Abstract
Carbon monoxide binding by myoglobin and hemoglobin has been studied under conditions of constant illumination. For hemoglobin, the homotropic heme-heme interaction (cooperativity) and the heterotropic Bohr effect are invariant with light intensity over a 1000-fold change of c½. The dissociation constant, measured as c½, increases linearly with light intensity, indicating that photodissociation is a one-quantum process. At sufficiently high illumination the apparent enthalpy of ligand binding becomes positive, although in the absence of light it is known to be negative. This finding indicates that light acts primarily by increasing the “off” constants by an additive factor. The invariance of both cooperativity and Bohr effect raises a perplexing issue. It would appear to demand either that the “off” constants for the various elementary steps are all alike (which is contrary to current ideas) or that the additive factor is in each case proportional to the particular “off” constant to which it is added (a seemingly improbable alternative).
Keywords: heme proteins, ligand binding, protein photochemistry
Full text
PDF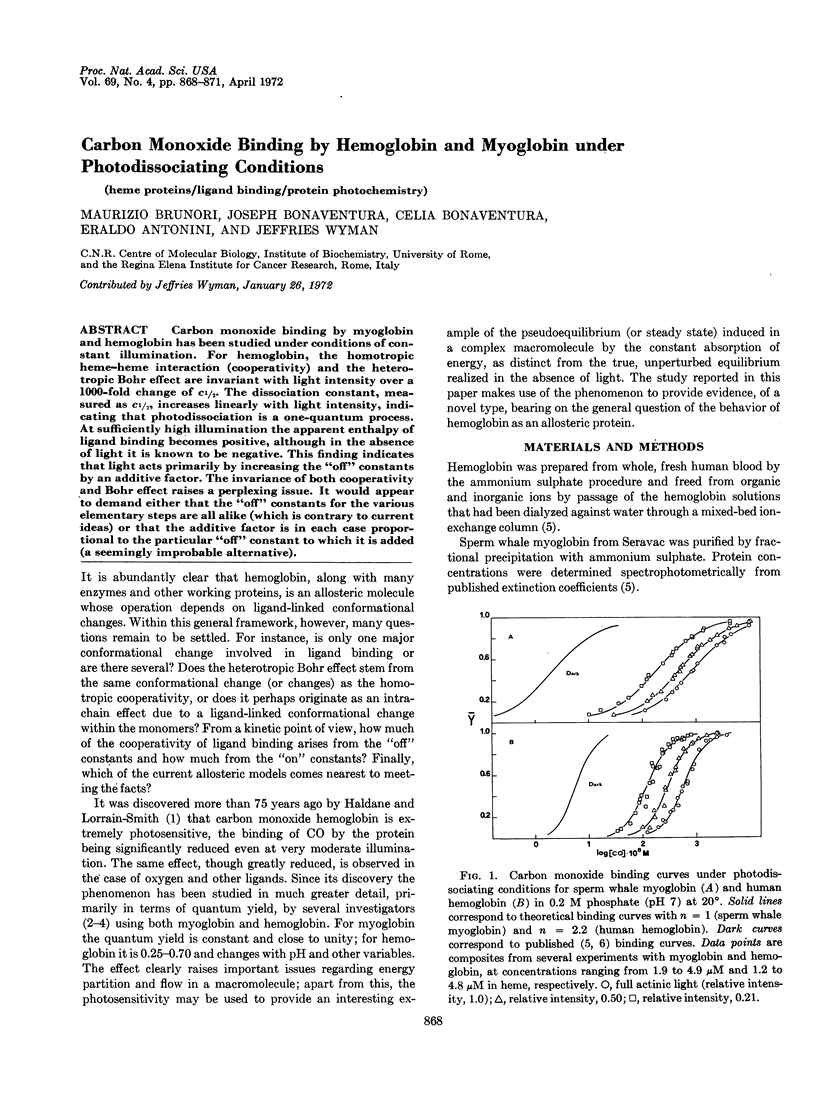


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. R., Antonini E. The binding of carbon monoxide by human hemoglobin. Proof of validity of the spectrophotometric method and direct determination of the equilibrium. J Biol Chem. 1968 Jun 10;243(11):2918–2920. [PubMed] [Google Scholar]
- Gibson Q. H. The reaction of oxygen with hemoglobin and the kinetic basis of the effect of salt on binding of oxygen. J Biol Chem. 1970 Jul 10;245(13):3285–3288. [PubMed] [Google Scholar]
- Haldane J., Smith J. L. The Oxygen Tension of Arterial Blood. J Physiol. 1896 Dec 3;20(6):497–520. doi: 10.1113/jphysiol.1896.sp000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. J., Shulman R. G., Ogawa S. An allosteric model of hemoglobin. I. Kinetics. J Mol Biol. 1971 Oct 28;61(2):425–443. doi: 10.1016/0022-2836(71)90391-3. [DOI] [PubMed] [Google Scholar]
- Noble R. W., Brunori M., Wyman J., Antonini E. Studies on the quantum yields of the photodissociation of carbon monoxide from hemoglobin and myoglobin. Biochemistry. 1967 Apr;6(4):1216–1222. doi: 10.1021/bi00856a035. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Roughton F. J. The equilibrium of carbon monoxide with human hemoglobin in whole blood. Ann N Y Acad Sci. 1970 Oct 5;174(1):177–188. doi: 10.1111/j.1749-6632.1970.tb49784.x. [DOI] [PubMed] [Google Scholar]
- WYMAN J. THE BINDING POTENTIAL, A NEGLECTED LINKAGE CONCEPT. J Mol Biol. 1965 Mar;11:631–644. doi: 10.1016/s0022-2836(65)80017-1. [DOI] [PubMed] [Google Scholar]


