Abstract
Collagen synthesis in chick-embryo frontal bone and 3T3 fibroblasts from mice was measured by incorporation in vitro of [14C]proline into collagenase-digestible material. About 15-25% of the collagen synthesized by the frontal bone in 60 min, and 60% of that synthesized by the fibroblasts in 2 hr, was found to be soluble in the culture medium. The microtubular disruptive drugs colchicine and vinblastine, at 10 μM, inhibited collagen secretion in both systems almost completely. Formation of collagen hydroxyproline from proline was not inhibited by these drugs. Cytochalasin B, which impairs microfilament function, had no effect on collagen secretion. Our results support the theory that collagen is transported in vesicles to the cell membrane, where it is secreted. This conclusion is based on the similarity of the collagen-secreting system to other systems in which the movement of secretory vesicles or storage granules is inhibited by microtubule disruption.
Keywords: synthesis, hydroxylation, procollagen, colchicine, vinblastine, cytochalasin B
Full text
PDF
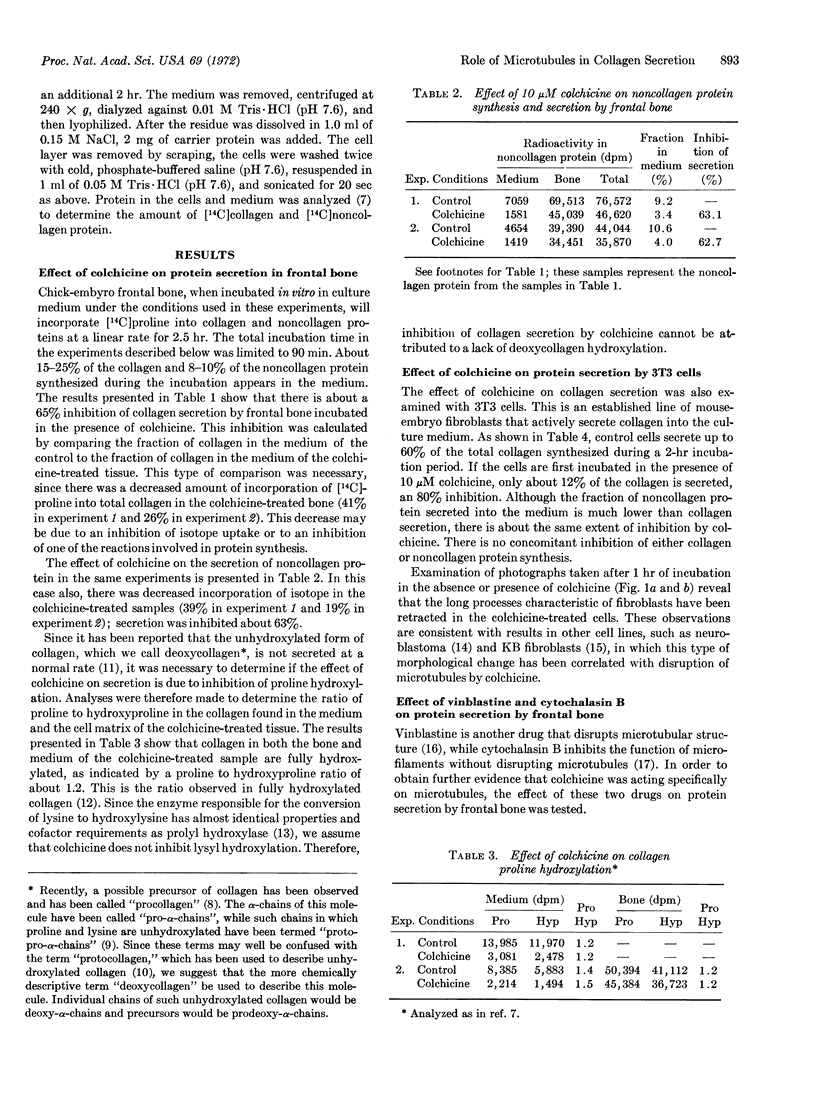
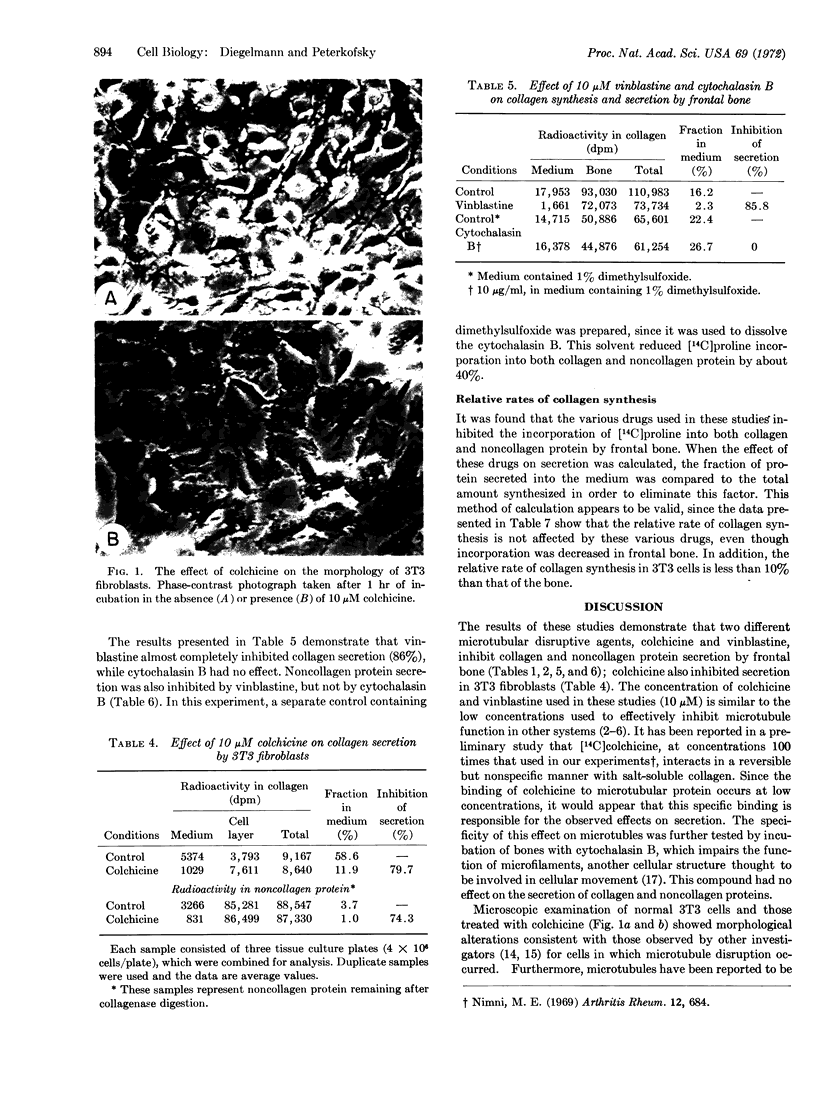


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Borisy G. G., Shelanski M. L., Weisenberg R. C., Taylor E. W. Cytoplasmic filaments and tubules. Fed Proc. 1968 Sep-Oct;27(5):1186–1193. [PubMed] [Google Scholar]
- Bellamy G., Bornstein P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1138–1142. doi: 10.1073/pnas.68.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K. G., Malawista S. E. Microtubular crystals in mammalian cells. J Cell Biol. 1969 Jan;40(1):95–107. doi: 10.1083/jcb.40.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar R. S., Kivirikko K. I., Prockop D. J. Studies on the synthesis and intracellular accumulation of protocollagen in organ culture of embryonic cartilage. Biochim Biophys Acta. 1968 Jan 22;154(1):196–207. doi: 10.1016/0005-2795(68)90272-9. [DOI] [PubMed] [Google Scholar]
- Butler W. T. Chemical studies on the cyanogen bromide peptides of rat skin collagen. The covalent structure of alpha 1-CB5, the major hexose-containing cyanogen bromide peptide of alpha 1. Biochemistry. 1970 Jan 6;9(1):44–50. doi: 10.1021/bi00803a006. [DOI] [PubMed] [Google Scholar]
- Church R. L., Pfeiffer S. E., Tanzer M. L. Collagen biosynthesis: synthesis and secretion of a high molecular weight collagen precursor (procollagen). Proc Natl Acad Sci U S A. 1971 Nov;68(11):2638–2642. doi: 10.1073/pnas.68.11.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlström A. Effect of colchicine on transport of amine storage granules in sympathetic nerves of rat. Eur J Pharmacol. 1968 Dec;5(1):111–113. doi: 10.1016/0014-2999(68)90165-9. [DOI] [PubMed] [Google Scholar]
- Dehm P., Jimenez S. A., Olsen B. R., Prockop D. J. A transport form of collagen from embryonic tendon: electron microscopic demonstration of an NH 2 -terminal extension and evidence suggesting the presence of cystine in the molecule (chick embryo-tropocollagen-gel filtration). Proc Natl Acad Sci U S A. 1972 Jan;69(1):60–64. doi: 10.1073/pnas.69.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBERG B., GREEN H. AN ANALYSIS OF COLLAGEN SECRETION BY ESTABLISHED MOUSE FIBROBLAST LINES. J Cell Biol. 1964 Jul;22:227–258. doi: 10.1083/jcb.22.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie E., Levine R. J., Malawista S. E. Histamine release from rat peritoneal mast cells: inhibition by colchicine and potentiation by deuterium oxide. J Pharmacol Exp Ther. 1968 Nov;164(1):158–165. [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Enzymatic hydroxylation of proline and lysine in protocollagen. Proc Natl Acad Sci U S A. 1967 Mar;57(3):782–789. doi: 10.1073/pnas.57.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E., Howell S. L., Young D. A., Fink C. J. New hypothesis of insulin secretion. Nature. 1968 Sep 14;219(5159):1177–1179. doi: 10.1038/2191177a0. [DOI] [PubMed] [Google Scholar]
- Lapière C. M., Lenaers A., Kohn L. D. Procollagen peptidase: an enzyme excising the coordination peptides of procollagen. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3054–3058. doi: 10.1073/pnas.68.12.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman D. L., McGoodwin E. B., Martin G. R. The nature of the collagen synthesized by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1971 Feb;68(2):454–458. doi: 10.1073/pnas.68.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAWISTA S. E. ON THE ACTION OF COLCHICINE, THE MELANOCYTE MODEL. J Exp Med. 1965 Aug 1;122:361–384. doi: 10.1084/jem.122.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J. Biochemical studies on the structure of chick bone collagen. Fed Proc. 1969 Nov-Dec;28(6):1839–1845. [PubMed] [Google Scholar]
- Miller R. L. Chromatographic separation of the enzymes required for hydroxylation of lysine and proline residues of protocollagen. Arch Biochem Biophys. 1971 Nov;147(1):339–342. doi: 10.1016/0003-9861(71)90342-0. [DOI] [PubMed] [Google Scholar]
- Müller P. K., McGoodwin E., Martin G. R. Studies on protocollagen: identification of a precursor of proto alpha 1. Biochem Biophys Res Commun. 1971 Jul 2;44(1):110–117. doi: 10.1016/s0006-291x(71)80165-1. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- REVEL J. P., HAY E. D. AN AUTORADIOGRAPHIC AND ELECTRON MICROSCOPIC STUDY OF COLLAGEN SYNTHESIS IN DIFFERENTIATING CARTILAGE. Z Zellforsch Mikrosk Anat. 1963 Oct 8;61:110–144. doi: 10.1007/BF00341524. [DOI] [PubMed] [Google Scholar]
- Ross R. The fibroblast and wound repair. Biol Rev Camb Philos Soc. 1968 Feb;43(1):51–96. doi: 10.1111/j.1469-185x.1968.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Wolff J. Possible role of microtubules in thyroid secretion. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1901–1908. doi: 10.1073/pnas.67.4.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]