Abstract
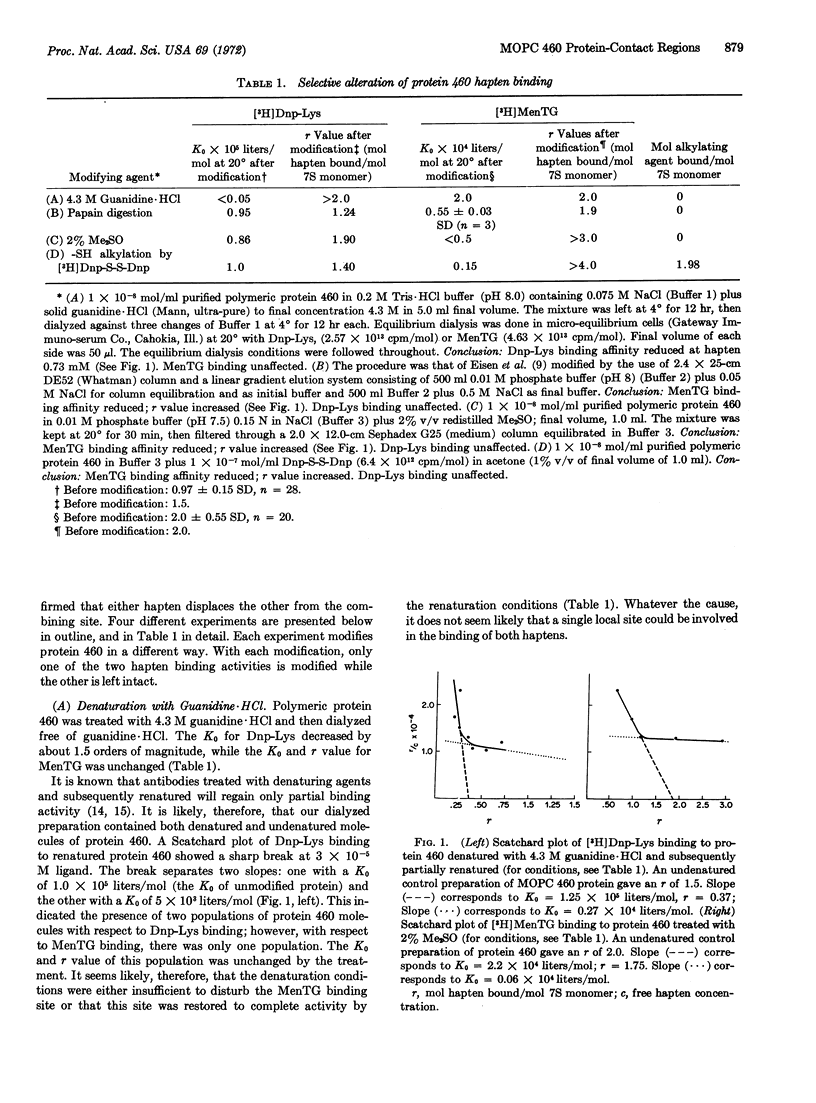
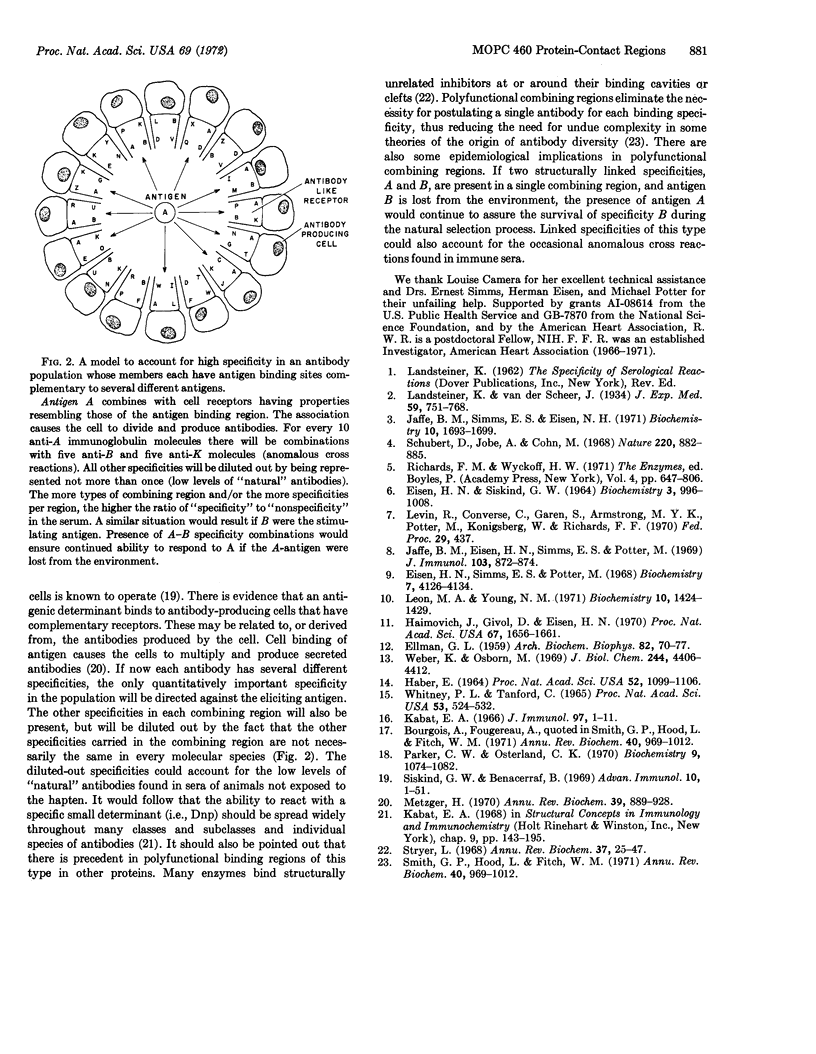
Protein 460 is a mouse myeloma γ A2 protein that competitively binds two small haptens, 2,4-ε-dinitrophenyl-L-lysine (Dnp-Lys) and 2-methyl-1:4-naphthaquinone thioglycollate (MenTG), to the antibody-combining region. The intact protein has a relatively inaccessible sulfhydryl group on each heavy chain. When it is substituted with a bulky reagent the binding affinity for MenTG decreases, while the binding of Dnp-Lys remains the same. Guanidine·HCl selectively reduces binding of Dnp-Lys; dimethylsulfoxide selectively reduces binding of MenTG. Papain digestion of protein 460 followed by column chromatography gave two fractions: one contained both binding activities and the other contained the sulfhydryl group. The affinity for Dnp-Lys of the first fraction is the same as that of the whole molecule, while affinity for MenTG is decreased. Since selective alteration of one or the other binding activity can occur in different ways, it seems likely that even though the haptens compete with each other, there is some spatial separation between the groups of contact amino-acid residues involved in the binding of these two haptens. These findings do not support the hypothesis that an immunoglobulin molecule carries combining sites complementary only to a single hapten or to a structurally related series of haptens, but rather suggests that the antibody-combining site may be a polyfunctional region capable of binding several structurally dissimilar haptens. We discuss a mechanism whereby polyfunctional combining sites can give rise to an antibody population (immune serum) that has a high degree of specificity to a single hapten.
Keywords: myeloma protein, combining site, sulfhydryl group, antibody heterogeneity
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Eisen H. N., Simms E. S., Potter M. Mouse myeloma proteins with antihapten antibody acitivity. The protein produced by plasma cell tumor MOPC-315. Biochemistry. 1968 Nov;7(11):4126–4134. doi: 10.1021/bi00851a048. [DOI] [PubMed] [Google Scholar]
- HABER E. RECOVERY OF ANTIGENIC SPECIFICITY AFTER DENATURATION AND COMPLETE REDUCTION OF DISULFIDES IN A PAPAIN FRAGMENT OF ANTIBODY. Proc Natl Acad Sci U S A. 1964 Oct;52:1099–1106. doi: 10.1073/pnas.52.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich J., Givol D., Eisen H. N. Affinity labeling of the heavy and light chains of a myeloma protein with anti-2,4-dinitrophenyl activity. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1656–1661. doi: 10.1073/pnas.67.4.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe B. M., Eisen H. N., Simms E. S., Potter M. Myeloma proteins with anti-hapten antibody activity: epsilon-2,4-dinitrophenyl lysine binding by the protein produced by mouse plasmacytoma MOPC-460. J Immunol. 1969 Oct;103(4):872–874. [PubMed] [Google Scholar]
- Jaffe B. M., Simms E. S., Eisen H. N. Specificity and structure of the myeloma protein produced by mouse plasmacytoma MOPC-460. Biochemistry. 1971 Apr 27;10(9):1693–1699. doi: 10.1021/bi00785a029. [DOI] [PubMed] [Google Scholar]
- Kabat E. A. The nature of an antigenic determinant. J Immunol. 1966 Jul;97(1):1–11. [PubMed] [Google Scholar]
- Leon M. A., Young N. M. Specificity for phosphorylcholine of six murine myeloma proteins reactive with Pneumococcus C polysaccharide and beta-lipoprotein. Biochemistry. 1971 Apr 13;10(8):1424–1429. doi: 10.1021/bi00784a024. [DOI] [PubMed] [Google Scholar]
- Metzger H. The antigen receptor problem. Annu Rev Biochem. 1970;39:889–928. doi: 10.1146/annurev.bi.39.070170.004325. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Osterland C. K. Hydrophobic binding sites on immunoglobulins. Biochemistry. 1970 Mar 3;9(5):1074–1082. doi: 10.1021/bi00807a004. [DOI] [PubMed] [Google Scholar]
- Schubert D., Jobe A., Cohn M. Mouse myelomas producing precipitating antibody to nucleic acid bases and-or nitrophenyl derivatives. Nature. 1968 Nov 30;220(5170):882–885. doi: 10.1038/220882a0. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Stryer L. Implications of X-ray crystallographic studies of protein structure. Annu Rev Biochem. 1968;37:25–50. doi: 10.1146/annurev.bi.37.070168.000325. [DOI] [PubMed] [Google Scholar]
- WHITNEY P. L., TANFORD C. RECOVERY OF SPECIFIC ACTIVITY AFTER COMPLETE UNFOLDING AND REDUCTION OF AN ANTIBODY FRAGMENT. Proc Natl Acad Sci U S A. 1965 Mar;53:524–532. doi: 10.1073/pnas.53.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]