Abstract
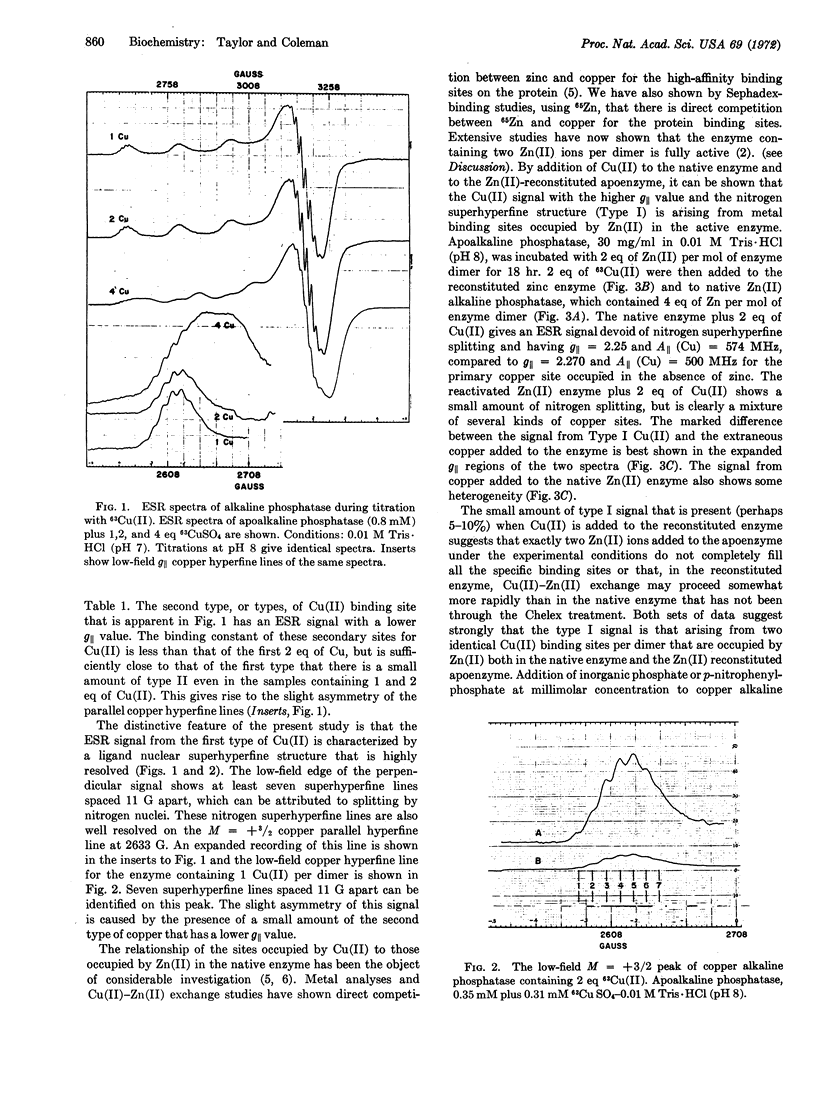
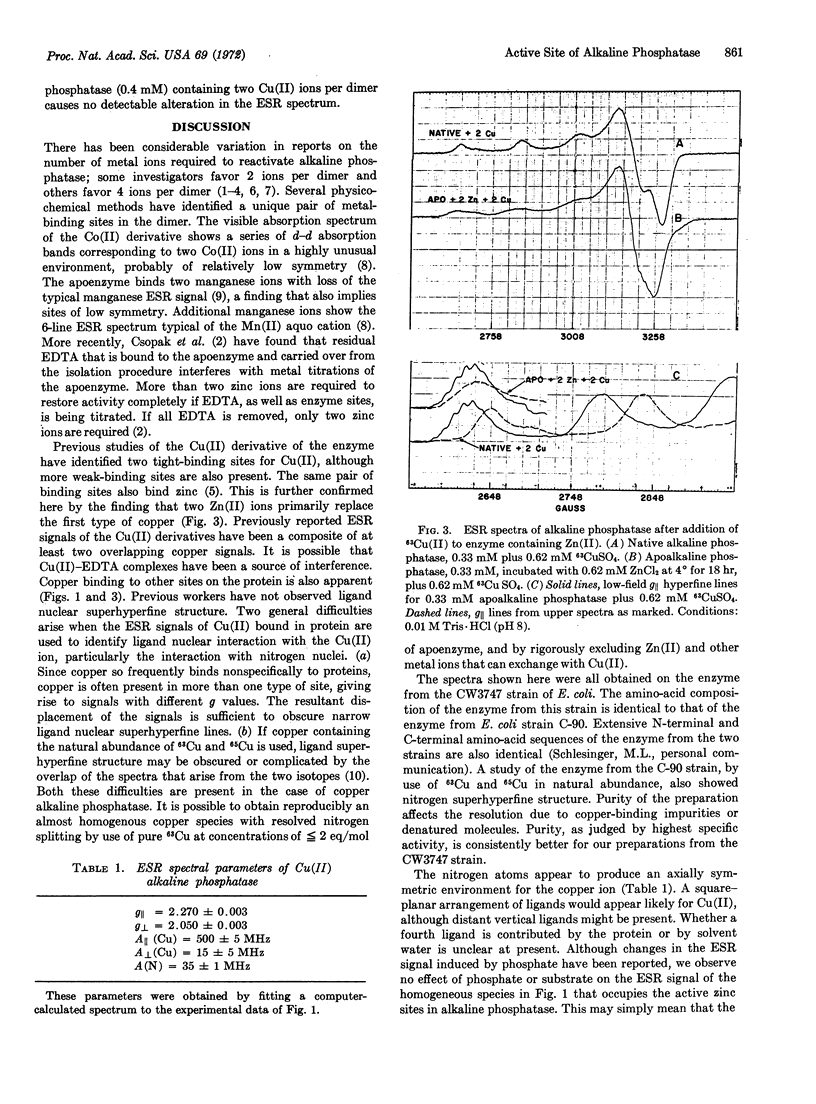
The two Zn(II) ions of native Escherichia coli alkaline phosphatase (EC 3.1.3.1) that are necessary for activity have been replaced by 63Cu(II). Titration of apoenzyme with up to 2 eq of Cu(II) gives a homogeneous species with an electron spin resonance typical for Cu(II) in an axially symmetric environment, with Az = 496 MHz, gz = gǁ = 2.27, and gx = gy = 2.05. At least seven nitrogen hyperfine lines, spaced 11 G apart, are clearly resolved on the M = +[unk] Cu(II) hyperfine peak in the parallel region. When more than 2 eq of Cu(II) are added, the electron spin resonance spectrum shows at least two types of Cu(II) binding sites; the additional site, or sites, are characterized by lower g and higher Az values. When Cu(II) is added to native Zn(II) alkaline phosphatase or to apoenzyme incubated with 2 eq of Zn(II), the electron spin resonance spectrum shows little or no trace of the species with higher g values and nitrogen splitting. These results indicate that the species with higher g represents copper bound at the site normally occupied by the 2 Zn (II) ions necessary for enzyme activity, and that the metal ion at this site has at least 3 equivalent nitrogen ligands, probably histidyl side chains.
Keywords: Zn, Cu, E. coli, electron spin resonance
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Coleman J. E. Escherichia coli alkaline phosphatase. Metal binding, protein conformation, and quaternary structure. J Biol Chem. 1969 Jan 25;244(2):308–318. [PubMed] [Google Scholar]
- Applebury M. L., Coleman J. E. Escherichia coli co (II) alkaline phsophatase. J Biol Chem. 1969 Feb 25;244(4):709–718. [PubMed] [Google Scholar]
- Applebury M. L., Johnson B. P., Coleman J. E. Phosphate binding to alkaline phosphatase. Metal ion dependence. J Biol Chem. 1970 Oct 10;245(19):4968–4976. [PubMed] [Google Scholar]
- Applebury M. L., Johnson B. P., Coleman J. E. Phosphate binding to alkaline phosphatase. Metal ion dependence. J Biol Chem. 1970 Oct 10;245(19):4968–4976. [PubMed] [Google Scholar]
- Csopak H., Falk K. E. The specific binding of copper(II) to alkaline phosphatase of E. coli. FEBS Lett. 1970 Apr 2;7(2):147–150. doi: 10.1016/0014-5793(70)80142-9. [DOI] [PubMed] [Google Scholar]
- Harris M. I., Coleman J. E. The biosynthesis of apo- and metalloalkaline phosphatases of Escherichia coli. J Biol Chem. 1968 Oct 10;243(19):5063–5073. [PubMed] [Google Scholar]
- Lazdunski C., Chappelet D., Petitclerc C., Leterrier F., Douzou P., Lazdunski M. The Cu2 plus-alkaline phosphatase of Escherichia coli. Eur J Biochem. 1970 Dec;17(2):239–245. doi: 10.1111/j.1432-1033.1970.tb01159.x. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Petitclerc C., Chappelet D., Leterrier F., Douzou P., Lazdunski M. Tight and loose metal binding sites in the apoalkaline phosphatase of E. coli. Reconstitution of the Ca2+--phosphatase from the apoenzyme EPR study of the Mn2+-phosphatase. Biochem Biophys Res Commun. 1970 Aug 11;40(3):589–593. doi: 10.1016/0006-291x(70)90943-5. [DOI] [PubMed] [Google Scholar]
- Lazdunski M., Petitclerc C., Chappelet D., Lazdunski C. Flip-flop mechanisms in enzymology. A model: the alkaline phosphatase of Escherichia coli. Eur J Biochem. 1971 May 11;20(1):124–139. doi: 10.1111/j.1432-1033.1971.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Tait G. H., Vallee B. L. Studies on the active center of alkaline phosphatase of E. coli. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1247–1251. doi: 10.1073/pnas.56.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. S., Coleman J. E. Electron spin resonance of metallocarbonic anhydrases. J Biol Chem. 1971 Nov 25;246(22):7058–7067. [PubMed] [Google Scholar]


