Abstract
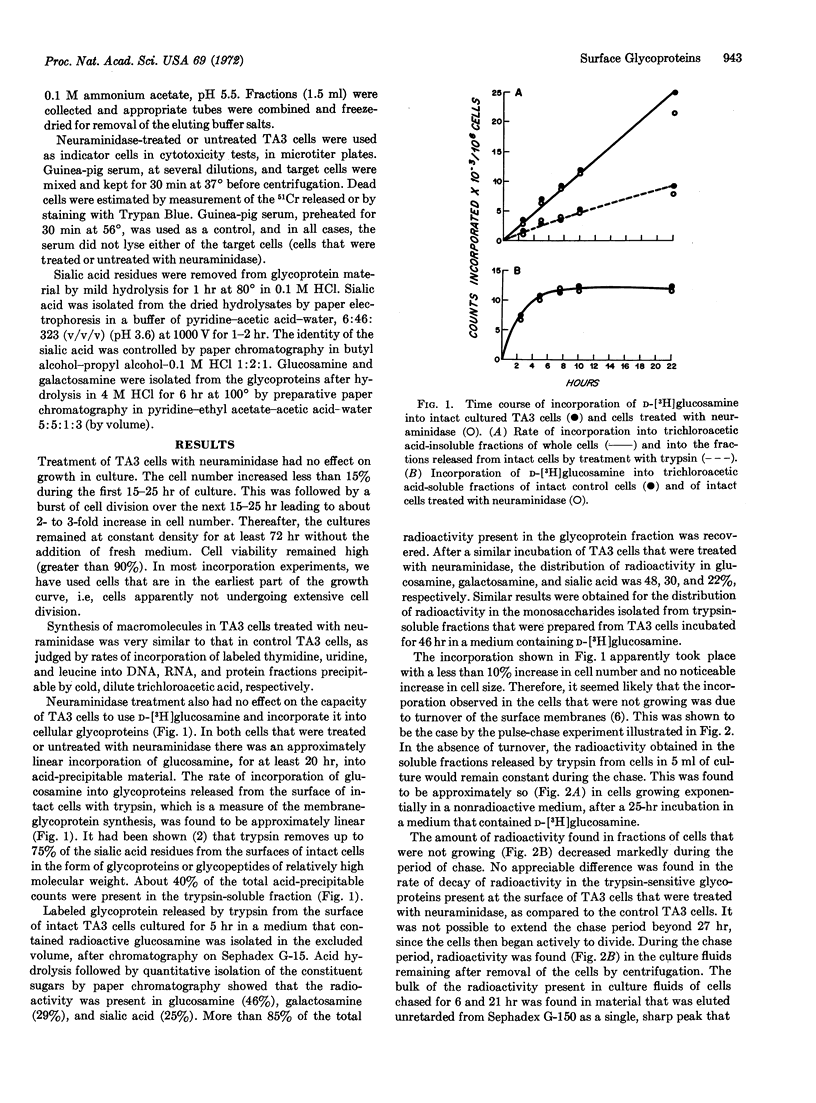
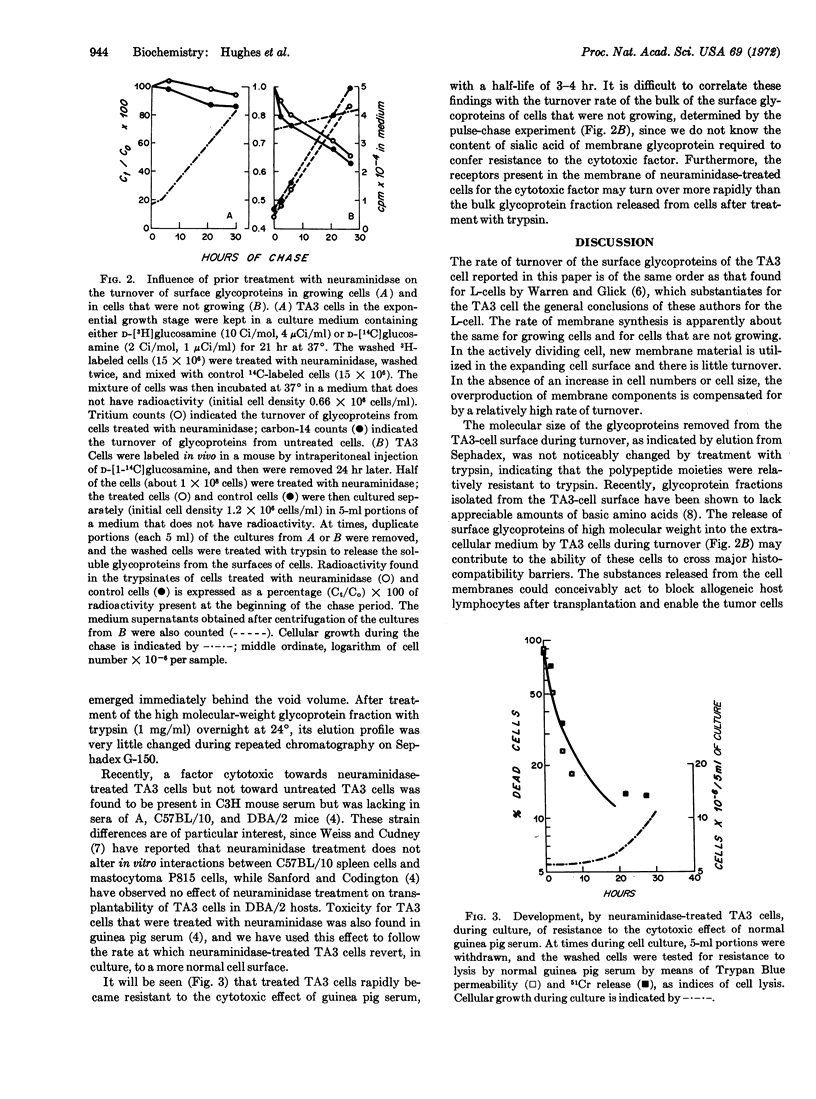
Strain A mouse ascites tumor cells that were treated with neuraminidase (EC 3.2.1.18) (these cells show decreased tumor-forming ability in allogeneic C3H mice) rapidly regenerate sialoglycoproteins at the cell surface during culture. The incorporation of labeled D-glucosamine into membrane glycoproteins of cells that were treated or untreated with neuraminidase proceeds at similar rates. Surface glycoproteins that contain sialic acid are synthesized de novo during culture of neuraminidase-treated cells, and in nondividing cells, synthesis is accompanied by turnover of the membrane glycoproteins. The rate of turnover of membrane glycoproteins that lack sialic acid residues is the same as that occurring in non-dividing cells that are not treated with neuraminidase and that are cultured under identical conditions. Turnover of the surface membrane of nondividing cells leads to the accumulation of glycoproteins in the supernatant medium of cell cultures. The rapid regeneration of cell surfaces that contain sialic acid by TA3 cells that were treated with neuraminidase, makes it unlikely that the rejection of these cells in vivo, in C3H mice, is due solely to the induction of a primary immune response to new or to previously concealed antigenic specificities that would be expressed on the surface of the sialic acid-depleted cell.
Keywords: sialic acid, cytotoxic factor
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Codington J. F., Sanford B. H., Jeanloz R. W. Glycoprotein coat of the TA 3 cell. I. Removal of carbohydrate and protein material from viable cells. J Natl Cancer Inst. 1970 Oct;45(4):637–647. [PubMed] [Google Scholar]
- Currie G. A., Van Doorninck W., Bagshawe K. D. Effect of neuraminidase on the immunogenicity of early mouse trophoblast. Nature. 1968 Jul 13;219(5150):191–192. doi: 10.1038/219191a0. [DOI] [PubMed] [Google Scholar]
- GASIC G., GASIC T. Removal and regeneration of the cell coating in tumour cells. Nature. 1962 Oct 13;196:170–170. doi: 10.1038/196170a0. [DOI] [PubMed] [Google Scholar]
- GASIC G., GASIC T. Removal of sialic acid from the cell coat in tumor cells and vascular endothelium, and its effects on metastasis. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1172–1177. doi: 10.1073/pnas.48.7.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka T. S., Weiss L., Holdridge B. A., Cudney T. L., Zumpft M., Planinsek J. A. Karyotypic and surface features of murine TA3 carcinoma cells during immunoselection in mice and rats. J Natl Cancer Inst. 1971 Aug;47(2):343–359. [PubMed] [Google Scholar]
- Law L. W., Appella E., Wright P. W., Strober S. Immunologic enhancement of allogeneic tumor growth with soluble histocompatibility-2 antigens. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3078–3082. doi: 10.1073/pnas.68.12.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. J Biol Chem. 1971 Aug 10;246(15):4825–4833. [PubMed] [Google Scholar]
- Sanford B. H. An alteration in tumor histocompatibility induced by neuraminidase. Transplantation. 1967 Sep 5;5(5):1273–1279. doi: 10.1097/00007890-196709000-00005. [DOI] [PubMed] [Google Scholar]
- Schwartz B. D., Nathenson S. G. Regeneration of transplantation antigens on mouse cells. Transplant Proc. 1971 Mar;3(1):180–182. [PubMed] [Google Scholar]
- Simmons R. L., Rios A. Immunotherapy of cancer: immunospecific rejection of tumors in recipients of neuraminidase-treated tumor cells plus BCG. Science. 1971 Nov 5;174(4009):591–593. doi: 10.1126/science.174.4009.591. [DOI] [PubMed] [Google Scholar]
- Warren L., Glick M. C. Membranes of animal cells. II. The metabolism and turnover of the surface membrane. J Cell Biol. 1968 Jun;37(3):729–746. doi: 10.1083/jcb.37.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins E., Jr, Ogata Y., Anderson L. L., Watkins E., 3rd, Waters M. F. Activation of host lymphocytes cultured with cancer cells treated with neuraminidase. Nat New Biol. 1971 May 19;231(20):83–85. doi: 10.1038/newbio231083a0. [DOI] [PubMed] [Google Scholar]
- Weiss L., Cudney T. L. Some effects of neuraminidase on the in vitro interactions between spleen and mastocytoma (P815) cells. Int J Cancer. 1971 Mar 15;7(2):187–197. doi: 10.1002/ijc.2910070202. [DOI] [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. LOCATION AND REGENERATION OF ENTERIOVIRUS RECEPTORS OF HELA CELLS. J Bacteriol. 1965 Apr;89:1097–1100. doi: 10.1128/jb.89.4.1097-1100.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


