Abstract
Objective
To evaluate pre-exposure prophylaxis (PrEP) efficacy for HIV-1 prevention among women using depot medroxyprogesterone acetate (DMPA) for contraception and men whose HIV-1 infected partners use DMPA.
Design
Secondary analysis of data from a randomized placebo-controlled trial of daily oral tenofovir and emtricitabine/tenofovir PrEP among heterosexual Kenyan and Ugandan HIV-1 serodiscordant couples
Methods
PrEP efficacy for HIV-1 prevention was compared among HIV-1 uninfected women using DMPA versus no hormonal contraception and among HIV-1 uninfected men whose HIV-1 infected female partners used DMPA versus no hormonal contraception.
Results
Of 4747 HIV-1 serodiscordant couples, 901 HIV-1 uninfected women used DMPA at some point during follow-up, 1422 HIV-1 uninfected women used no hormonal contraception, 1568 HIV-1 uninfected men had female partners who used DMPA, and 2626 men had female partners who used no hormonal contraception. PrEP efficacy estimates for HIV-1 prevention, compared to placebo, were similar among women using DMPA and those using no hormonal contraception (64.7% and 75.5%, adjusted interaction p=0.65). Similarly, for men whose female partners used DMPA, PrEP efficacy did not differ from men whose partners used no hormonal contraception (90.0% versus 81.7%, adjusted interaction p=0.52).
Conclusions
PrEP is efficacious for HIV-1 prevention among women using DMPA and men whose partners use DMPA, suggesting PrEP could mitigate the potential increased HIV-1 acquisition and transmission risks that have been associated with DMPA use. Women at risk for HIV-1 choosing DMPA could maintain this contraceptive method and add PrEP to achieve prevention of unintended pregnancy and HIV-1.
Keywords: HIV-1 prevention, pre-exposure prophylaxis efficacy, hormonal contraception, DMPA
Introduction
Safe and effective contraception and HIV-1 protection are imperative for women's health. In some observational studies, the injectable contraceptive depot medroxyprogesterone acetate (DMPA) has been associated with elevated risk of HIV-1 acquisition in women and HIV-1 transmission from women with HIV-1 to their male partners but the totality of evidence remains inconclusive, with a number of other studies demonstrating no increased HIV-1 risk.[1–6] Biological mechanisms to provide insight into potential increased HIV-1 risk have not been definitively identified but hypotheses include structural and immunologic changes, such as increases in HIV-1 cell receptors and genital viral levels that would expose men to a higher inoculum.[5, 7, 8] Studies linking DMPA and HIV-1 risk are particularly concerning because DMPA is the most common method of contraception used in many countries with high HIV-1 rates.[9] While alternative contraceptives with similar degrees of pregnancy protection exist, uptake of hormonal implants and intrauterine devices lags behind injectable methods, adherence to oral contraceptives is often suboptimal, and client preferences and provider biases often favor the efficiency and discretion of injectable methods.[10–13]
Pre-exposure prophylaxis (PrEP) – using the oral antiretroviral agent tenofovir disoproxil fumarate (TDF), alone or in combination with emtricitabine (FTC) – is highly effective for HIV-1 prevention.[14–16] Limited studies have examined potential drug-drug interactions between hormonal contraceptives and antiretrovirals.[17] A small pharmacokinetic study suggested that women using DMPA might have lower serum tenofovir concentrations but the threshold concentration of tenofovir needed to impart HIV-1 protection remains unclear.[18] PrEP does not diminish the contraceptive efficacy of DMPA, and a combined DMPA-PrEP approach could allow women to continue using their preferred contraceptive method while reducing their HIV-1 risk. [19] Strategies such as this are important while the overall body of evidence about DMPA and HIV-1 risk remains inconclusive.[6]
No clinical studies have specifically assessed whether co-administration of DMPA and tenofovir-based PrEP results in a drug-drug interaction that reduces PrEP efficacy for HIV-1 prevention. In a secondary analysis of data from a randomized, placebo-controlled trial of PrEP, we evaluated PrEP efficacy among women using DMPA and among men whose HIV-1 infected female partners used DMPA.
Methods
Study population and procedures
This analysis includes data from participants in the Partners PrEP Study, a placebo-controlled, randomized trial of daily oral TDF and FTC/TDF among HIV-1 serodiscordant couples to assess its efficacy for HIV-1 prevention.[20, 21] Nine sites in Kenya and Uganda followed 4747 couples between 2008 and 2011. At enrollment, couples were sexually active and planning to remain as a couple for at least two years. HIV-1 uninfected partners were randomized in a 1:1:1 fashion to daily oral TDF:FTC/TDF:placebo. HIV-1 infected partners were not eligible for antiretroviral therapy (ART) at study entry (per national guidelines of the time) but were actively referred for ART if they became eligible during follow up.
During follow up visits, couples received an HIV-1 prevention package including counseling and free condoms and completed interviewer-administered questionnaires to assess demographic and partnership characteristics, sexual behavior, and medical history. HIV-1 uninfected partners were seen monthly for HIV-1 (two rapid tests done in parallel, seroreactivity confirmed by EIA testing) and pregnancy testing (urine β-hCG), and study medication dispensation. Adherence to study medication was monitored by in-clinic pill counts and participant self-report; subsequent analyses of archived blood samples and other objective measures of adherence confirmed high PrEP adherence in the study population.[15, 22] Study medication was withheld from HIV-1 uninfected women during pregnancy since PrEP was investigational and without demonstrated benefit at the time the study was being conducted. HIV-1 infected partners were seen quarterly to assess clinical disease progression. For HIV-1 seroconversions, analysis of HIV-1 pol sequences from both members of a couple was used to establish whether transmission was linked within the partnership. [23, 24]
In July 2011, the study's independent Data and Safety Monitoring Board recommended discontinuation of the trial's placebo arm due to definitive protection being provided by both PrEP medications (compared to placebo, TDF efficacy=67%, FTC/TDF efficacy=75%)[20]; this analysis includes data collected before July 2011. The study protocol was approved by human subjects committees at the University of Washington and all study sites. All participants provided written informed consent.
Contraceptive use
All women were offered free contraceptives on site, but contraception was not a study requirement. Contraceptive use was measured by a structured interviewer-administered questionnaire during regular study visits. In Kenya and Uganda, DMPA was the only type of injectable contraceptive available during the time of this study.
Statistical methods
We used Cox proportional hazards regression to estimate PrEP efficacy for HIV-1 prevention, compared to placebo, during visits when women used DMPA, and we compared this to efficacy estimates from visits when women were not using hormonal contraception. The comparison group using no hormonal contraception excluded women using a copper IUD (17 HIV-1 uninfected and 21 HIV-1 infected) and women with a hysterectomy or tubal ligation (124 HIV-1 uninfected and 121 HIV-1 infected). An interaction term was included to assess whether DMPA use significantly modified the association between PrEP use and HIV-1 acquisition (i.e., whether DMPA use acted as an effect modifier). DMPA use was a time dependent factor and follow up time from women who switched methods during the study was divided among each different method. Study participants randomized to TDF and FTC/TDF were combined for all analyses since the two PrEP medications had comparable efficacy for HIV-1 prevention in the trial.[25]
We separately computed estimates of PrEP efficacy for HIV-1 prevention among men during visits when they were exposed to DMPA use by their female partners and compared these efficacy estimates to those from visits when men were not exposed to hormonal contraceptive use by their female partners. Only seroconversions that were linked between study partners were included, to reduce misclassification. In the group of men with female partners using DMPA and who were themselves using active PrEP, there were no incident HIV-1 infections and thus we used incidence ratios to confirm the subgroup-specific hazard ratios and exact logistic regression to estimate the interaction p-value for this comparison.[26]
We determined a priori that our statistical models would adjust for baseline age of the HIV-1 uninfected partner as well as several time-varying measures: PrEP adherence during each study month when study drug was dispensed (taking <80% or ≥80% of expected doses based on clinic pill counts), sexual behavior (coital frequency and any unprotected sex with the study partner during the past month), and the plasma HIV-1 RNA concentration of the HIV-1 infected partner. SAS 9.3 (Cary, NC) and Stata 11.2 (College Station, TX) were used for analysis.
Results
Participant characteristics
The Partners PrEP Study enrolled 1785 HIV-1 uninfected women and 2962 men (Table 1). At enrollment, HIV-1 uninfected women had a median age of 33 years (IQR 28–38) and HIV-1 uninfected men had a median age of 34 years (IQR 28–41). Nearly all couples were married and most had at least 1 child. In the month prior to study enrollment, 22% of women and 29% of men reported at least one unprotected sex act.
Table 1.
Enrollment characteristics of HIV-1 uninfected women and men, N (%) or Median (IQR).
|
HIV-1 uninfected women N=1785 |
HIV-1 uninfected men N=2962 |
|
|---|---|---|
| Active arm (TDF and FTC/TDF combined) | 1164 (65.2) | 1999 (67.5) |
| Contraceptive use at enrollment * | ||
| DMPA | 486 (27.2) | 565 (19.1) |
| Oral contraceptives | 123 (6.9) | 129 (4.4) |
| Implant | 88 (4.9) | 100 (3.4) |
| IUD | 17 (1.0) | 21 (0.7) |
| Other contraceptive method | 161 (9.0) | 470 (15.9) |
| No contraception | 910 (51.0) | 1677 (56.6) |
| Demographic characteristics | ||
| Age, yrs | 33 (28–38) | 34 (28–41) |
| Married | 1770 (99.2) | 2907 (98.1) |
| Partnership duration, yrs | 11.9 (6.0–18.5) | 5.7 (2.4–11.0) |
| Number of children | 3 (1–5) | 1 (0–3) |
| Behavioral characteristics | ||
| Number of sex acts with study partner, past month | 4 (2–8) | 4 (3–8) |
| Number of unprotected sex acts with study partner, past month | 0 (0–0) | 0 (0–1) |
| Any unprotected sex with study partner, past month | 406 (22.7) | 861 (29.1) |
| Any sex with additional partner, past month | 8 (0.4) | 398 (13.4) |
| Clinical characteristics | ||
| Infected with Neisseria gonorrhoeae, Chlamydia trachomatis, or Trichomonas vaginalis | 144 (8.4) | 164 (5.6) |
| Infected with herpes simplex virus type 2 | 1476 (84.8) | 1807 (62.4) |
| CD4 count, HIV-1 infected partner (cells/mm3) | 457 (354–596) | 528 (396–704) |
| Plasma HIV-1 RNA concentration, HIV-1 infected partner (log10 copies/mL) | 4.1 (3.4–4.7) | 3.7 (3.1–4.4) |
For men, contraceptive use refers to methods used by their HIV-1 infected female partners. Other contraceptive methods includes surgical, natural, or traditional methods, or a male partner having a vasectomy. Condom use was assessed separately as a measure to prevent HIV/STI.
At enrollment, 486 HIV-1 uninfected women were using DMPA and 910 were not using contraception. Among HIV-1 uninfected men, 565 had partners using DMPA and 1677 had partners who were not using hormonal contraception. During follow up, an additional 415 HIV-1 uninfected women and 1003 uninfected men had periods of time when they were using or were exposed to DMPA and 512 HIV-1 uninfected women and 949 men had periods of time when they or their female partners were not using hormonal contraception.
HIV-1 incidence and PrEP efficacy among women using DMPA
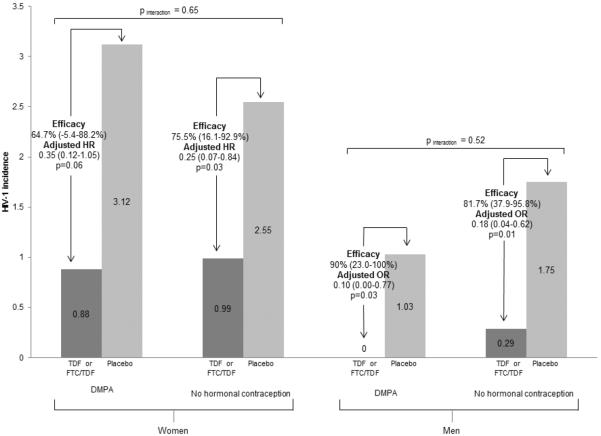
HIV-1 incidence among women using DMPA was 0.88 per 100 person years (5 infections/565.60 person years) among those randomized to active PrEP (Figure 1) and 3.12 per 100 person years (10 infections/320.30 person years) among those assigned placebo. PrEP efficacy for HIV-1 prevention was 64.7% (from an adjusted hazard ratio [aHR] of 0.35, 95% CI 0.12–1.05). Among women not using a hormonal contraceptive method, HIV-1 incidence rates were 0.99 per 100 person years (7 infections/704.50 person years) among women assigned active PrEP and 2.55 per 100 person years (8 infections/313.34 person years) among women assigned to placebo. In this group, PrEP efficacy was 75.5% (aHR=0.25, 95% CI 0.07–0.84). HIV-1 protection by PrEP, compared to placebo, did not differ statistically for women using DMPA and women using no hormonal contraception (adjusted pinteraction=0.65, comparing aHR 0.35 versus aHR 0.25).
Figure 1. HIV-1 incidence among women and men exposure and unexposed to DMPA.
HIV-1 incidence among women and men with exposure to DMPA or no hormonal contraception and randomized to PrEP (dark grey bars) or placebo (light grey bars). 901 HIV-1 uninfected women used DMPA at some point during follow-up, 1422 HIV-1 uninfected women used no hormonal contraception, 1568 HIV-1 uninfected men had female partners who used DMPA, and 2626 men had female partners who used no hormonal contraception. Hazard ratios compare HIV-1 incidence between participants using PrEP versus placebo and are adjusted for the number of sex acts with study partner, any unprotected sex with study partner, adherence to study drug, HIV-1 RNA concentration of the HIV-1 infected partner (time dependent variables), and age. Interaction p-values compare the adjusted HR for HIV-1 protection from PrEP between participants with DMPA exposure versus no exposure to hormonal contraception.
HIV-1 incidence and PrEP efficacy among men with female partners using DMPA
There were no HIV-1 seroconversions among men using active PrEP who were exposed to DMPA use by their female partners (0 infections/784.79 person years). Among men assigned to the placebo arm whose partners were using DMPA, HIV-1 incidence was 1.03 per 100 person years (4 infections/390.01 person years). Among men with partners using DMPA, PrEP efficacy was 90.0% (adjusted odds ratio (aOR) 0.10 (95% CI 0.00–0.77). Among men whose partners did not use hormonal contraception, PrEP efficacy was 81.7% (aOR 0.18, 95% CI 0.04–0.62). These HIV-1 risk estimates were similar for men with partners using DMPA and men whose partners were not using hormonal contraception (adjusted pinteraction=0.52, comparing aOR 0.10 versus aOR 0.18).
Discussion
PrEP efficacy to prevent HIV-1 acquisition was high among women using DMPA and men whose female partners were using DMPA. For women with high HIV-1 risk and who choose to use DMPA, PrEP would offer substantial HIV-1 protection and would counteract the potentially detrimental effect of DMPA on HIV-1 risk.
Data linking DMPA use and increased HIV-1 risk are inconclusive.[6] In our study population, HIV-1 incidence among women using DMPA was only marginally higher than those not using hormonal contraception (in the absence of PrEP), but there were few HIV-1 infections and statistical power to assess whether DMPA was associated with increased HIV-1 risk was limited. Nonetheless, the findings we present here provide strong evidence that PrEP is efficacious for HIV-1 prevention for women using DMPA and partners of HIV-1 infected women using DMPA. The World Health Organization recommends that women using DMPA for contraception who are at high risk for HIV-1 be counseled especially to use condoms for HIV-1 protection.[17] Our results suggest that PrEP could be an HIV-1 prevention strategy for such women, without reduced contraceptive effectiveness for pregnancy prevention [19] or reduced PrEP effectiveness for preventing HIV-1, and provide an alternative for women who face any of the well documented challenges with consistent condom use. Combining PrEP use with DMPA use could be an important risk-mitigation strategy in countries with high HIV-1 risk and concerns about DMPA possibly increasing HIV-1 risk.[9]
Critical work remains to improve contraceptive and HIV-1 prevention options for women, especially options that are co-formulated, controlled by women, and easy to use. Multipurpose products that are co-formulated to prevent pregnancy and HIV-1 acquisition are under development.[27] Their production as low cost injections, rings, or other long acting user-independent devices is critical, especially for countries with high HIV-1 rates and long histories of injectables as the preferred contraceptive method. Further research is also needed to understand factors that influence women's adherence to PrEP, including drivers of HIV-1 risk perception and influential societal beliefs. [28–31]
For women with and at high risk of acquiring HIV-1 who prefer to use DMPA for contraception, daily oral PrEP in combination with DMPA provides a strategy to decrease HIV-1 acquisition/transmission risk, overcoming any potential DMPA-related increases in HIV-1 risk, while continuing to provide protection against unintended pregnancy. Integration of family planning and HIV-1 prevention services is paramount. Programs working towards this integration are well-suited to initiate discussions with women about fertility plans, HIV-1 risk, contraceptive choices, and benefits and risks (including the potential increased HIV-1 risk with DMPA use) of contraceptive methods. For women choosing to use DMPA in settings with high HIV-1 incidence, PrEP could be offered to offset any potential increase in HIV-1 risk from DMPA.
Acknowledgements
We thank the couples who participated in this study and the teams at the study sites and the University of Washington for work on data collection and management. The study team also acknowledges the Director KEMRI for his support.
Funding: Eunice Kennedy Shriver National Institute of Child Health and Development (grants K99HD076679 and R21HD074439) and the Bill & Melinda Gates Foundation (research grant OPP47674), and University of Washington Center for AIDS Research, (NIH, P30AI027757). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Preliminary data from this analysis were presented at the 21st Conference on Retroviruses and Opportunistic Infections. Boston, MA. Poster #950.
References
- 1.Baeten JM, Benki S, Chohan V, Lavreys L, McClelland RS, Mandaliya K, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007;21:1771–1777. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 2.Morrison CS, Chen PL, Kwok C, Richardson BA, Chipato T, Mugerwa R, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24:1778–1781. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCoy SI, Zheng W, Montgomery ET, Blanchard K, van der Straten A, de Bruyn G, et al. Oral and injectable contraception use and risk of HIV acquisition among women in sub-Saharan Africa. AIDS. 2013;27:1001–1009. doi: 10.1097/QAD.0b013e32835da401. [DOI] [PubMed] [Google Scholar]
- 5.Polis CB, Phillips SJ, Curtis KM. Hormonal contraceptive use and female-to-male HIV transmission: a systematic review of the epidemiologic evidence. AIDS. 2013;27:493–505. doi: 10.1097/QAD.0b013e32835ad539. [DOI] [PubMed] [Google Scholar]
- 6.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013;13:797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 7.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev. 2010;31:79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achilles SL, Hillier SL. The complexity of contraceptives: understanding their impact on genital immune cells and vaginal microbiota. AIDS. 2013;27(Suppl 1):S5–15. doi: 10.1097/QAD.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler AR, Smith JA, Polis CB, Gregson S, Stanton D, Hallett TB. Modelling the global competing risks of a potential interaction between injectable hormonal contraception and HIV risk. AIDS. 2013;27:105–113. doi: 10.1097/QAD.0b013e32835a5a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngure K, Heffron R, Mugo NR, Celum C, Cohen CR, Odoyo J, et al. Contraceptive method and pregnancy incidence among women in HIV-1-serodiscordant partnerships. AIDS. 2012;26:513–518. doi: 10.1097/QAD.0b013e32834f981c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Early end for FEM-PrEP HIV prevention trial. AIDS Patient Care STDS. 2011;25:383. doi: 10.1089/apc.2011.9874. [DOI] [PubMed] [Google Scholar]
- 12.United Nations . World Contraceptive Patterns 2013. Department of Economic and Social Affairs, United Nations; New York, USA: 2013. [Google Scholar]
- 13.Gutin SA, Mlobeli R, Moss M, Buga G, Morroni C. Survey of knowledge, attitudes and practices surrounding the intrauterine device in South Africa. Contraception. 2011;83:145–150. doi: 10.1016/j.contraception.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . In: Hormonal contraceptive methods for women at high risk of HIV and living with HIV. WHO Department of Reproductive Health and Research, editor. WHO; Geneva, Switzerland: 2014. [PubMed] [Google Scholar]
- 18.Coleman J, Chaturveda A, Hendrix C. MTN-001 Protocol Team. Method of hormonal contraception is associated with lower tenofovir concentration in healthy women (MTN-001): implications for pre-exposure prophylaxis. Abstract FRLBC03. XIX International AIDS Conference; Washington, DC, USA. 2012. [Google Scholar]
- 19.Murnane PM, Heffron R, Ronald A, Bukusi EA, Donnell D, Mugo NR, et al. Pre-exposure prophylaxis for HIV-1 prevention does not diminish the pregnancy prevention effectiveness of hormonal contraception. AIDS. 2014;28:1825–1830. doi: 10.1097/QAD.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mujugira A, Baeten JM, Donnell D, Ndase P, Mugo NR, Barnes L, et al. Characteristics of HIV-1 Serodiscordant Couples Enrolled in a Clinical Trial of Antiretroviral Pre-Exposure Prophylaxis for HIV-1 Prevention. PLoS ONE. 2011;6:e25828. doi: 10.1371/journal.pone.0025828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haberer JE, Baeten JM, Campbell J, Wangisi J, Katabira E, Ronald A, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell MS, Mullins JI, Hughes JP, Celum C, Wong KG, Raugi DN, et al. Viral linkage in HIV-1 seroconverters and their partners in an HIV-1 prevention clinical trial. PLoS One. 2011;6:e16986. doi: 10.1371/journal.pone.0016986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eshleman SH, Hudelson SE, Redd AD, Wang L, Debes R, Chen YQ, et al. Analysis of genetic linkage of HIV from couples enrolled in the HIV Prevention Trials Network 052 trial. J Infect Dis. 2011;204:1918–1926. doi: 10.1093/infdis/jir651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeten JM, Partners PrEP Study Team Single agent TDF versus combination FTC/TDF PrEP among heterosexual men and women. Abstract. Conference on Retroviruses and Opportunistic Infections 2014; Boston, USA. 2014. [Google Scholar]
- 26.Mehta CR, Patel NR. Exact logistic regression: theory and examples. Stat Med. 1995;14:2143–2160. doi: 10.1002/sim.4780141908. [DOI] [PubMed] [Google Scholar]
- 27.Friend DR, Clark JT, Kiser PF, Clark MR. Multipurpose prevention technologies: products in development. Antiviral Res. 2013;100(Suppl):S39–47. doi: 10.1016/j.antiviral.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marrazzo J, Ramjee G, Nair G, Palanee T, Mkhize B, Nakabiito C, et al. Pre-exposure Prophylaxis for HIV in Women: Daily Oral Tenofovir, Oral Tenofovir/Emtricitabine, or Vaginal Tenofovir Gel in the VOICE Study (MTN 003). Abstract #26LB. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 30.van der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, et al. Women's experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS One. 2014;9:e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corneli AL, Deese J, Wang M, Taylor D, Ahmed K, Agot K, et al. FEM-PrEP: Adherence Patterns and Factors Associated With Adherence to a Daily Oral Study Product for Pre-exposure Prophylaxis. J Acquir Immune Defic Syndr. 2014;66:324–331. doi: 10.1097/QAI.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]