Abstract
Electric impulses are capable of inducing long-lived conformational changes in (metastable) biopolymers. Results of experiments with poly(A)·2 poly(U) and ribosomal RNA, which are known to develop metastabilities, are reported. A polarization mechanism is proposed to explain the structural transitions observed in the biopolymers exposed to the impulses. In accordance with this idea, the applied electric field (of about 20 kV/cm and decaying exponentially, with a decay time of about 10 μsec) induces large dipole moments by shifting the ionic atmosphere of multistranded polynucleotide helices. This shift, in turn, causes strand repulsion and partial unwinding. The fields used in our experiments are of the same order of magnitude as those in nerve impulses. The significance of the impulse experiments with regard to the question of biological memory recording is briefly discussed.
Keywords: memory recording, metastability, biopolyelectrolytes, poly(A)/poly(U)
Full text
PDF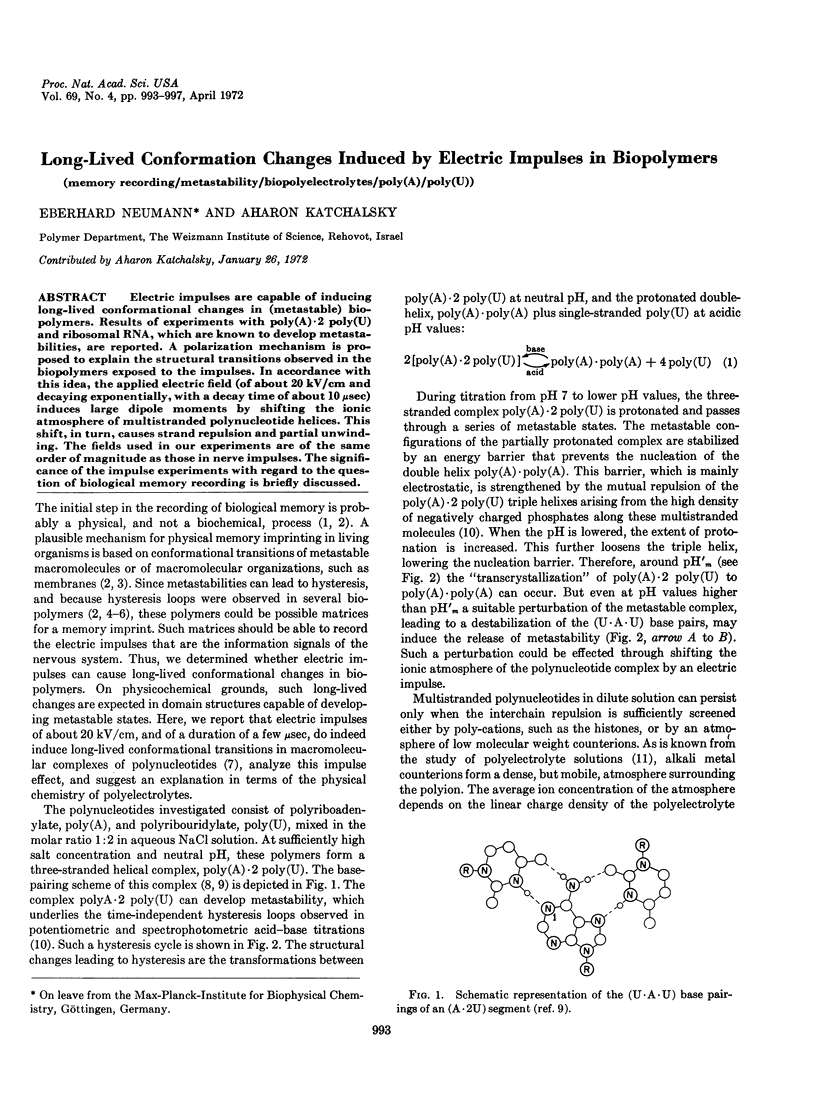


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COX R. A., JONES A. S., MARSH G. E., PEACOCKE A. R. On hydrogen bonding and branching in a bacterial ribonucleic acid. Biochim Biophys Acta. 1956 Sep;21(3):576–577. doi: 10.1016/0006-3002(56)90198-6. [DOI] [PubMed] [Google Scholar]
- Cohen R. J., Crothers D. M. Rate of unwinding small DNA. J Mol Biol. 1971 Nov 14;61(3):525–542. doi: 10.1016/0022-2836(71)90063-5. [DOI] [PubMed] [Google Scholar]
- Eisenberg H., Felsenfeld G. Studies of the temperature-dependent conformation and phase separation of polyriboadenylic acid solutions at neutral pH. J Mol Biol. 1967 Nov 28;30(1):17–37. doi: 10.1016/0022-2836(67)90240-9. [DOI] [PubMed] [Google Scholar]
- FELSENFELD G., RICH A. Studies on the formation of two- and three-stranded polyribonucleotides. Biochim Biophys Acta. 1957 Dec;26(3):457–468. doi: 10.1016/0006-3002(57)90091-4. [DOI] [PubMed] [Google Scholar]
- Hornick C., Weill G. Electrooptical study of the electric polarizability of rodlike fragments of DNA. Biopolymers. 1971 Nov;10(11):2345–2358. doi: 10.1002/bip.360101124. [DOI] [PubMed] [Google Scholar]
- Inners L. D., Felsenfeld G. Conformation of polyribouridylic acid in solution. J Mol Biol. 1970 Jun 14;50(2):373–389. doi: 10.1016/0022-2836(70)90199-3. [DOI] [PubMed] [Google Scholar]
- MILES H. T. THE STRUCTURE OF THE THREE-STRANDED HELIX, POLY (A+2U). Proc Natl Acad Sci U S A. 1964 Jun;51:1104–1109. doi: 10.1073/pnas.51.6.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörschke D., Eigen M. Co-operative non-enzymic base recognition. 3. Kinetics of the helix-coil transition of the oligoribouridylic--oligoriboadenylic acid system and of oligoriboadenylic acid alone at acidic pH. J Mol Biol. 1971 Dec 14;62(2):361–381. doi: 10.1016/0022-2836(71)90433-5. [DOI] [PubMed] [Google Scholar]
- Roberts R. B., Flexner L. B. The biochemical basis of long-term memory. Q Rev Biophys. 1969 May;2(2):135–173. doi: 10.1017/s0033583500000937. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Triebel H. Studies on conformational changes in the DNA structure induced by protonation: reversible and irreversible acid titrations and sedimentation measurments. Biopolymers. 1969;8(5):573–593. doi: 10.1002/bip.1969.360080503. [DOI] [PubMed] [Google Scholar]


