and denatured DNA were both effective in quenching fluorescence. Thus, a bihelical conformation is not required for fluorescence quenching. Nearly all of these properties are shared with proflavine. In contrast, acridine orange, which stains most areas of chromosome preparations, shows enhanced fluorescence in the presence of all members of a series of natural DNAs. These data suggest that base-pairs composed of AT (rather than GC) residues are responsible for the observed fluorescence of specific chromosome regions after treatment with quinacrine, and support the proposal of Ellison and Barr (Chromosoma, in press) that the highly localized quinacrine fluorescence in their cytological preparations reflects the presence of DNA that has a high (A + T)/(G + C) ratio.
Keywords: double-stranded DNA, heterochromatin, proflavine, acridine orange, fluorescence
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr H. J., Ellison J. R. Quinacrine staining of chromosomes and evolutionary studies in Drosophila. Nature. 1971 Sep 17;233(5316):190–191. doi: 10.1038/233190a0. [DOI] [PubMed] [Google Scholar]
- Blumenfled M., Forrest H. S. Is Drosophila dAT on the Y chromosome? Proc Natl Acad Sci U S A. 1971 Dec;68(12):3145–3149. doi: 10.1073/pnas.68.12.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersson T., Farber S., Foley G. E., Kudynowski J., Modest E. J., Simonsson E., Wagh U., Zech L. Chemical differentiation along metaphase chromosomes. Exp Cell Res. 1968 Jan;49(1):219–222. doi: 10.1016/0014-4827(68)90538-7. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Zech L., Modest E. J., Foley G. E., Wagh U., Simonsson E. DNA-binding fluorochromes for the study of the organization of the metaphase nucleus. Exp Cell Res. 1969 Nov;58(1):141–152. doi: 10.1016/0014-4827(69)90124-4. [DOI] [PubMed] [Google Scholar]
- Craig-Holmes A. P., Shaw M. W. Polymorphism of human constitutive heterochromatin. Science. 1971 Nov 12;174(4010):702–704. doi: 10.1126/science.174.4010.702. [DOI] [PubMed] [Google Scholar]
- Ellison J. R., Barr H. J. Chromosome variation within Drosophila simulans detected by quinacrine staining. Genetics. 1971 Sep;69(1):119–122. doi: 10.1093/genetics/69.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein T., Weinstein I. B. Proflavine binding to transfer ribonucleic acid, synthetic ribonucleic acids, and deoxyribonucleic acid. J Biol Chem. 1967 Sep 10;242(17):3763–3768. [PubMed] [Google Scholar]
- Harwood S. J., Schendel P. F., Wells R. D. Micrococcus luteus deoxyribonucleic acid polymerase. Studies of the enzymic reaction and properties of the deoxyribonucleic acid product. J Biol Chem. 1970 Nov 10;245(21):5614–5624. [PubMed] [Google Scholar]
- Löber G., Achtert G. On the complex formation of acridine dyes with DNA. VII. Dependence of the binding on the dye structure. Biopolymers. 1969;8(5):595–608. doi: 10.1002/bip.1969.360080504. [DOI] [PubMed] [Google Scholar]
- Pearson P. L., Bobrow M., Vosa C. G., Barlow P. W. Quinacrine fluorescence in mammalian chromosomes. Nature. 1971 Jun 4;231(5301):326–329. doi: 10.1038/231326a0. [DOI] [PubMed] [Google Scholar]
- TUBBS R. K., DITMARS W. E., Jr, VANWINKLE Q. HETEROGENEITY OF THE INTERACTION OF DNA WITH ACRIFLAVINE. J Mol Biol. 1964 Aug;9:545–557. doi: 10.1016/s0022-2836(64)80226-6. [DOI] [PubMed] [Google Scholar]
- Thomes J. C., Weill G., Daune M. Fluorescence of proflavine--DNA complexes: heterogeneity of binding sites. Biopolymers. 1969;8(5):647–669. doi: 10.1002/bip.1969.360080507. [DOI] [PubMed] [Google Scholar]
- Vosa C. G. The discriminating fluorescence patterns of the chromosomes of Drosophila melanogaster. Chromosoma. 1970;31(4):446–451. doi: 10.1007/BF00285835. [DOI] [PubMed] [Google Scholar]


