Abstract
BACKGROUND
Phase 3 studies of bevacizumab in patients with advanced pancreatic cancer (APCA) demonstrated no improvement in outcome. To the authors’ knowledge, no validated predictive biomarkers for bevacizumab exist, although emerging data suggest that subsets of patients with APCA may benefit from treatment with bevacizumab. The authors evaluated baseline serum albumin (b-alb) as a predictive biomarker in a pooled analysis from 7 prospective clinical trials of gemcitabine-based therapy with or without bevacizumab.
METHODS
Data were collected from individual databases from 7 prospective clinical trials. Patients were grouped by exposure to bevacizumab and by b-alb level (≥ 3.4 g/L or < 3.4 g/dL). Overall survival (OS), time to disease progression (TTP), overall response rate, and disease control rate (overall response rate plus stable disease lasting ≥ 16 weeks) were compared between groups. Univariate and multivariable analyses of prognostic factors were performed.
RESULTS
A total of 264 patients were included. The median age was 59 years (range, 31 years-85 years) and all patients had stage IV disease per TNM staging. Normal b-alb was associated with significantly improved median OS (10.2 months vs 4.1 months; P =.0001), median TTP (6.2 months vs 3.7 months; P = 0.0488), and disease control rate (71% vs 46%; P =.007) for patients receiving bevacizumab, but not for those treated without bevacizumab. Multivariable analysis revealed a significant influence of normal b-alb on OS (P =.0008) and TTP (P =.033).
CONCLUSIONS
Patients with APCA with normal b-alb derive benefit from treatment with bevacizumab. Future prospective investigations of bevacizumab in patients with APCA should consider selecting patients with normal b-alb to maximize potential benefit.
Keywords: pancreatic cancer, albumin, bevacizumab, predictive biomarker
INTRODUCTION
Pancreatic cancer (PCA) remains the fourth leading cause of cancer death in United States.1 The prognosis for patients with advanced disease is poor, with the majority surviving < 6 months with standard gemcitabine therapy.2 Although a 4-month survival benefit was recently reported with the combination of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX),3 the toxicities of this regimen prohibit its use in the majority of patients with metastatic disease. Recently presented data indicate a less impressive but significant 1.8-month survival benefit from the addition of nabpaclitaxel to gemcitabine in patients with metastatic PCA.4 Despite these advances, there is a continuous need to further improve survival through the investigation of molecularly targeted agents.
Bevacizumab is a recombinant humanized monoclonal immunoglobulin G1 antibody that binds to vascular endothelial growth factor (VEGF) and prevents it from interacting with its receptors (VEGFR).5 Although preclinical data have suggested VEGF as a promising therapeutic target in PCA,6-8 3 phase 3 trials of gemcitabine plus antiangiogenic therapy with bevacizumab9,10 or the VEGF receptor tyrosine kinase in hibitor axitinib11 failed to reach their primary endpoint of overall survival (OS) in unselected patients.
Efforts have been made to identify predictive bio-markers for bevacizumab efficacy in patients with PCA; however, to the best of our knowledge none have been validated to date. Exploratory analyses from the AViTA trial9 suggested that a subset of patients with elevated VEGFA or VEGFR2 levels may benefit from bevacizumab.12 These results suggest that angiogenesis remains an interesting therapeutic target in patients with PCA and further investigation is needed to identify subsets of patients who may benefit from this treatment approach.
Pharmacokinetic analyses of bevacizumab have shown that patients with a low baseline serum albumin (b-alb) experience a 15% to 20% increase in the rate of bevacizumab clearance.13Although the clinical implications of this phenomenon are not well understood, exposure to lower therapeutic levels of bevacizumab could adversely impact clinical outcome. We recently reported a significant improvement in OS and time to disease progression (TTP) for patients with advanced PCA (APCA) and a b-alb level ≥ 3.4 g/dL who were treated on a phase 2 study of gemcitabine, infusional 5-fluorouracil, and bevacizumab.16 Our data suggested that this subset of patients may derive significant benefit from bevacizumab, and that the potential predictive value of b-alb should be further investigated in patients with APCA.
To further investigate the role of b-alb as a bio-marker for bevacizumab efficacy in patients with APCA, we evaluated clinical outcomes according to b-alb using pooled data from 7 prospective studies of gemcitabine-based therapy with or without bevacizumab.
MATERIALS AND METHODS
Study Design
The current study was an analysis of pooled data from 7 prospective, single-arm, phase 1/2 or phase 2 trials of gemcitabine-based regimens conducted at the Ohio State University, University of Michigan, Roswell Park Cancer Institute, University of California at San Francisco, The University of Texas MD Anderson Cancer Center, and the University of Oklahoma (Table 1).16-22 Raw data were collected from each clinical trial database before pooled analysis, including patient demographics, known prognostic factors (including disease stage, Eastern Cooperative Oncology Group [ECOG] performance status, baseline CA 19-9, and change in CA 19-9 with treatment), b-alb level (< 3.4 g/dL or ≥ 3.4 g/dL) measured within 7 days of treatment initiation, and clinical outcome measures (including objective response rate [ORR], disease control rate [DCR; ORR plus stable disease lasting≥ 16 weeks], TTP, and OS) for all patients. Patients were grouped according to treatment with bevacizumab (group 1) or no bevacizumab (group 2) and clinical outcomes of interest were assessed within each group and for all patients according to b-alb. The primary objectives of the current study were to determine the predictive and/or prognostic value of b-alb in patients with APCA and specifically those treated with bevacizumab.
TABLE 1.
Studies Included in the Pooled Analysis
| Study | Phase | No. of Patients | Chemotherapy | Median TTP/PFS | Median OS |
|---|---|---|---|---|---|
| Ko 200617 | 2 | 51 | FDR gemcitabine + cisplatin | 3.9 mo (TTP) | 7.1 mo |
| Ko 200818 | 2 | 52 | FDR gemcitabine + cisplatin + bevacizumab | 6.6 mo (TTP) | 8.2 mo |
| Javle 200919 | 2 | 50 | Gemcitabine + capecitabine+ bevacizumab | 5.8 mo (PFS) | 4.8 mo |
| Fogelman 201120 | 2 | 50 | FDR gemcitabine + oxaliplatin + bevacizumab | 4.9 mo (PFS) | 11.9 mo |
| Hill 201121 | 1 | 21 | FDR gemcitabine + capecitabine + docetaxel | 5.8 mo (PFS) | 7.4 mo |
| Martin 201216 | 2 | 42 | FDR gemcitabine + infusional 5-FU + bevacizumab | 5.9 mo (PFS) | 7.4 mo |
| Ko 201222 | 1 | 45 | FDR gemcitabine + capecitabine | 5.5 mo (TTP) | 9.8 mo |
Abbreviations: 5-FU, 5-fluorouracil; FDR, fixed dose rate; OS, overall survival; PFS, progression-free survival; TTP, time to disease progression.
Eligibility
Studies selected for pooled analysis were required to include patients with APCA proven by cytology or histology. To limit potential confounding factors for clinical outcomes, studies for pooled analysis (both bevacizumab and nonbevacizumab studies) had strict inclusion criteria, including the availability of b-alb data. Patients included in the raw database were required to have stage IV disease, an ECOG performance status of 0 to 1, no prior treatment for metastatic disease, and a b-alb value collected within 7 days of treatment initiation. Treatment was required to be gemcitabine-based for inclusion in the current analyses and would include bevacizumab at a dose of 5 mg/kg/week with the various dosing schedules. All studies included were approved by the respective Institutional Review Boards at each institution.
Statistical Analysis
Clinical outcomes were defined as follows: OS was defined as the time from first treatment until death from any cause, TTP was defined as the time from first treatment until disease progression, ORR was defined as the percentage of patients achieving a complete or partial response by Response Evaluation Criteria In Solid Tumors (RECIST) criteria used in each individual study, and DCR was defined as the percentage of patients achieving an objective response or stable disease for ≥ 16 weeks. Patients who were lost to follow-up or who were still alive were censored at the date of their last visit. Patient characteristics were summarized using descriptive statistics and graphical analyses as part of exploratory data analyses. Factors were compared between groups of interest (eg, protocol treatment, bevacizumab vs not, low b-alb vs not) using 2-sample Student t tests for continuous measures and chi-square tests for categorical markers or their nonparametric equivalents in the cases in which assumptions did not hold. The clinical outcomes described above were compared between groups of interest. For dichotomous outcomes such as ORR and DCR, univariate and multivariable logistic regression models were used to evaluate differences. Goodness of fit for logistic regression models was assessed based on the methods of Hosmer and Leme-show.23 For TTP and OS, univariate and multivariable Cox regression models24 were used to assess the prognostic influence of clinical factors. Proportional hazards were tested using the methods of Therneau and Grambsch.25 Kaplan-Meier26 methods were also used to assess differences in these distributions graphically and log-rank tests27 were used to quantitatively evaluate differences in survival distributions. Statistical significance was declared for P values < .05.
RESULTS
Characteristics of Included Clinical Trials
Seven prospective clinical trials were included in these analyses (Table 1).16-22 All trials involved gemcitabine-based therapy, and 4 trials included bevacizumab. Only 2 of these trials met their primary endpoint.16,19 All studies included patients with APCA. Patient characteristics by study included in these analyses demonstrated some differences in clinical factors, including ECOG performance status distribution (P = .0003), sex distribution (P = .03), and percentage of patients with a b-alb level < 3.4 g/dL (P = .014). Median age and CA 19-9 level were found to be similar among the studies.
Patient Characteristics
A total of 311 patients were identified in the pooled database. Forty-seven patients were excluded for the following reasons: stage III disease (28 patients), ECOG performance status of 2 (11 patients), prior therapy for advanced disease (1 patient), or lack of an available b-alb value (7 patients). A total of 264 patients were therefore included in the raw data analysis. Patient characteristics are outlined in Table 2, and were balanced between groups 1 and 2 (bevacizumab vs no bevacizumab, respectively). Continuous measures of b-alb were found to be similar in both treatment groups (measure 1: 3.8 g/dL; and measure 2: 3.8 g/dL [P = .7]). The percentage of patients with low b-alb (< 3.4 g/dL) was also similar in groups 1 and 2 (21% vs 16%; P = .47) (Table 2).
TABLE 2.
Patient Characteristics (N = 264)
| Characteristics | Group 1: Bevacizumab | Group 2: No Bevacizumab | P |
|---|---|---|---|
| No. (%) | 167 | 97 | |
| Median age (range), y | 60 (31-85) | 58 (33-78) | .17 |
| Sex | .96 | ||
| Male | 78 (47) | 47 (48) | |
| Female | 88 (53) | 50 (52) | |
| ECOG performance status | |||
| 0 | 72 (43) | 43 (44) | .64 |
| 1 | 92 (55) | 47 (48) | |
| 0 or 1 (not specified) | 3 (2) | 7 (8) | |
| Stage | |||
| IV | 136 (100) | 97 (100) | 1.00 |
| CA 19-9 ≥2× ULN | |||
| Yes | 122 (73) | 76 (78) | .46 |
| No | 44 (26) | 21 (22) | |
| Not available | 1 (1) | 0 (0) | |
| Baseline albumin, g/dL | |||
| ≥3.4 | 132 (75) | 81 (83) | .15 |
| <3.4 | 35 (25) | 16(17) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ULN, upper limit of normal.
Clinical Outcomes According to b-alb Level
When combining all patients, a b-alb level ≥ 3.4 g/dL was found to be associated with significantly improved OS (median, 9.6 months vs 4.9 months; hazards ratio [HR], 0.8 [P = .0005]), TTP (median 5.8 months vs 3.4 months; HR, 0.69 [P = .04]), and ORR and DCR (ORR: 24% vs 10% [P = .043]; DCR: 65% vs 43% [P = .007]). Using multivariable analysis adjusted for known clinical prognostic factors and receipt of bevacizumab, b-alb remained a significant independent predictor of OS (P = .0009) (Fig. 1 Top), TTP (P = .04) (Fig. 1 Bottom), and DCR (P = .008) and was found to have borderline significance for ORR (P = .053).
Figure 1.
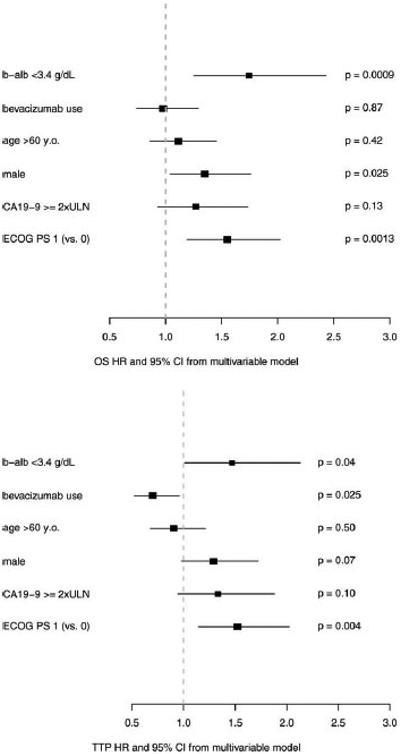
Using multivariable analysis adjusted for known clinical prognostic factors and receipt of bevacizumab, baseline serum albumin (b-alb) remained a significant independent predictor of (Top) overall survival (OS) (P =.0009) and (Bottom) time to disease progression (TTP) (P =.04). ULN indicates upper limit of normal; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazards ratio; 95% CI, 95% confidence interval.
In patients specifically treated with bevacizumab, a b-alb level ≥ 3.4 g/dL was found to be associated with a significant improvement in OS (median, 10.2 months vs 4.1 months; HR, 2.1 [P = .0001]) (Fig. 2 Top), TTP (median 6.2 months vs 3.7 months; HR, 1.8 [P = .00488]) (Fig. 2 Bottom), and DCR (71% vs 46%; P = .007), with a trend toward improved ORR (28% vs 11%; P = .051). Multivariable analysis for OS and TTP revealed b-alb (P = .0004 and P = .049, respectively), ECOG performance status (P = .03 and P = .0007, respectively), and sex (P = .02 and P = .017, respectively) to be independent predictors of OS and TTP.
Figure 2.
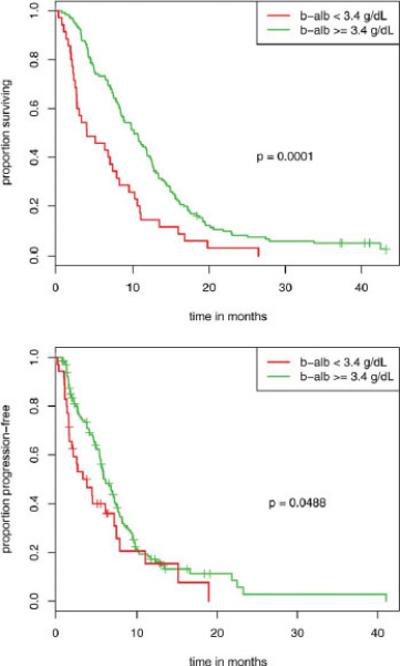
In patients specifically treated with bevacizumab, a baseline serum albumin (b-alb) level ≥ 3.4 g/dL was found to be associated with a significant improvement in (Top) overall survival (median, 10.2 months vs 4.1 months; hazards ratio, 2.1 [P =.0001]) and (Bottom) time to disease progression (median 6.2 months vs 3.7 months; hazards ratio, 1.8 [P = .00488]).
For patients who did not receive bevacizumab, there were no significant differences noted with regard to OS (median, 8.9 months vs 5.1 months; HR, 1.26 [P = .43]), TTP (median, 4.7 months vs 3.4 months; HR, 1.54 [P = .17]), ORR (18% vs 6%; P = .28), or DCR (56% vs 44%; P = .36) according to b-alb (Table 3).
TABLE 3.
Clinical Outcomes According to Baseline Albumin (N=264)
| Outcome (95% CI) |
||||
|---|---|---|---|---|
| Group | Median OS, Months | Median TTP, Months | ORR, % | DCR, %a |
| All patients (N=264) | ||||
| b-alb ≥3.4 g/dL | 9.6 (8.5-11.1) | 5.8 (5.1-6.6) | 24 (18-30) | 65 (58-71) |
| b-alb <3.4 g/dL | 4.9 (3.4-8.0) | 3.4 (2.2-7.3) | 10 (3-21) | 43 (29-58) |
| P b | .0005 | .04 | .043 | .007 |
| Group 1 (B) (N = 167) | ||||
| b-alb ≥3.4 g/dL | 10.2 (8.6-11.9) | 6.2 (5.6-7.6) | 28 (21-37) | 71 (62-78) |
| b-alb <3.4 g/dL | 4.1 (2.8-8.4) | 3.7 (2.0-7.6) | 11 (3-27) | 46 (29-63) |
| P b | .0001 | .0488 | .051 | .007 |
| Group 2 (no B) (N=97) | ||||
| b-alb ≥3.4 g/dL | 8.9 (7.8-11.1) | 4.7 (3.6-6.4) | 18 (10-28) | 56 (45-67) |
| b-alb <3.4 g/dL | 5.1 (3.8-16.0) | 3.4 (1.8-NA) | 6 (0.2-30) | 44 (20-70) |
| P b | .43 | .17 | .28 | .36 |
Abbreviations: 95% CI, 95% confidence interval; B, bevacizumab, b-alb, baseline serum albumin; DCR, disease control rate; NA, not available; ORR, overall response rate; OS, overall survival; TTP, time to disease progression.
DCR indicates partial response and stable disease with a duration of ≥16 weeks.
Bold type indicates statistical significance.
DISCUSSION
APCA has proven to be a relatively chemoresistant disease and new approaches with targeted therapies are needed. A large volume of preclinical evidence has implicated angio-genesis, and VEGF in particular, as relevant and promising therapeutic targets in patients with APCA6; however, phase 3 studies of antiangiogenic agents including bevacizumab have had negative results in unselected patients.9-11 Increasing evidence suggests that proper patient selection through the identification and use of predictive biomarkers may maximize the efficacy of targeted anticancer therapies. To the best of our knowledge, no such predictive biomarkers for bevacizumab have been validated in any advanced malignancy, although recent exploratory data have suggested baseline VEGFA levels may correlate with clinical outcomes.12 Our previous investigations indicated that a balb level ≥ 3.4 g/dL may be predictive of bevacizumab efficacy and warranted further investigation.
The results of the current study suggest a predictive role for b-alb in patients with APCA receiving bevacizumab, independent of other prognostic factors. Our analyses revealed that b-alb significantly influenced clinical outcome in those patients with regimens that included bevacizumab, but did not appear to impact survival outcomes in those patients who did not receive bevacizumab. In addition, in our analysis of patients with APCA who were treated with bevacizumab, a b-alb level < 3.4 g/dL was found to remain a strong independent predictor of inferior outcomes for patients with APCA receiving bevacizumab, after adjusting for other clinical covariates. The current study data suggest that b-alb has a prognostic role in patients with APCA who are treated with bevacizumab, and is a potential predictive marker for survival outcomes in those patients despite combination treatments with other agents. The literature suggests that this finding may be confounded by the association between low b-alb with other factors associated with poor prognosis, including older age, poor ECOG performance status, more aggressive or advanced disease, poor nutritional status, or hepatic dysfunction. In the analysis cohort in the current study, b-alb proved to be significantly associated with clinical outcomes even when we adjusted for ECOG performance status and patient age. Therefore, despite the previously reported prognostic significance of b-alb regardless of treatment, the results of the current study indicate a novel use for b-alb as a predictive biomarker in patients with APCA receiving bevacizumab. Furthermore, to the best of our knowledge, the current study is the first report of the predictive or prognostic value of b-alb specifically in patients with APCA.
The predictive value of b-alb may be explained by a pharmacokinetic description of bevacizumab. A small exploratory analysis suggested a 15% increased rate of bevacizumab clearance for patients with colon cancer with lower b-alb who were treated with bevacizumab. This was independent of prognostic variables such as age, sex, tumor stage, and ECOG performance status, all of which were found to have no impact on bevacizumab clearance.13 A larger study using pooled data from 8 clinical trials found that patients with a b-alb level ≥ 2.9 g/dL experienced a 20% increase in their rate of bevacizumab clearance.14
The interpretation of the findings of the current study is limited by their retrospective nature and the relatively small sample size of the group not treated with bevacizumab, although these results were strengthened by the choice of study design. Unlike a meta-analysis, a pooled analysis includes individual patient data that were prospectively collected within the context of a clinical trial, which improves the strength and statistical significance of the final results. An additional potential weakness of the current study is that relatively small numbers of patients had low b-alb levels. However, the overall results are strengthened by the relatively large sample size of the study population enrolled at multiple participating institutions, including a meaningful control population of patients treated without bevacizumab. Finally, although there was some heterogeneity with regard to treatment and prognostic factors among the 7 individual studies included, we demonstrated the absence of a significant difference in these prognostic factors between groups in our pooled data set.
In conclusion, we identified b-alb as a potential predictive biomarker for the efficacy of bevacizumab in patients with APCA. In addition, the results of the current study suggest a likely prognostic role for b-alb in patients with APCA who are being treated with bevacizumab, regardless of other agents being used in combination with bevacizumab. Future prospective investigations of bevacizumab in patients with APCA should continue and should consider selecting patients with normal b-alb to maximize potential benefit. Finally, although the current study focused on patients with APCA, the findings have potential applicability to other advanced malignancies in which bevacizumab is currently under study or represents a standard of care.
Acknowledgments
CONFLICT OF INTEREST DISCLOSURES
Dr. Pant has received prior honorarium from Genentech (2011). Dr. Geyer has received a grant from the Mayo Clinic as a subcon-tract for work on the Alliance for Clinical Trials in Oncology cooperative group, for which Mayo Clinic is the statistics and data center. She has also received grants from the National Institutes of Health and the US Food and Drug Administration for biostatistical collaborations on projects and grants. Dr. Iyer has received a grant from Genentech and the National Comprehensive Cancer Network. Genentech also supplied bevacizumab. Dr. Ko has received a grant from Genentech to the University of California at San Francisco for work related to the current study and has also acted as a paid member of the Advisory Board of Genentech. Dr. Bekaii-Saab received a grant from Genentech for work related to the current study and has acted as a paid consultant for Genentech.
Footnotes
FUNDING SUPPORT
No specific funding was disclosed.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33(suppl 1):S18–S22. doi: 10.1016/s0959-8049(96)00324-3. [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genentech . Avastin Prescribing Information. Genentech Inc; South San Francisco, CA: 2013. [Google Scholar]
- 6.Buchler P, Reber HA, Ullrich A, et al. Pancreatic cancer growth is inhibited by blockade of VEGF-RII. Surgery. 2003;134:772–782. doi: 10.1016/S0039-6060(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 7.Baker CH, Solorzano CC, Fidler IJ. Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res. 2002;62:1996–2003. [PubMed] [Google Scholar]
- 8.Bruns CJ, Shrader M, Harbison MT, et al. Effect of the vascular endothelial growth factor receptor-2 antibody DC101 plus gemcitabine on growth, metastasis and angiogenesis of human pancreatic cancer growing orthotopically in nude mice. Int J Cancer. 2002;102:101–108. doi: 10.1002/ijc.10681. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 10.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem E, Jayson G, Dive C, et al. Analysis of blood plasma factors in the AVITA Phase III randomized study of bevacizumab (bev) with gemcitabine-erlotinib (GE) in patients (pts) with meta-static pancreatic cancer (mPC) [abstract]. Eur J Cancer. 2011;47(suppl 1):S95–S96. [Google Scholar]
- 13.Gaudreault J, Lieberman G, Kabbinavar E, et al. Pharmacokinetics of bevacizumab (BV) in colorectal cancer (CRC). Clin Pharmacol Ther. 2001;69:25. [Google Scholar]
- 14.Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–786. doi: 10.1007/s00280-007-0664-8. [DOI] [PubMed] [Google Scholar]
- 15.Zondor SD, Medina PJ. Bevacizumab: an angiogenesis inhibitor with efficacy in colorectal and other malignancies. Ann Pharmac-other. 2004;38:1258–1264. doi: 10.1345/aph.1D470. [DOI] [PubMed] [Google Scholar]
- 16.Martin LK, Li X, Kleiber B, et al. VEGF remains an interesting target in advanced pancreas cancer (APCA): results of a multi-institutional phase II study of bevacizumab, gemcitabine, and infusional 5-fluorouracil in patients with APCA. Ann Oncol. 2012;23:2812–2820. doi: 10.1093/annonc/mds134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko AH, Dito E, Schillinger B, Venook AP, Bergsland EK, Tempero MA. Phase II study of fixed dose rate gemcitabine with cisplatin for metastatic adenocarcinoma of the pancreas. J Clin Oncol. 2006;24:379–385. doi: 10.1200/JCO.2005.01.8267. [DOI] [PubMed] [Google Scholar]
- 18.Ko AH, Dito E, Schillinger B, et al. A phase II study evaluating bevacizumab in combination with fixed-dose rate gemcitabine and low-dose cisplatin for metastatic pancreatic cancer: is an anti-VEGF strategy still applicable? Invest New Drugs. 2008;26:463–471. doi: 10.1007/s10637-008-9127-2. [DOI] [PubMed] [Google Scholar]
- 19.Javle M, Yu J, Garrett C, et al. Bevacizumab combined with gemcitabine and capecitabine for advanced pancreatic cancer: a phase II study. Br J Cancer. 2009;100:1842–1845. doi: 10.1038/sj.bjc.6605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogelman D, Jafari M, Varadhachary GR, et al. Bevacizumab plus gemcitabine and oxaliplatin as first-line therapy for metastatic or locally advanced pancreatic cancer: a phase II trial. Cancer Chemother Pharmacol. 2011;68:1431–1438. doi: 10.1007/s00280-011-1601-4. [DOI] [PubMed] [Google Scholar]
- 21.Hill ME, Li X, Kim S, et al. A phase I study of the biomodulation of capecitabine by docetaxel and gemcitabine (mGTX) in previously untreated patients with metastatic adenocarcinoma of the pancreas. Cancer Chemother Pharmacol. 2011;67:511–517. doi: 10.1007/s00280-010-1348-3. [DOI] [PubMed] [Google Scholar]
- 22.Ko AH, Espinoza AM, Jones KA, et al. Optimizing the administration of fixed-dose rate gemcitabine plus capecitabine using an alternating-week schedule: a dose finding and early efficacy study in advanced pancreatic and biliary carcinomas. Am J Clin Oncol. 2012;35:411–417. doi: 10.1097/COC.0b013e3182185888. [DOI] [PubMed] [Google Scholar]
- 23.Hosmer DW, Lemeshow S. Applied Logistic Regression. John Wiley & Sons; New York: 2000. [Google Scholar]
- 24.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer-Verlag; New York: 2000. [Google Scholar]
- 25.Therneau GA, Grambsch PM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Harrington DP, Fleming TR. A class of rank test procedures for censored survival data. Biometrika. 1982;69:553–566. [Google Scholar]


