Abstract
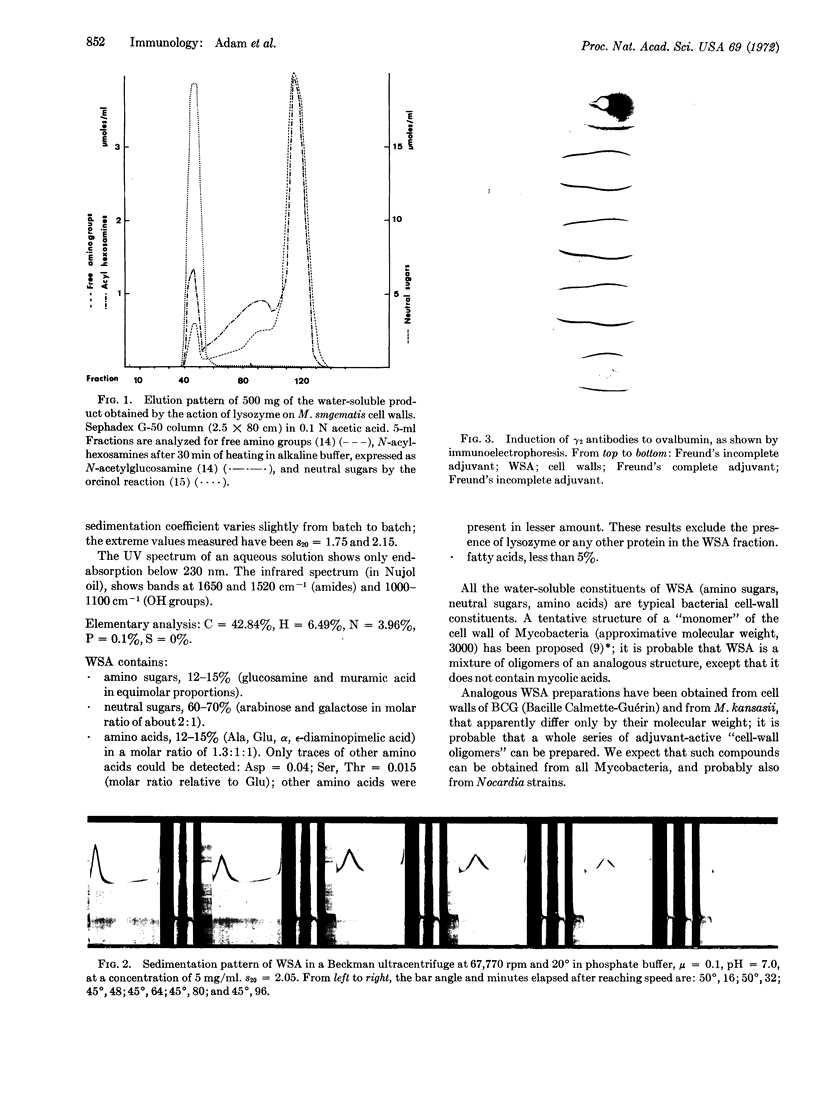
Trypsin- and chymotrypsin-treated delipidated cell walls of Mycobacterium smegmatis were digested overnight with lysozyme. The water-soluble products thus obtained were filtered on a column of Sephadex G-50; the first peak, excluded from the column, has immunological adjuvant activity. The material of the excluded peak is obtained after lyophilization as a snow-white, fluffy material, soluble in water and insoluble in organic solvents. It behaves as a slightly polydisperse macromolecule in an ultracentrifuge, with an approximate molecular weight of 20,000. All the constituents of this material are typical bacterial cell-wall constituents; thus, the water-soluble adjuvant is considered to be an “oligomer” of the cell wall.
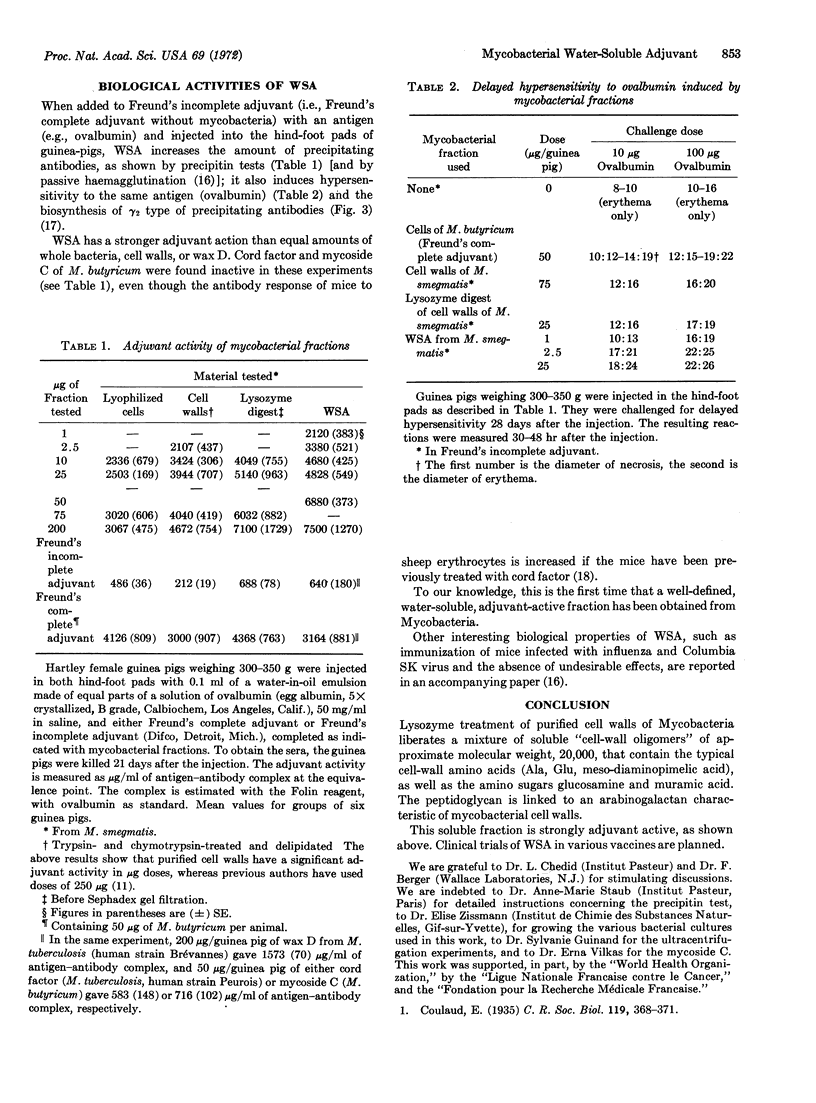
When added to Freund's incomplete adjuvant with an antigen (e.g., ovalbumin) and injected into hind-foot pads of guinea pigs, this water-soluble adjuvant increases the amount of precipitating antibodies and induces hypersensitivity to ovalbumin and the biosynthesis of γ2-type precipitating antibodies. The water-soluble material has a stronger adjuvant activity than equal amounts of whole bacteria, cell walls, or wax D, and seems to be the first well-defined, water-soluble, adjuvant-active fraction isolated from Mycobacteria.
Keywords: Sephadex chromatography, cell-wall oligomer, wax D, Freund's adjuvant, guinea pig
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma I., Kishimoto S., Yamamura Y., Petit J. F. Adjuvanticity of mycobacterial cell walls. Jpn J Microbiol. 1971 Mar;15(2):193–197. doi: 10.1111/j.1348-0421.1971.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Bekierkunst A., Levij I. S., Yarkoni E., Vilkas E., Lederer E. Cellular reaction in the footpad and draining lymph nodes of mice induced by mycobacterial fractions and BCG bacilli. Infect Immun. 1971 Sep;4(3):245–255. doi: 10.1128/iai.4.3.245-255.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Parant M., Parant F., Gustafson R. H., Berger F. M. Biological study of a nontoxic, water-soluble immunoadjuvant from mycobacterial cell walls. Proc Natl Acad Sci U S A. 1972 Apr;69(4):855–858. doi: 10.1073/pnas.69.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWIJS H., JOLLES P. CELL WALLS OF THREE STRAINS OF MYCOBACTERIA (MYCOBACTERIUM PHLEI, MYCOBACTERIUM FORTUITUM AND MYCOBACTERIUM KANSASII). PREPARATION, ANALYSIS AND DIGESTION BY LYSOZYMES OF DIFFERENT ORIGINS. Biochim Biophys Acta. 1964 Nov 1;83:326–332. [PubMed] [Google Scholar]
- FREUND J. The mode of action of immunologic adjuvants. Bibl Tuberc. 1956;(10):130–148. [PubMed] [Google Scholar]
- KOTANI S., HASHIMOTO S., MATSUBARA T., KATO K., HARADA K., KOGAMI J. LYSIS OF ISOLATED BCG CELL WALLS WITH ENZYMES. 2. DEMONSTRATION OF 'BOUND WAX D' AS A COMPONENT OF BCG CELL WALLS. Biken J. 1963 Oct;6:181–196. [PubMed] [Google Scholar]
- Kanetsuna F., Blas G. S. Chemical analysis of a mycolic acid-arabinogalactan-mucopeptide complex of mycobacterial cell wall. Biochim Biophys Acta. 1970 Jun;208(3):434–443. doi: 10.1016/0304-4165(70)90216-3. [DOI] [PubMed] [Google Scholar]
- Lederer E. The mycobacterial cell wall. Pure Appl Chem. 1971;25(1):135–165. doi: 10.1351/pac197125010135. [DOI] [PubMed] [Google Scholar]
- Markovits J., Vilkas E., Lederer E. Sur la structure chimique des cires D, peptidoglycolipides macromoléculaires des souches humaines de Mycobacterium tuberculosis. Eur J Biochem. 1971 Jan;18(2):287–291. doi: 10.1111/j.1432-1033.1971.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Migliore D., Jolles P. Contribution to the study of the structure of adjuvant active waxes D from mycobacteria: Isolation of a peptidoglycan. FEBS Lett. 1968 Nov;2(1):7–9. doi: 10.1016/0014-5793(68)80085-7. [DOI] [PubMed] [Google Scholar]
- Petit J. F., Adam A., Wietzerbin-Falszpan J., Lederer E., Ghuysen J. M. Chemical structure of the cell wall of Mycobacterium smegmatis. I. Isolation and partial characterization of the peptidoglycan. Biochem Biophys Res Commun. 1969 May 22;35(4):478–485. doi: 10.1016/0006-291x(69)90371-4. [DOI] [PubMed] [Google Scholar]
- Rimington C. Seromucoid and the bound carbohydrate of the serum proteins. Biochem J. 1940 Jun;34(6):931–940. doi: 10.1042/bj0340931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R. G., BERNSTOCK L., JOHNS R. G., LEDERER E. The influence of components of M. tuberculosis and other Mycobacteria upon antibody production to ovalbumin. Immunology. 1958 Jan;1(1):54–66. [PMC free article] [PubMed] [Google Scholar]
- WHITE R. G. FACTORS AFFECTING THE ANTIBODY RESPONSE. Br Med Bull. 1963 Sep;19:207–213. doi: 10.1093/oxfordjournals.bmb.a070058. [DOI] [PubMed] [Google Scholar]
- WHITE R. G., JOLLES P., SAMOUR D., LEDERER E. CORRELATION OF ADJUVANT ACTIVITY AND CHEMICAL STRUCTURE OF WAX D FRACTIONS OF MYCOBACTERIA. Immunology. 1964 Mar;7:158–171. [PMC free article] [PubMed] [Google Scholar]




