Abstract
The binding of elongation factor EF G to ribosomes inhibits the subsequent reaction of the ribosomes with the ternary complex aminoacyl-tRNA·EF Tu·GTP. Both the hydrolysis of GTP and the binding of aminoacyl-tRNA to ribosomes are nearly abolished by the previous binding of factor EF G to ribosomes in the presence of either fusidic acid plus either GTP or a nonhydrolyzable analog of GTP. The results suggest that each elongation factor binds to the same region on the ribosome. The GTPase activities of both factors EF G and EF Tu may be activated by interaction at the same ribosomal site, as has been previously suggested by others.
Keywords: aminoacyl-tRNA binding, GTP hydrolysis, fusidic acid, chloramphenicol, GMPPCP
Full text
PDF
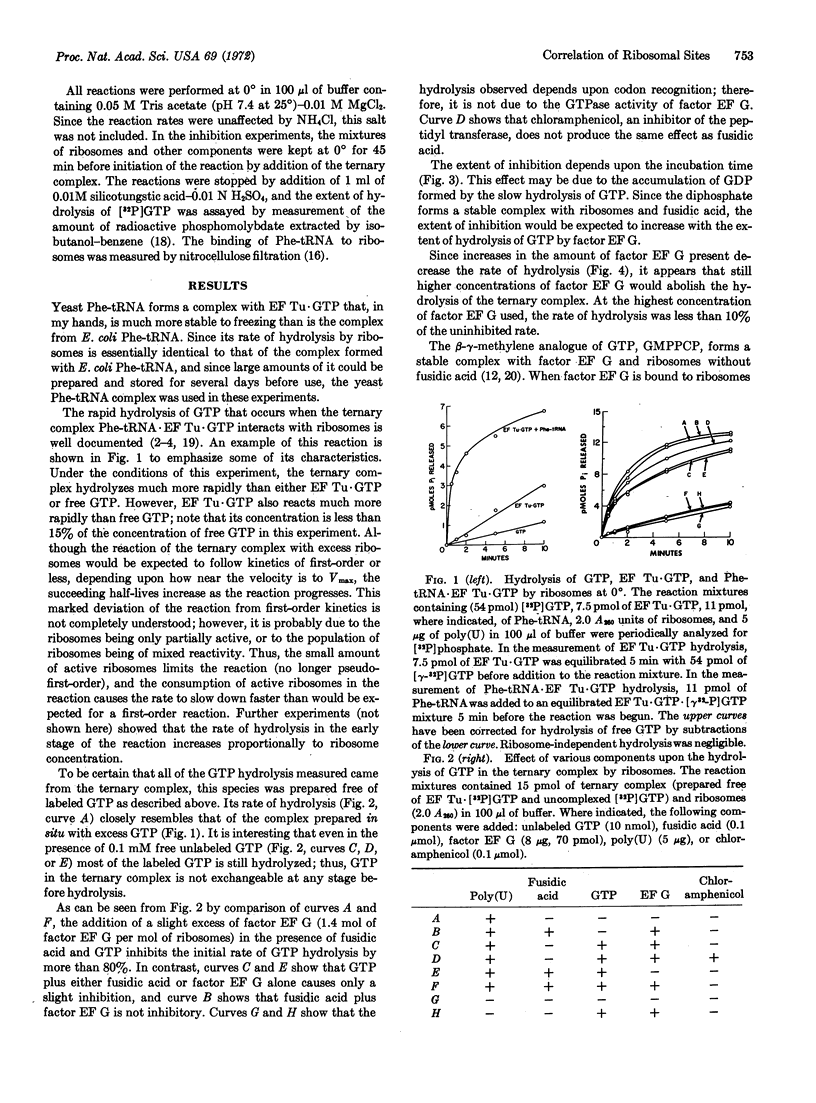
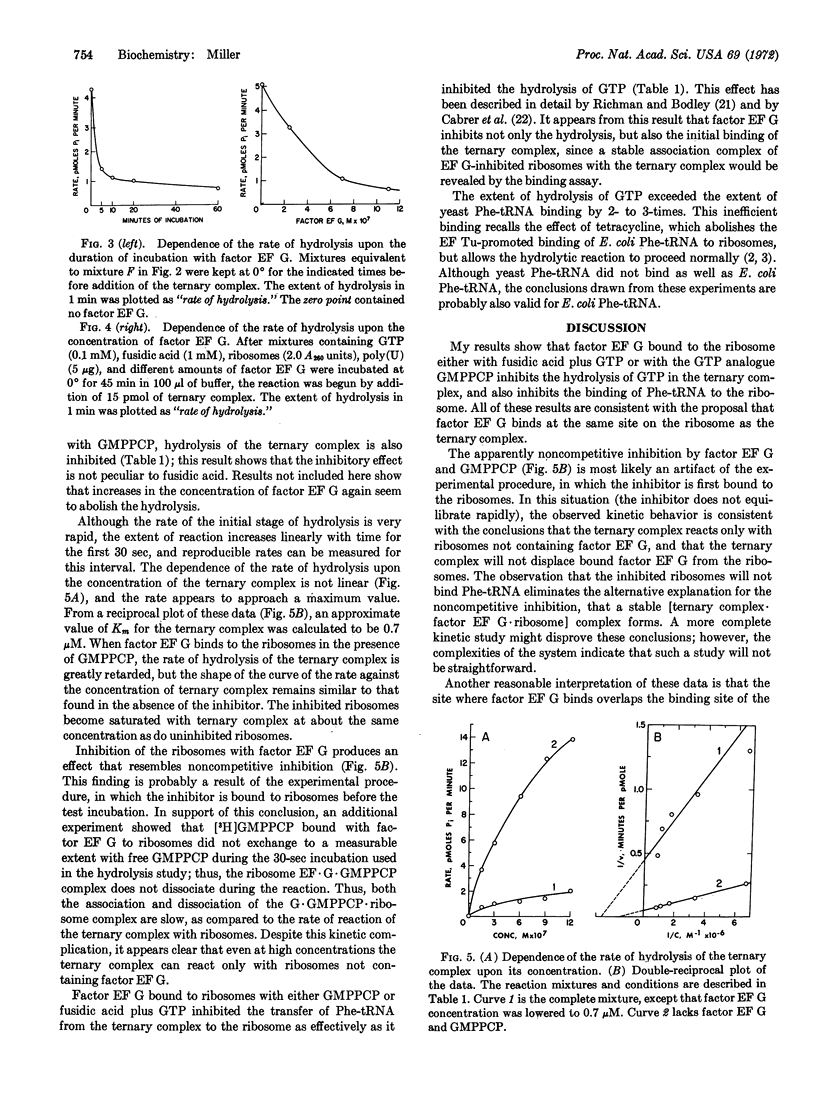

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodley J. W., Zieve F. J., Lin L. Studies on translocation. IV. The hydrolysis of a single round of guanosine triphosphate in the presence of fusidic acid. J Biol Chem. 1970 Nov 10;245(21):5662–5667. [PubMed] [Google Scholar]
- Brot N., Spears C., Weissbach H. The formation of a complex containing ribosomes, transfer factor G and A guanosine nucleotide. Biochem Biophys Res Commun. 1969 Mar 31;34(6):843–848. doi: 10.1016/0006-291x(69)90257-5. [DOI] [PubMed] [Google Scholar]
- Brot N., Spears C., Weissbach H. The interaction of transfer factor G, ribosomes, and guanosine nucleotides in the presence of fusidic acid. Arch Biochem Biophys. 1971 Mar;143(1):286–296. doi: 10.1016/0003-9861(71)90211-6. [DOI] [PubMed] [Google Scholar]
- Brot N., Yamasaki E., Redfield B., Weissbach H. The binding of aminoacyl-tRNA and poly U to a soluble factor (S) extracted from ribosomes. Biochem Biophys Res Commun. 1970 Aug 11;40(3):698–707. doi: 10.1016/0006-291x(70)90960-5. [DOI] [PubMed] [Google Scholar]
- Cabrer B., Vázquez D., Modolell J. Inhibition by elongation factor EF G of aminoacyl-tRNA binding to ribosomes. Proc Natl Acad Sci U S A. 1972 Mar;69(3):733–736. doi: 10.1073/pnas.69.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. Hydrolysis of guanosine 5'-triphosphate associated wh binding of aminoacyl transfer ribonucleic acid to ribosomes. J Biol Chem. 1969 Oct 25;244(20):5680–5686. [PubMed] [Google Scholar]
- Gupta S. L., Waterson J., Sopori M. L., Weissman S. M., Lengyel P. Movement of the ribosome along the messenger ribonucleic acid during protein synthesis. Biochemistry. 1971 Nov 23;10(24):4410–4421. doi: 10.1021/bi00800a010. [DOI] [PubMed] [Google Scholar]
- Highland J. H., Lin L., Bodley J. W. Protection of ribosomes from thiostrepton inactivation by the binding of G factor and guanosine diphosphate. Biochemistry. 1971 Nov 23;10(24):4404–4409. doi: 10.1021/bi00800a009. [DOI] [PubMed] [Google Scholar]
- Ishitsuka H., Kuriki Y., Kaji A. Release of transfer ribonucleic acid from ribosomes. A G factor and guanosine triphosphate-dependent reaction. J Biol Chem. 1970 Jul 10;245(13):3346–3351. [PubMed] [Google Scholar]
- Kinoshita T., Liou Y., Tanaka N. Inhibition by thiopeptin of ribosomal functions associated with T and G factors. Biochem Biophys Res Commun. 1971 Aug 20;44(4):859–863. doi: 10.1016/0006-291x(71)90790-x. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Studies on the purification and properties of factor Tu from E. coli. Arch Biochem Biophys. 1970 Nov;141(1):26–37. doi: 10.1016/0003-9861(70)90102-5. [DOI] [PubMed] [Google Scholar]
- Modolell J., Cabrer B., Parmeggiani A., Vazquez D. Inhibition by siomycin and thiostrepton of both aminoacyl-tRNA and factor G binding to ribosomes. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1796–1800. doi: 10.1073/pnas.68.8.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmeggiani A., Gottschalk E. M. Properties of the crystalline amino acid polymerization factors from Escherichia coli: binding of G to ribosomes. Biochem Biophys Res Commun. 1969 Jun 27;35(6):861–867. doi: 10.1016/0006-291x(69)90703-7. [DOI] [PubMed] [Google Scholar]
- Richman N., Bodley J. W. Ribosomes cannot interact simultaneously with elongation factors EF Tu and EF G. Proc Natl Acad Sci U S A. 1972 Mar;69(3):686–689. doi: 10.1073/pnas.69.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz D., Goodwin F., Weissbach H. Inhibition by aminochromes of in vitro polypeptide synthesis in Escherichia coli. Arch Biochem Biophys. 1969 Nov;134(2):478–485. doi: 10.1016/0003-9861(69)90308-7. [DOI] [PubMed] [Google Scholar]
- Shorey R. L., Ravel J. M., Garner C. W., Shive W. Formation and properties of the aminoacyl transfer ribonucleic acid-guanosine triphosphate-protein complex. J Biol Chem. 1969 Sep 10;244(17):4555–4564. [PubMed] [Google Scholar]
- Skoultchi A., Ono Y., Waterson J., Lengyel P. Peptide chain elongation; indications for the binding of an amino acid polymerization factor, guanosine 5'-triphosphate--aminoacyl transfer ribonucleic acid complex to the messenger-ribosome complex. Biochemistry. 1970 Feb 3;9(3):508–514. doi: 10.1021/bi00805a009. [DOI] [PubMed] [Google Scholar]
- Thach S. S., Thach R. E. Translocation of messenger RNA and "accommodation" of fMet-tRNA. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1791–1795. doi: 10.1073/pnas.68.8.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach H., Miller D. L., Hachmann J. Studies on the role of factor Ts in polypeptide synthesis. Arch Biochem Biophys. 1970 Mar;137(1):262–269. doi: 10.1016/0003-9861(70)90433-9. [DOI] [PubMed] [Google Scholar]
- Weissbach H., Redfield B., Brot N. Aminoacyl-tRNA-Tu-GTP interaction with ribosomes. Arch Biochem Biophys. 1971 Aug;145(2):676–684. doi: 10.1016/s0003-9861(71)80028-0. [DOI] [PubMed] [Google Scholar]


