Abstract
Cognitive deficits are commonly observed in patients with schizophrenia. Converging lines of evidence suggest that these deficits are associated with impaired long-term potentiation (LTP). In our systematic review, this hypothesis is evaluated using neuroimaging literature focused on proton magnetic resonance spectroscopy, positron emission tomography, and single-photon emission computed tomography. The review provides evidence for abnormal dopaminergic, GABAergic, and glutamatergic neurotransmission in antipsychotic-naive/free patients with schizophrenia compared with healthy controls. The review concludes with a model illustrating how these abnormalities could lead to impaired LTP in patients with schizophrenia and consequently cognitive deficits.
Key words: schizophrenia, long-term potentiation, glutamate, dopamine, GABA, MRS, PET, SPECT
Background
Schizophrenia is a psychiatric disorder that affects 1% of the world population.1,2 Cognitive deficits such as learning and memory impairments are considered core features of the illness.3,4 Long-term potentiation (LTP) is a key determinant of learning and memory function5,6 and may be a key neurophysiological mechanism underlying cognitive impairment in schizophrenia.
LTP is defined as an activity dependent long lasting enhancement in synaptic efficacy.7 LTP is typically dependent on the glutamatergic N-methyl-d-aspartate (NMDA) receptor.8,9 Glutamate activates NMDA receptors allowing calcium (Ca+2) entry, which in turn acts on calmodulin-dependent protein kinases (CaM Kinases) II and IV and leads to the upregulation of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors.10
LTP is modulated by the dopaminergic11 and GABAergic systems.12,13 Dopaminergic modulation of LTP depends on the type of receptors. Dopamine D1 receptor activation enhances LTP,14,15 while dopamine D2/3 receptor activation suppresses NMDA activity and GABA activity.16,17 GABAergic modulation of LTP also depends on the subtype of GABA receptor. Antagonism of GABAA receptor facilitates LTP.18 Activation of GABAB receptor modulates GABAA receptor through presynaptic autoinhibition of interneurons which facilitates LTP.19,20
A number of imaging studies using proton magnetic resonance spectroscopy (1H MRS), positron emission tomography (PET), and single-photon emission computed tomography (SPECT) assessed these systems (glutamatergic, dopaminergic, and GABAergic) in patients. To date, there has been 1 meta-analysis, and 1 review paper on glutamate 1H MRS studies,21,22 2 meta-analyses on dopamine PET and SPECT studies,23,24 and 1 narrative review on imaging studies assessing dopamine, serotonin, GABA, and glutamate systems in schizophrenia.25 This last review was performed more than a decade ago and included patient with and without exposure to antipsychotic treatment. Thus, our aim was to perform a systematic review on imaging studies assessing these 3 neurotransmitter systems, focusing only on antipsychotic-naive or antipsychotic-free patients with schizophrenia. Assessing this subgroup helps to disentangle changes in neurochemistry related to illness compared to changes related to medications. Differences between medicated and unmedicated patients are also highlighted throughout the review only for comparison purposes. Lastly, we present a model linking these systems to abnormal LTP and cognitive deficits associated with schizophrenia.
Methods
A literature search was performed on November 18, 2013 using PubMed with no date limits and the following terms were used: schizo* AND drug naiv* OR antipsychotic naiv* OR untreat* OR unmedicat* OR never treat* OR neuroleptic free OR antipsychotic free OR first episod* AND glutamate OR GABA OR dopamine. The inclusion criteria were determined a priori and were (1) in vivo human studies, (2) imaging studies, and (3) studies including antipsychotic-free and/or antipsychotic-naive patients with schizophrenia or schizoaffective disorder. In total, 2383 publications were identified. Articles were excluded after reviewing titles and abstracts, leaving 63 studies. Considering there was only one study for GABA, we summarized the characteristics and findings of each study into 2 separate tables: for dopamine and glutamate (See supplementary tables 1 and 2).
Results
Our search identified 16 studies on the glutamatergic system, 44 studies on the dopaminergic system, and 3 studies on the GABAergic system.
Glutamatergic System
Several 1H MRS studies and one SPECT study demonstrated altered glutamatergic activity in antipsychotic-naive or antipsychotic-free patients. Changes were reported in the concentrations of glutamate, glutamine, a precursor of glutamate26 and/or GLX, a combination of both. We summarize the findings below and have chosen to divide these findings based on various regions of the brain due to intrinsic variations that exist in the healthy brain.27
Medial Prefrontal Cortex.
Two studies assessed glutamatergic activity in the medial prefrontal cortex (MPFC) of antipsychotic-free/naive patients with schizophrenia compared with healthy controls. One of these studies reported a 30% increase in GLX levels of 9 antipsychotic-naive and 7 antipsychotic-free patients (M = 11, F = 5) (mean age: 32 years) compared with 22 healthy controls (M = 14, F = 8) and 16 medicated patients (M = 11, F = 5).28 The authors proposed that antipsychotics may have normalized GLX levels in the MPFC of medicated patients. Elevated GLX levels are also evident in the right MPFC of 20 adolescents (M = 7, F = 13) (mean age: 16.4 years), who are at high-risk for developing schizophrenia by having a parent with schizophrenia.29 Since glutamine concentration is 40%–60% lower than that of glutamate,30,31 it can be inferred that elevated GLX levels mostly reflect elevated glutamate concentrations.32,33 These findings suggest that high-risk adolescents and young patients with schizophrenia have elevated levels of glutamate in the MPFC early in the illness. However, a study assessing both glutamine and glutamate levels independently reported an increase in only glutamine levels in the MPFC of 10 antipsychotic-naive patients (M = 8, F = 2).34 The authors concluded that schizophrenia may be associated with an abnormal conversion of glutamine to glutamate, resulting in elevated glutamine levels.34 Alternatively, this finding may be explained by experimental limitations. To accurately measure glutamate and glutamine levels separately, specialized 1H MRS techniques (eg, high-magnetic field (>3 T) with short echo and long acquisition time) or editing techniques (eg, J-editing) are necessary due to glutamine and glutamate’s analogous signals.35–37 In this study, a 1.5 T magnetic field without editing techniques was used, which could be unreliable in distinguishing peaks arising from glutamine and glutamate independently, potentially confounding the results. While glutamine level in antipsychotic-free/naive patients might be still elusive, a meta-analysis including medicated and unmedicated patients indicated that glutamine is higher in patients than healthy controls.21
In contrast, a study comparing glutamate levels in the MPFC of older 12 patients with schizophrenia (M = 7, F = 5) (medication status unknown; mean age: 49.5 years) and their unaffected twin with healthy controls (M = 12, F = 9) found that both patients and their unaffected twins had decreased glutamate levels.38 Taken together, these studies suggest that patients have elevated glutamate levels in the MPFC early in their illness but then experience a decline in glutamate concentrations as they age. This age-related change in glutamate levels in schizophrenia was shown by a recent meta-analysis describing a drop below healthy controls after the age of 35. Since some of the studies included in this meta-analysis include medicated patients, medication effects cannot be ruled out and therefore little is known about glutamate changes over the course of the illness in unmediated patients.21
Dorsolateral Prefrontal Cortex.
Four studies assessed the dorsolateral prefrontal cortex (DLPFC) of antipsychotic-free/naive patients compared with healthy controls. A study using a 3 T MRS found no difference in GLX levels in the DLPFC of antipsychotic-free patients (M = 11, F = 5).28 This finding is in line with other studies that reported similar results in antipsychotic-naive patients,39–41 high-risk individuals,42 and childhood-onset patients.43 However, a study that assessed 23 chronic antipsychotic-free patients using 1.5 T MRS found significantly greater combination of glutamate and GABA levels in patients than healthy controls.44 Inconsistent results may be explained by the differences in acquisition and analysis techniques employed in these studies. In contrast, decrease in GLX levels were noted when 20 chronic medicated patients (M = 14, F = 6) were compared with 20 healthy controls (M = 13, F = 7),41 suggesting either an aging or chronicity (including chronic exposure to antipsychotics) effect. As such, further studies using more specific 1H MRS acquisition and quantification techniques are required.
Thalamus.
Three different studies comparing antipsychotic-naive patients with healthy controls reported elevated glutamine levels in the thalamus of patients.45–47 The first study assessed 21 antipsychotic-naive patients (M = 14, F = 7) and reported elevated glutamine levels in the left thalamus.45 In contrast, a follow-up study conducted in 21 chronic medicated patients with schizophrenia (M = 20, F = 1) detected reduced glutamine levels in the left thalamus of patients.48 This finding was replicated and extended in a cohort of 16 antipsychotic-naive patients (M = 14, F = 2), which included 12 patients from the earlier study.46 Baseline glutamine levels in the left thalamus remained elevated until 30 months of antipsychotic treatment.46 Another study also found high glutamine levels in antipsychotic-naive patients (M = 14, F = 3), which decreased over 80 months.47 These findings may suggest an aging or treatment effect. In contrast, another study detected no difference in glutamine/glutamate (Gln/Glu) ratio between 14 (M = 12, F = 2) minimally treated patients and 10 healthy controls (M = 12, F = 2).49 Medication effects could have played a role in this inconsistent finding, since these patients had some, albeit minimal exposure to antipsychotics, lasting less than 3 weeks. On the other hand, glutamate levels were found to be decreased in the thalamus of 27 high-risk adolescents (M = 14, F = 13).50 However, recently Tandon et al (2013) reported increased GLX in the thalamus of 23 high-risk adolescents (M = 10, F = 13).51
These findings support a dysfunctional glutamate-glutamine cycle in the brains of patients. It is postulated that an abnormal conversion of glutamine to glutamate would result in high glutamine and low glutamate levels, consistent with the majority of the above-mentioned findings.
Basal Ganglia.
Two studies assessed the basal ganglia (BG) in antipsychotic-naive/free patients compared to healthy controls. A study looking at the precommissural dorsal caudate (PCDC) of 14 antipsychotic-free patients detected elevated glutamate/creatine (Glu/Cr) ratio, suggesting elevated glutamate levels.52 Another study that assessed the PCDC of first episodes antipsychotic-free (N = 18) (M = 10, F = 8) and ultra-high risk for psychosis patients (N = 18) (M = 14, F = 4) detected elevated glutamate levels in both groups.53 A longitudinal study of 24 antipsychotic-naive patients (M = 13, F = 11) reported elevated glutamate in the PCDC of patients.54 This study also showed that after 4 weeks of exposure to antipsychotics, glutamate levels in PCDC decreased to similar levels as controls. The same group followed 19 ultra-high-risk subjects for 2 years and showed that transition to psychosis was associated with higher glutamate levels in the PCDC. Another study including 23 ultra-high-risk subjects (M = 13, F = 10) reported increases in GLX in the caudate nucleus.51 When 40 high-risk adolescents were assessed, a gender effect was noted, that is elevated glutamate and GLX levels was detected in the BG of only male adolescents (N = 18).55 Overall, these results suggest that high glutamate and GLX levels in the BG precede the onset of schizophrenia, predict the onset of the first episode of psychosis, and remain elevated until patients are treated with antipsychotics.
Anterior Cingulate.
Three publications reported increased glutamine levels in the anterior cingulate of high-risk adolescents (mean age: 16) or antipsychotic-naive first-episode patients (mean age: 21).29,45,50 In contrast, a study assessing the anterior cingulate of 17 antipsychotic-naive patients found no difference in glutamate or glutamine levels.47 Another group reported increased Gln/Glu ratio but did not find elevated glutamine levels in the anterior cingulate of 14 minimally treated patients (M = 12, F = 2) (mean age: 27).49 A study assessing chronic medicated patients found decreased glutamate and glutamine levels in the anterior cingulate of patients.46 Overall, these findings suggest that the levels of glutamine and glutamate are abnormal in the anterior cingulate of high-risk adolescents and first-episode antipsychotic-naive patients. Such findings suggest that the glutamate-glutamine cycle may be dysfunctional in anterior cingulate, resulting in excessive glutamine levels that decline as the disease progresses. The reason for the decline in glutamine level is still elusive.
Occipital, Parietal, and Hippocampal Regions.
Several imaging studies have focused on glutamatergic activity in the occipital, parietal, and temporal regions of antipsychotic-naive or antipsychotic-free patients. A study looking at the hippocampus of 10 male patients (7 antipsychotic-free and 3 antipsychotic medicated) found elevated GLX/Cho levels in patients.56 It is important to note that although in this study GLX is a combination of glutamate, glutamine, and GABA, the contribution of GABA and glutamine are almost negligible. A recent study assessing 27 patients (M = 20, F = 7) (11 antipsychotic-naive and 16 antipsychotic-free) found elevated GLX in the hippocampus of patients compared with healthy controls.57 In contrast, no differences in glutamate or glutamine were found in studies that assessed the medial temporal lobes of 11 antipsychotic-naive patients (M = 9, F = 2),58 or glutamate in 14 twins discordant for schizophrenia..38 It is important to note that the first study used a higher MRS field strength (3 T) and a larger sample size compared to the second study. No difference in GLX levels were reported when assessing the temporal gyri of 28 youths with childhood-onset schizophrenia (M = 15, F = 13).43 Also, one study found elevated GLX levels in the inferior parietal lobe of only high-risk male adolescents (M = 18, F = 22).55 In keeping with the glutamatergic dysfunction hypothesis, one SPECT study found reduced NMDA binding in the medial temporal lobe of antipsychotic-free patients, but not in antipsychotic medicated patients compared to healthy controls, suggesting that antipsychotic medication may have a normalizing effect.59 Taken together, these studies suggest increased glutamatergic activity in the occipital and parietal region and in the medial temporal lobes of antipsychotic-naive or antipsychotic-free patients when compared with healthy controls.
Cerebellum.
When assessing the cerebellum, 2 studies did not find difference in glutamatergic levels and 1 reported increased glutamate and GLX. The first negative study included first-episode antipsychotic-free patient and looked at the Glu/Cr ratio.52 The second negative study included 18 antipsychotic-naive patients (M = 14, F = 4) and 18 patients with ultra-high risk for psychosis (M = 14, F = 4).53 In contrast, a third study, which included only 24 antipsychotic-naive first-episode patients (M = 13, F = 11), reported increased glutamate and GLX levels. Interestingly, glutamate levels normalized after 4 weeks of antipsychotic treatment and GLX remained increased.54 Glutamine could not be quantified to understand its contribution in the GLX signal.
Summary of Glutamatergic System Findings.
The above sections evaluated studies that assessed glutamate, glutamine, or GLX levels in the brains of antipsychotic-free or antipsychotic-naive patients with schizophrenia and patients at high-risk of psychosis compared with healthy controls. Overall, our findings revealed the following: elevated GLX levels in the MPFC, parietal, anterior cingulate, thalamus, BG, and occipital region; elevated glutamine levels in the MPFC, thalamus, and anterior cingulate; elevated glutamate levels in the BG; decreased glutamate levels in the thalamus; and no differences or uncertainty in glutamatergic metabolites in the DLPFC and temporal and cerebellum regions. These results support the notion that the pathophysiology of schizophrenia may stem from dysfunctional glutamate and glutamine neurotransmission.
Dopaminergic System
Several PET and SPECT imaging studies assessed dopamine levels and receptors in different regions of the brains of antipsychotic-naive and antipsychotic-free patients with schizophrenia. This section will review the literature pertaining to abnormalities in the dopamine D1 and D2/3 receptors, because these dopamine receptors are highly relevant to LTP modulation,60–62 as well as, dopamine synthesis, and release.
Dopamine D1 Receptor Studies
Four studies assessed dopamine D1 receptor binding in the prefrontal cortex of patients. One of these studies using the PET radiotracer [11C]-NNC112 reported greater dopamine D1 receptor binding in 7 antipsychotic-naive and 9 antipsychotic-free patients (M = 13, F = 3).63 In a follow-up study, an elevation in dopamine D1 receptor binding was detected in the prefrontal cortex of only antipsychotic-naive patients (N = 12) (M = 5, F = 7), and not in that of antipsychotic-free patients (N = 13) (M = 11, F = 2) when compared with healthy controls (N = 24).64 On the contrary, studies using the radiotracer [11C]-SCH23390 reported decreased65 or no change66 in the dopamine D1 receptor binding in the prefrontal cortex of antipsychotic-naive or antipsychotic-free patients. Discrepancies between studies might be accounted for by differences in demographic, clinical characteristics, previous antipsychotic exposure, and PET radiotracers ([11C]-NNC112 vs [11C]-SCH23390). Dopamine depletion studies in rodents showed increased [11C]-NNC112 binding and decreased [11C]-SCH23390 binding,67 indicating opposite sensitivity for dopamine levels. In addition, 5-HT2A binding was shown to contribute to the cortical binding of both radiotracers in nonhuman primates68 and in humans for [11C]-NNC112 only.69 As such, these limitations should be taken into consideration when evaluating the aforementioned studies. Regarding other brain regions, no difference in dopamine D1 binding was found in the striatal, limbic, and thalamic regions when patients were compared with healthy controls.63–65 Taken together, these results illustrate inconsistent differences in dopamine D1 receptor binding in the DLPFC and no difference in D1 receptor binding in the striatum, limbic, and thalamic regions of antipsychotic-naive/antipsychotic-free patient.
Dopamine D2/3 Receptor Studies.
Striatum and Substantia Nigra
Studies Without Pharmacological Challenges
Fourteen publications assessing dopamine D2/3 receptor binding reported no difference between patients and healthy controls in the striatum.65,70–85 In contrast, one study reported reduced D2/3 binding in 23 acutely ill patients (M = 19; F = 4) compared with healthy controls.86 In the above-mentioned studies, patients had in general mild to moderate symptoms. The mean scores on the Positive and Negative Symptom Scale (PANSS) positive subscale ranged from 18 to 21.9 and on the Brief Psychiatric Rating Scale (BPRS) ranged from 28.8 to 60. One exception was a study in which patients had a mean PANSS positive subscale score of 30.92.75 In contrast, the publication showing reduced D2/3 binding in patients included patients with severe symptoms (PANSS positive score = 21.9; PANSS general score = 60.4; BPRS score = 73.6).86 As such, lower dopamine D2/3 receptor binding may be a result of greater endogenous dopamine concentrations which compete with the D2/3 receptor ligand, resulting in reduced D2/3 binding.87 Thus, given that there is an inverse correlation between severity of psychosis and D2/3 binding potential,88 the differences in severity of patients’ symptoms may account for the differences detected in D2/3 binding among these studies.
On the contrary, 3 studies reported increased D2/3 receptors binding in the striatal region.89–92 Corripio et al (2011) found that D2/3 striatal/frontal binding ratio was increased in 25 first-episode antipsychotic-naive patients (compared with 12 healthy controls and 12 patients with a psychotic disorder different to schizophrenia) using123 I-IBZM SPECT.90 Increased D2/3 receptor binding was also reported in 11 patients (M = 6, F = 5) compared with 18 healthy controls (M = 10, F = 8) using123 I-IBZM SPECT.91 This finding is in line with an earlier study that reported increased D2/3 receptor striatal binding in 25 antipsychotic-naive and antipsychotic-free chronic patients (M = 17, F = 8).92 Notwithstanding, a meta-analysis by Laruelle reported approximately 12% elevation in D2/3 receptor binding in antipsychotic-free patients with schizophrenia compared to healthy controls.24
Studies that assessed the caudate or putamen independently reported inconsistent findings that seemed to be influenced by the radiotracer. For instance, when [11C]-raclopride was used to separately assess the caudate and putamen of 18 antipsychotic-naive patients (M = 10, F = 8), elevation in D2/3 receptor binding was not detected.80 In contrast, 2 other studies that used [11C]-methylspiperone reported greater D2/3 receptor binding in the caudate nucleus of 10 antipsychotic-naive and antipsychotic-free patients (M = 8, F = 2)93 and 22 antipsychotic-naive patients (M = 13, F = 9).94 The radiotracer[11C]-methylspiperone has been shown to be less sensitive to endogenous dopamine and binds to dopamine D4 receptors unlike [11C]-raclopride.95,96 Therefore, considering that [11C]-raclopride and [11C]-methylspiperone have different pharmacological properties, it may be difficult to compare results obtained with these 2 radiotracers. Nevertheless, a study comparing antipsychotic-free (N = 16) (M = 13, F = 3) and antipsychotic-naive patients (N = 12) (M = 5, F = 7) detected no difference in D2/3 receptor binding in the striatum between the 2 groups of patients.97
Lastly, 3 studies employing the dopamine D2/3 receptor high affinity radiotracers [123I]epidepride (SPECT)98 and [18F]fallypride (PET)79 and the agonist [11C]-(+)-PHNO81 assessed the substantia nigra and reported inconsistent results. The study employing [123I]epidepride detected decreased D2/3 receptor binding, the study employing [18F]fallypride detected greater D2/3 receptor binding, and the study employing [11C]-(+)-PHNO did not find any difference in antipsychotic-free patients with schizophrenia in comparison with controls. The reason for the discrepancy in results is still elusive and could be due to differences in the radiotracers employed and/or differences in the characteristics of the clinical population. Thus, the majority of the present results reveal no difference in D2/3 receptor binding in the striatum, however a meta-analysis reported an elevation in D2 receptors24 and the results in the substantia nigra require further exploration.
Studies Assessing Dopamine Synthesis Capacity
In addition to changes in D2/3 receptor, several PET studies performed on antipsychotic-naive and antipsychotic-free patients reported increased dopamine synthesis capacity in the striatum. Three studies found greater dopamine synthesis in the caudate nucleus and putamen of patients.99–101 Specifically, Nozaki et al found significantly greater dopamine synthesis in only the left caudate of 14 antipsychotic-naive and 4 antipsychotic-free patients who were 3-month antipsychotic-free (M = 10, F = 3). Another study revealed increased dopamine synthesis in the striatum of 8 male antipsychotic-free/antipsychotic-naive patients (N = 3 antipsychotic-naive and N = 5 antipsychotic-free for at least 6 months).102 This difference was nearly 2-fold, the greatest biochemical difference reported to date. In contrast, one study found no difference between 6 untreated male patients (2 antipsychotic-naive) and 7 male healthy controls.103 Contradictory findings may be explained by age, type of schizophrenia, and gender, as patients in this study were generally younger (mean age: 26 years), more catatonic compared with the other studies (30+ years), and consisted exclusively of male patients. Comparable results were also evident in the high-risk individuals (N = 30) (M = 17, F = 13)104 and dopamine synthesis in these individuals determined their clinical outcome 3 years later. The psychotic transition group (N = 9) had greater dopamine synthesis in the striatum (effect size = 1.18) compared with the healthy control (N = 29) (M = 20, F = 9) and the nontransition group (N = 15). This finding is consistent with another study that reported elevated dopamine levels in the striatum of high-risk individuals.105 One study reported significantly higher dopamine synthesis in only the putamen, with no difference found in the caudate.106 Overall, the evidence shows that patients with schizophrenia and individuals at high-risk for psychosis have increased dopamine release in the striatum and may be related to the illness severity.
Studies Under Dopamine Release Conditions
To study dopamine release, investigators used the amphetamine-challenge method, as amphetamine has been shown to be linked to psychosis.107 These studies reported elevated dopamine release in the striatum of antipsychotic-free patients71,108,109 and a sample of antipsychotic-naive and antipsychotic-free patients.76 Overall, these results illustrate increased dopamine release in patients with schizophrenia.
Studies Under Dopamine Depletion Conditions
To investigate indirectly the dopamine levels at the synaptic cleft, a few studies have used alpha-methyl-para-tyrosine (AMPT) to inhibit transiently the synthesis of dopamine. The first study compared 18 antipsychotic-naive and antipsychotic-free patients (M = 11, F = 7) to 18 healthy controls (M = 11, F = 7).72 They demonstrated that patients have greater amounts of dopamine occupying the D2/3 receptors in the striatum. In a follow-up study, the same group assessed only 6 antipsychotic-naive patients (M = 2, F = 4) with schizophrenia and demonstrated greater increase in dopamine D2/3 binding in the striatum, suggesting greater dopamine levels at the synaptic cleft in the striatum compared to 8 healthy controls (M = 6, F = 2).110
Furthermore, another study that used [11C]-raclopride after dopamine depletion with AMPT found greater D2/3 receptor binding in the PCDC of 18 antipsychotic-naive and antipsychotic-free patients (M = 13, F = 5).111 It is important to note that among the 18 patients assessed in this study, 12 were chronically ill and previously medicated.
In summary, based on the presented evidence, antipsychotic-naive and antipsychotic-free patients with schizophrenia present increased dopamine synthesis capacity, release after amphetamine challenge, and baseline dopamine levels in the striatum after dopamine depletion.
Thalamus
Nine studies assessed the thalamus, one of these studies using [18F]fallypride PET found increase binding in 6 antipsychotic-naive and 12 antipsychotic-free (M = 14, F = 7).78 Another study using the same technique assessing 15 antipsychotic-naive patients (M = 10, F = 5), however, reported reduced D2/3 receptor binding, with the greatest difference in the left medial dorsal nucleus and left pulvinar.112 Several other studies also reported decreased D2/3 receptor binding in the thalamus.74,79,110,111 Talvik et al demonstrated decreased D2/3 receptor binding in the right medial thalamus113; Yasuno et al., in the central medial114 and posterior subregion of the thalamus; and Kessler et al, in the left medial thalamus.79 A later study by Talvik and colleagues confirmed their earlier findings by demonstrating lower D2/3 receptor binding in the right thalamus of patients compared with healthy controls, but they detected no difference in the left thalamus.74 In contrast, 4 studies found no overall difference in D2/3 receptor binding in the thalamus.81,98,115,116 One assessed only 11 antipsychotic-naive male patients using [11C]FLB 457 PET115 and the other assessed 25 antipsychotic-naive patients (M = 2, F = 4) using 123I-epidepride SPECT, the largest sample to date.116 Although most of the evidence suggests reduced D2/3 receptor binding in the thalamus of patients with schizophrenia, the only study that performed partial volume correction found increased D2/3 binding in this region.78 As such, inconsistency in findings for the thalamus warrants further studies.
Temporal, Limbic, and Frontal Regions
Studies comparing D2/3 receptor binding between patients and healthy controls found patients had equal amounts of D2/3 receptors in the limbic, sensorimotor, temporal, and frontal regions.78,79,113 In contrast, a study specifically assessing the amygdala, cingulate gyrus, and temporal cortices reported reduced D2/3 receptor binding in these regions.112 Furthermore, a study that assessed the anterior cingulate of 11 antipsychotic-naive male patients reported a 12.5 % reduction in D2/3 binding in patients.115 As such, discrepancies may be attributed to sample and sex differences.
A study that assessed dopaminergic synthesis capacity in the limbic and temporal regions reported elevated dopamine levels.102 In this study, a 50% increase in FDOPA clearance was detected in the amygdala of 8 male patients.102 Greater dopamine synthesis capacity was also detected in the MPFC of 12 patients (M = 12, F = 2).101 Thus, although further investigations are needed, preliminary results demonstrate reduced D2/3 receptor binding and potentially elevated dopaminergic synthesis capacity in temporal and limbic regions of patients with schizophrenia compared with healthy controls.
In conclusion, evidence revealed no difference in D2/3 receptor binding, increased dopamine synthesis capacity, increased dopamine release, increased dopamine occupying the D2/3 receptors in the striatum, reduced D2/3 receptor binding in the thalamus, and potentially increased dopamine synthesis capacity in the temporal and limbic regions. Inconsistent results were reported in the anterior cingulate and substantia nigra. The findings pertaining to D1 receptor binding were inconsistent and further studies are needed to clarify inconclusive results.
GABAergic System
Presently, only one study has compared GABA levels independently between antipsychotic-free patients and healthy controls. The study reported elevated GABA concentrations in MPFC of 32 patients (M = 11, F = 5).28 This study, albeit preliminary, suggests the involvement of the GABAergic anomalies in schizophrenia. MRS studies assessing medicated patients compared with healthy controls reported increased GABA/Cr in the medial frontal and parieto-occipital regions,117 reduced GABA/Cr concentrations in the left BG but no difference in the frontal or occipital-parietal regions of early-stage patients with schizophrenia,118 lower GABA/Cr levels in the occipital region of patients,119 but no difference from the medial prefrontal and left BG,120 and increased GABA/Cr in medial frontal and parieto-occipital regions.121 Furthermore, as suggested by a recent study, GABA levels were elevated in younger patients compared with older patients with schizophrenia, suggesting an association between the stage of illness and GABA levels.122
Discussion
A number of studies revealed abnormalities in the glutamatergic system in antipsychotic-naive or antipsychotic-free patients with schizophrenia. In brief, studies focusing on the glutamatergic system demonstrated that among individuals at high risk for psychosis or during the first-episode of schizophrenia, GLX, glutamine, and glutamate levels are elevated in most regions of the brain. In contrast, studies looking at patients who were older than 35 years of age or labeled as chronic showed low GLX levels, which may be a medication effect. In addition, one study reported decreased NMDA binding in the hippocampus of antipsychotic-free patients.59
Studies focusing on the dopaminergic system demonstrated a decrease in the dopamine D2/3 receptor binding in the thalamus, an increase in dopamine synthesis capacity in the striatum, enhanced dopamine release, and increased dopamine at baseline. Lastly, one study reported elevation of GABA levels in MPFC of antipsychotic-free patients. Below we describe a model that could explain these various findings.
It has been proposed that at the onset of the disorder, hypofunctioning NMDA receptors on GABAergic interneurons lead to excessive release of glutamate from pyramidal neurons.123 Excessive glutamate levels lead to excitotoxicity-mediated neuronal death.124 As a precursor for glutamine, some of the glutamate is converted to glutamine within astrocytes125 and result in high levels glutamine as demonstrated by in vivo imaging studies. Elevated glutamate levels may also over stimulate dopaminergic neurons resulting in high levels of dopamine, as suggested by striatal studies and yet to be confirmed in the cortex.126,127 Further, given that glutamate is a precursor to GABA and that the current literature suggests high levels of GABA early in the course of the illness, our model proposes that high levels of GABA are driven by high levels of glutamate and that glutamate-to-GABA conversion is intact. Another possible scenario is that excessive glutamatergic activity stimulates interneurons to release more GABA. Finally, elevated GABA levels could be independent of high glutamate levels as they could reflect abnormal GABA reuptake by transporters. This finding is supported by postmortem studies that reported reduced presynaptic GAT1 transporters found in patients lead to increased GABAergic transmission at the synapse due to diminished reuptake.128 As a compensatory measure, postsynaptic GABAA receptors are upregulated, followed by the downregulation of GAD67 and parvalbumin-positive interneurons,13,117,129,130 eventually leading to reduced GABAergic activity. Irrespective of the underlying mechanism, high levels of GABA could be contributing to the relative stability of the excitation-inhibition system.
Dopamine effect on LTP facilitation depends on the concentration and activated subreceptors. Dopaminergic receptors are in close proximity to glutamatergic receptors and appear to have a major role in synaptic modulation, by affecting the phosphorylation of glutamatergic NMDA and AMPA receptors (figures 1 and 2).131 The relationship between dopamine levels and LTP facilitation is reported as an inverted “U” shape dose-response curve.132,133 Low levels of dopamine preferentially activate presynaptic D2/3 receptors, which reduces the release of dopamine and essentially LTP facilitation. On the other hand, high levels equally activate postsynaptic D1 and D2/3 receptors, counteracting each other’s effect. However, at optimal dopamine levels, D1 postsynaptic receptors are stimulated and LTP facilitated. That is, insufficient or excessive dopamine levels impair LTP facilitation and optimal facilitation is achieved at moderate concentrations. Thus, high levels of dopamine in the striatum and potentially in the cortex of patients with schizophrenia likely result in impaired LTP because excessive dopamine may lead to the upregulation of the D2/3 receptors134 or the functional sensitization of the D2/3 receptors.135 Presynaptic D2/3 receptors on interneurons enable LTP facilitation by suppressing GABAergic inhibition on pyramidal neurons.60 Low levels of dopamine in the cortex can also result in impaired LTP. When D2/3 receptors are hypofunctioning, understimulated pyramidal neurons are not sufficiently suppressed, thereby leading to excessive excitation. When D1 receptors are stimulated, LTP activity is facilitated and resting glutamatergic neurons increase their production of neurotransmitters and receptors by stimulating the CAMP/protein-kinase A pathway.62,136 As such, dopamine regulates both glutamatergic excitatory and GABAergic inhibitory circuits137 and the balanced concentration of dopamine and interplay between excitation and inhibition facilitate the induction of LTP.138 Several studies have demonstrated in vivo evidence for impaired LTP in patients with schizophrenia. Using transcranial direct current stimulation, Hasan et al (2011) showed that multiepisode patients had reduced LTP-like plasticity compared to healthy controls and recent-onset patients.139 LTP impairments have also been revealed in the motor cortex and DLPFC of patients using paired associative stimulation.140,141 LTP plasticity was also showed to be impaired in both medicated and unmedicated patients using transcranial magnetic stimulation.142,143 Lastly, impaired LTP has been demonstrated in the visual cortex using high frequency stimulation.144
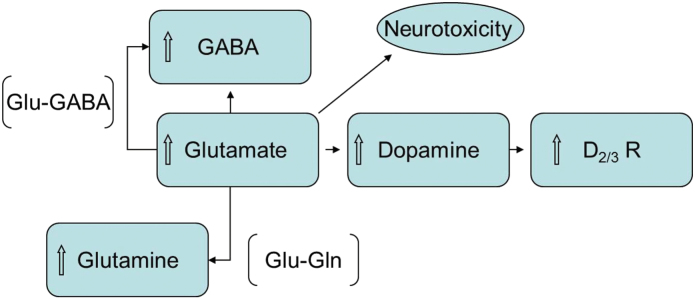
Fig. 1.
Hypoactive NMDA receptor causes downstream hyperglutamatergic activity, which leads to the conversion of glutamate to glutamine by the enzyme glutaminase, as such increasing glutamine levels.130 Glutamine is a molecule which cannot exert neurotoxic effects.148 To balance out excitatory activity with inhibitory activity, glutamate is converted into GABA, the main inhibitory neurotransmitter. Extracellular dopamine is regulated by NMDA receptors located on the dopaminergic neuron. Hypoactive NMDA receptors on cortico-brainstem pathway reduce inhibition of tonic dopamine neurons of the mesocortical pathway, which leads to increase in DA release.130,149 To attenuate the dopamine release, D2/3 receptor density is upregulated.
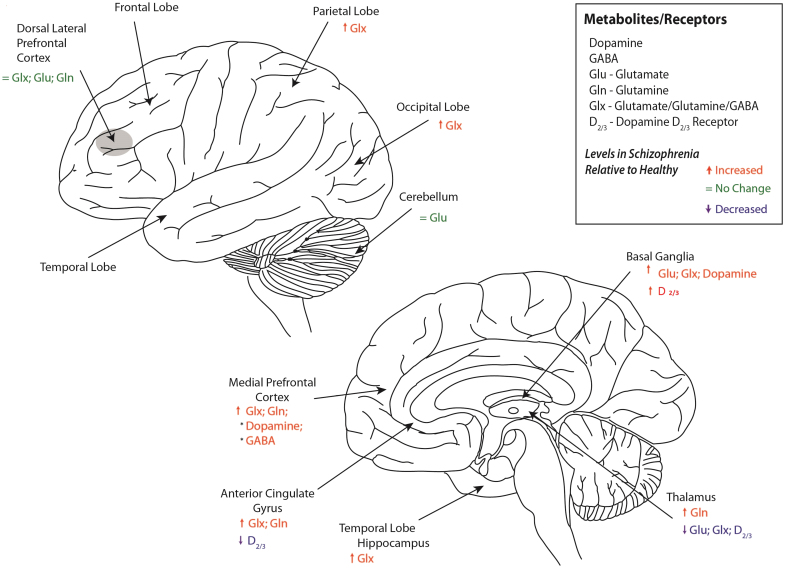
Fig. 2.
Neurochemicals and receptors in patients with schizophrenia relative to healthy controls in different brain regions. *Evidence is based on one study.
Limitations
First, discrepancies in findings may be accounted for by the difference in patient population, such as sex. Not all the studies included in this review assessed antipsychotic-naive patients, as some assessed antipsychotic-free; therefore, the effects of antipsychotics cannot be completely discounted, as studies in animals suggest that even minimal exposure to antipsychotics can modulate glutamatergic activity.12 Second, the interpretations of GABA and GLX measurements present another limitation. The validity of early 1H MRS studies may be less compared with recent studies, which employed better 1H MRS technology including acquisition and quantification that allows the separation of overlapping resonance signals arising from glutamate, glutamine, and GABA. Third, MRS is capable of detecting total concentration of a neurochemical and currently cannot distinguish between intracellular and extracellular glutamate, glutamine, or GABA.145 However, one study showed a relationship between MRS-derived measures of GABA and glutamate and behavior, suggesting that what is measured by MRS is associated with neurotransmission.146 Fourth, discrepancies among PET studies may have resulted from the differences in the selectivity and affinity of the radiotracers used. For instance, [11C]-N-methylspiperone binds to D2/3 receptors and 5-HT2 serotonin receptors in vivo and has affinity for dopamine D4 receptors in vitro.96 The increase in D2/3 binding detected with this tracer may include the binding of serotonin receptors, which are not detected using other ligands.147 Also, not all radioligands have the same affinity for D2/3 receptors, presenting a major limitation when comparing one study to another. Lastly, since the sample size in most of the studies was small and heterogeneous, larger homogenous samples are needed to verify such findings. Therefore, future studies using better 1H MRS technology, more selective PET ligands, and large homogenous samples are necessary in order to verify these observations.
Conclusion
LTP is a neuronal mechanism mediating learning and memory. This review presented evidence highlighting abnormal glutamatergic, dopaminergic, and GABAergic systems in antipsychotic-naive and antipsychotic-free patients with schizophrenia. As these systems are essential for LTP facilitation, cognitive impairments associated with schizophrenia may be explained by impaired LTP formation. This proposed model does not negate that these same systems could be mediating other dimensions of schizophrenia, eg, positive and negative symptoms, and not necessarily through LTP impairments. Lastly, it is important to note that medicated patients also experience cognitive deficits and that understanding the neurochemical abnormalities underlying these deficits among these patients could lead to better remediation interventions.
Supplementary Material
Supplementary material (reference 150 is cited in the supplementary material) is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Bahar Salavati received support from Ontario Graduate Scholarship, and currently receives support from Ontario Mental Health Foundation (OMHF) Scholarship. Dr. Rajji receives research support from Brain Canada, Brain and Behavior Research Foundation, Canadian Foundation for Innovation, Canadian Institutes of Health Research (CIHR), Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, the US National Institute of Health (NIH), and the W. Garfield Weston Foundation. Dr. Graff-Guerrero receives support from NIH, CIHR, OMHF, CONACyT, ICyTDF, Brain & Behavior Research Foundation (NARSAD) and Janssen. He has served as consultant for Abbott Laboratories, Gedeon Richter Plc, and Eli Lilly. Yinming Sun receives support from CIHR. Dr. Daskalakis, in the last 5 years received research and equipment in-kind support for an investigator-initiated study through BrainswayInc and a travel allowance through Merck. He also received speaker funding through SepracorInc, AstraZeneca and served on the advisory board for Hoffmann-La Roche Limited and Merck and received speaker support from Eli Lilly.
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. [DOI] [PubMed] [Google Scholar]
- 2. Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738–1749. [DOI] [PubMed] [Google Scholar]
- 3. Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:286–293. [DOI] [PubMed] [Google Scholar]
- 4. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. [DOI] [PubMed] [Google Scholar]
- 5. Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. [DOI] [PubMed] [Google Scholar]
- 6. Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: John Wiley and Sons; 1949. [Google Scholar]
- 7. Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. [DOI] [PubMed] [Google Scholar]
- 8. Coan EJ, Collingridge GL. Characterization of an N-methyl-D-aspartate receptor component of synaptic transmission in rat hippocampal slices. Neuroscience. 1987;22:1–8. [DOI] [PubMed] [Google Scholar]
- 9. Errington ML, Lynch MA, Bliss TV. Long-term potentiation in the dentate gyrus: induction and increased glutamate release are blocked by D(-)aminophosphonovalerate. Neuroscience. 1987;20:279–284. [DOI] [PubMed] [Google Scholar]
- 10. Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100:433–442. [DOI] [PubMed] [Google Scholar]
- 11. Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522:69–75. [DOI] [PubMed] [Google Scholar]
- 12. López-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. [DOI] [PubMed] [Google Scholar]
- 13. Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. [DOI] [PubMed] [Google Scholar]
- 14. Bailey CP, Andrews N, McKnight AT, Hughes J, Little HJ. Prolonged changes in neurochemistry of dopamine neurones after chronic ethanol consumption. Pharmacol Biochem Behav. 2000;66:153–161. [DOI] [PubMed] [Google Scholar]
- 15. Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci USA. 1995;92:2446–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Z, Ito K, Fujii S, et al. Roles of dopamine receptors in long-term depression: enhancement via D1 receptors and inhibition via D2 receptors. Recept Channels. 1996;4:1–8. [PubMed] [Google Scholar]
- 17. Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruiz A, Campanac E, Scott RS, Rusakov DA, Kullmann DM. Presynaptic GABAA receptors enhance transmission and LTP induction at hippocampal mossy fiber synapses. Nat Neurosci. 2010;13:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. [DOI] [PubMed] [Google Scholar]
- 20. Deisz RA. GABA(B) receptor-mediated effects in human and rat neocortical neurones in vitro. Neuropharmacology. 1999;38:1755–1766. [DOI] [PubMed] [Google Scholar]
- 21. Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of ¹H-MRS studies. Schizophr Bull. 2013;39:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poels EM, Kegeles LS, Kantrowitz JT, et al. Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Mol Psychiatry. 2014;19:20–29. [DOI] [PubMed] [Google Scholar]
- 23. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42:211–221. [PubMed] [Google Scholar]
- 25. Soares JC, Innis RB. Neurochemical brain imaging investigations of schizophrenia. Biol Psychiatry. 1999;46:600–615. [DOI] [PubMed] [Google Scholar]
- 26. Bradford HF, Thomas AJ. Metabolism of glucose and glutamate by synaptosomes from mammalian cerebral cortex. J Neurochem. 1969;16:1495–1504. [DOI] [PubMed] [Google Scholar]
- 27. Sailasuta N, Ernst T, Chang L. Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magn Reson Imaging. 2008;26:667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex ?-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. [DOI] [PubMed] [Google Scholar]
- 29. Tibbo P, Hanstock C, Valiakalayil A, Allen P. 3-T proton MRS investigation of glutamate and glutamine in adolescents at high genetic risk for schizophrenia. Am J Psychiatry. 2004;161:1116–1118. [DOI] [PubMed] [Google Scholar]
- 30. Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. [DOI] [PubMed] [Google Scholar]
- 31. Jensen JE, Licata SC, Ongür D, et al. Quantification of J-resolved proton spectra in two-dimensions with LCModel using GAMMA-simulated basis sets at 4 Tesla. NMR Biomed. 2009;22:762–769. [DOI] [PubMed] [Google Scholar]
- 32. Bradford HF, Ward HK, Thomas AJ. Glutamine–a major substrate for nerve endings. J Neurochem. 1978;30:1453–1459. [DOI] [PubMed] [Google Scholar]
- 33. Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol Aging. 2005;26:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bartha R, Williamson PC, Drost DJ, et al. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54:959–965. [DOI] [PubMed] [Google Scholar]
- 35. Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond, B, Biol Sci. 1999;354:1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn Reson Med. 2008;60:964–969. [DOI] [PubMed] [Google Scholar]
- 37. Hurd R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn Reson Med. 2004;51:435–440. [DOI] [PubMed] [Google Scholar]
- 38. Lutkenhoff ES, van Erp TG, Thomas MA, et al. Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 2010;15:308–318. [DOI] [PubMed] [Google Scholar]
- 39. Ohrmann P, Siegmund A, Suslow T, et al. Evidence for glutamatergic neuronal dysfunction in the prefrontal cortex in chronic but not in first-episode patients with schizophrenia: a proton magnetic resonance spectroscopy study. Schizophr Res. 2005;73:153–157. [DOI] [PubMed] [Google Scholar]
- 40. Stanley JA, Williamson PC, Drost DJ, et al. An in vivo proton magnetic resonance spectroscopy study of schizophrenia patients. Schizophr Bull. 1996;22:597–609. [DOI] [PubMed] [Google Scholar]
- 41. Ohrmann P, Siegmund A, Suslow T, et al. Cognitive impairment and in vivo metabolites in first-episode neuroleptic-naive and chronic medicated schizophrenic patients: a proton magnetic resonance spectroscopy study. J Psychiatr Res. 2007;41:625–634. [DOI] [PubMed] [Google Scholar]
- 42. Yoo SY, Yeon S, Choi CH, et al. Proton magnetic resonance spectroscopy in subjects with high genetic risk of schizophrenia: investigation of anterior cingulate, dorsolateral prefrontal cortex and thalamus. Schizophr Res. 2009;111:86–93. [DOI] [PubMed] [Google Scholar]
- 43. Seese RR, O’Neill J, Hudkins M, et al. Proton magnetic resonance spectroscopy and thought disorder in childhood schizophrenia. Schizophr Res. 2011;133:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choe BY, Kim KT, Suh TS, et al. 1H magnetic resonance spectroscopy characterization of neuronal dysfunction in drug-naive, chronic schizophrenia. Acad Radiol. 1994;1:211–216. [DOI] [PubMed] [Google Scholar]
- 45. Théberge J, Bartha R, Drost DJ, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. [DOI] [PubMed] [Google Scholar]
- 46. Théberge J, Williamson KE, Aoyama N, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334. [DOI] [PubMed] [Google Scholar]
- 47. Aoyama N, Théberge J, Drost DJ, et al. Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psychiatry. 2011;198:448–456. [DOI] [PubMed] [Google Scholar]
- 48. Théberge J, Al-Semaan Y, Williamson PC, et al. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160:2231–2233. [DOI] [PubMed] [Google Scholar]
- 49. Bustillo JR, Rowland LM, Mullins P, et al. 1H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psychiatry. 2010;15:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stone JM, Day F, Tsagaraki H, et al. ; OASIS. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66:533–539. [DOI] [PubMed] [Google Scholar]
- 51. Tandon N, Bolo NR, Sanghavi K, et al. Brain metabolite alterations in young adults at familial high risk for schizophrenia using proton magnetic resonance spectroscopy. Schizophr Res. 2013;148:59–66. [DOI] [PubMed] [Google Scholar]
- 52. de la Fuente-Sandoval C, Favila R, Alvarado P, et al. Glutamate increase in the associative striatum in schizophrenia: a longitudinal magnetic resonance spectroscopy preliminary study. Gac Med Mex. 2009;145:109–113. [PubMed] [Google Scholar]
- 53. de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology. 2011;36:1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMAPsychiatry. 2013;70:1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Keshavan MS, Dick RM, Diwadkar VA, Montrose DM, Prasad KM, Stanley JA. Striatal metabolic alterations in non-psychotic adolescent offspring at risk for schizophrenia: a (1)H spectroscopy study. Schizophr Res. 2009;115:88–93. [DOI] [PubMed] [Google Scholar]
- 56. Kegeles LS, Shungu DC, Anjilvel S, et al. Hippocampal pathology in schizophrenia: magnetic resonance imaging and spectroscopy studies. Psychiatry Res. 2000;98:163–175. [DOI] [PubMed] [Google Scholar]
- 57. Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bartha R, al-Semaan YM, Williamson PC, et al. A short echo proton magnetic resonance spectroscopy study of the left mesial-temporal lobe in first-onset schizophrenic patients. Biol Psychiatry. 1999;45:1403–1411. [DOI] [PubMed] [Google Scholar]
- 59. Pilowsky LS, Bressan RA, Stone JM, et al. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry. 2006;11:118–119. [DOI] [PubMed] [Google Scholar]
- 60. Xu TX, Yao WD. D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proc Natl Acad Sci USA. 2010;107:16366–16371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Granado N, Ortiz O, Suárez LM, et al. D1 but not D5 dopamine receptors are critical for LTP, spatial learning, and LTP-Induced arc and zif268 expression in the hippocampus. Cereb Cortex. 2008;18:1–12. [DOI] [PubMed] [Google Scholar]
- 62. Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Abi-Dargham A. Recent evidence for dopamine abnormalities in schizophrenia. Eur Psychiatry. 2002;17(suppl 4):341s–347s. [DOI] [PubMed] [Google Scholar]
- 64. Abi-Dargham A, Xu X, Thompson JL, et al. Increased prefrontal cortical D1 receptors in drug naive patients with schizophrenia: a PET study with [¹¹C]NNC112. J Psychopharmacol (Oxford). 2012;26:794–805. [DOI] [PubMed] [Google Scholar]
- 65. Okubo Y, Suhara T, Suzuki K, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. [DOI] [PubMed] [Google Scholar]
- 66. Karlsson P, Farde L, Halldin C, Sedvall G. PET study of D(1) dopamine receptor binding in neuroleptic-naive patients with schizophrenia. Am J Psychiatry. 2002;159:761–767. [DOI] [PubMed] [Google Scholar]
- 67. Guo N, Hwang DR, Lo ES, Huang YY, Laruelle M, Abi-Dargham A. Dopamine depletion and in vivo binding of PET D1 receptor radioligands: implications for imaging studies in schizophrenia. Neuropsychopharmacology. 2003;28:1703–1711. [DOI] [PubMed] [Google Scholar]
- 68. Ekelund J, Slifstein M, Narendran R, et al. In vivo DA D(1) receptor selectivity of NNC 112 and SCH 23390. Mol Imaging Biol. 2007;9:117–125. [DOI] [PubMed] [Google Scholar]
- 69. Catafau AM, Searle GE, Bullich S, et al. Imaging cortical dopamine D1 receptors using [11C]NNC112 and ketanserin blockade of the 5-HT 2A receptors. J Cereb Blood Flow Metab. 2010;30:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yang YK, Yu L, Yeh TL, Chiu NT, Chen PS, Lee IH; SPECT study. Associated alterations of striatal dopamine D2/D3 receptor and transporter binding in drug-naive patients with schizophrenia: a dual-isotope SPECT study. Am J Psychiatry. 2004;161:1496–1498. [DOI] [PubMed] [Google Scholar]
- 71. Abi-Dargham A, Gil R, Krystal J, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155:761–767. [DOI] [PubMed] [Google Scholar]
- 72. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pilowsky LS, Costa DC, Ell PJ, Verhoeff NP, Murray RM, Kerwin RW. D2 dopamine receptor binding in the basal ganglia of antipsychotic-free schizophrenic patients. An 123I-IBZM single photon emission computerised tomography study. Br J Psychiatry. 1994;164:16–26. [DOI] [PubMed] [Google Scholar]
- 74. Talvik M, Nordström AL, Okubo Y, et al. Dopamine D2 receptor binding in drug-naïve patients with schizophrenia examined with raclopride-C11 and positron emission tomography. Psychiatry Res. 2006;148:165–173. [DOI] [PubMed] [Google Scholar]
- 75. Schmitt GJ, Dresel S, Frodl T, et al. Dual-isotope SPECT imaging of striatal dopamine: a comparative study between never-treated and haloperidol-treated first-episode schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 2012;262:183–191. [DOI] [PubMed] [Google Scholar]
- 76. Breier A, Su TP, Saunders R, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94:2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Knable MB, Egan MF, Heinz A, et al. Altered dopaminergic function and negative symptoms in drug-free patients with schizophrenia. [123I]-iodobenzamide SPECT study. Br J Psychiatry. 1997;171:574–577. [DOI] [PubMed] [Google Scholar]
- 78. Kegeles LS, Slifstein M, Xu X, et al. Striatal and extrastriatal dopamine D2/D3 receptors in schizophrenia evaluated with [18F]fallypride positron emission tomography. Biol Psychiatry. 2010;68:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kessler RM, Woodward ND, Riccardi P, et al. Dopamine D2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biol Psychiatry. 2009;65:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Farde L, Wiesel FA, Stone-Elander S, et al. D2 dopamine receptors in neuroleptic-naive schizophrenic patients. A positron emission tomography study with [11C]raclopride. Arch Gen Psychiatry. 1990;47:213–219. [DOI] [PubMed] [Google Scholar]
- 81. Graff-Guerrero A, Mizrahi R, Agid O, et al. The dopamine D2 receptors in high-affinity state and D3 receptors in schizophrenia: a clinical [11C]-(+)-PHNO PET study. Neuropsychopharmacology. 2009;34:1078–1086. [DOI] [PubMed] [Google Scholar]
- 82. Hietala J, West C, Syvälahti E, et al. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl). 1994;116:285–290. [DOI] [PubMed] [Google Scholar]
- 83. Martinot JL, Paillère-Martinot ML, Loc’h C, et al. Central D2 receptors and negative symptoms of schizophrenia. Br J Psychiatry. 1994;164:27–34. [DOI] [PubMed] [Google Scholar]
- 84. Martinot JL, Paillère-Martinot ML, Loc’h C, et al. The estimated density of D2 striatal receptors in schizophrenia. A study with positron emission tomography and 76Br-bromolisuride. Br J Psychiatry. 1991;158:346–350. [DOI] [PubMed] [Google Scholar]
- 85. Nordström AL, Farde L, Eriksson L, Halldin C. No elevated D2 dopamine receptors in neuroleptic-naive schizophrenic patients revealed by positron emission tomography and [11C]-N-methylspiperone. Psychiatry Res. 1995;61:67–83. [DOI] [PubMed] [Google Scholar]
- 86. Schmitt GJ, Meisenzahl EM, Frodl T, et al. Increase of striatal dopamine transmission in first episode drug-naive schizophrenic patients as demonstrated by [(123)I]IBZM SPECT. Psychiatry Res. 2009;173:183–189. [DOI] [PubMed] [Google Scholar]
- 87. Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(suppl 1):S1–S5. [DOI] [PubMed] [Google Scholar]
- 88. Tuppurainen H, Kuikka J, Viinamäki H, Husso-Saastamoinen M, Bergström K, Tiihonen J. Extrastriatal dopamine D 2/3 receptor density and distribution in drug-naive schizophrenic patients. Mol Psychiatry. 2003;8:453–455. [DOI] [PubMed] [Google Scholar]
- 89. Pearlson GD, Wong DF, Tune LE, et al. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Arch Gen Psychiatry. 1995;52:471–477. [DOI] [PubMed] [Google Scholar]
- 90. Corripio I, Escartí MJ, Portella MJ, et al. Density of striatal D2 receptors in untreated first-episode psychosis: an I123-IBZM SPECT study. Eur Neuropsychopharmacol. 2011;21:861–866. [DOI] [PubMed] [Google Scholar]
- 91. Corripio I, Pérez V, Catafau AM, Mena E, Carrió I, Alvarez E. Striatal D2 receptor binding as a marker of prognosis and outcome in untreated first-episode psychosis. Neuroimage. 2006;29:662–666. [DOI] [PubMed] [Google Scholar]
- 92. Tune LE, Wong DF, Pearlson G, et al. Dopamine D2 receptor density estimates in schizophrenia: a positron emission tomography study with 11C-N-methylspiperone. Psychiatry Res. 1993;49:219–237. [DOI] [PubMed] [Google Scholar]
- 93. Wong DF, Wagner HN, Jr, Tune LE, et al. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science. 1986;234:1558–1563. [DOI] [PubMed] [Google Scholar]
- 94. Wong DF, Singer HS, Brandt J, et al. D2-like dopamine receptor density in Tourette syndrome measured by PET. J Nucl Med. 1997;38:1243–1247. [PubMed] [Google Scholar]
- 95. Ishiwata K, Hayakawa N, Ogi N, et al. Comparison of three PET dopamine D2-like receptor ligands, [11C]raclopride, [11C]nemonapride and [11C]N-methylspiperone, in rats. Ann Nucl Med. 1999;13:161–167. [DOI] [PubMed] [Google Scholar]
- 96. Seeman P, Guan HC, Van Tol HH. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993;365:441–445. [DOI] [PubMed] [Google Scholar]
- 97. Lomeña F, Catafau AM, Parellada E, et al. Striatal dopamine D2 receptor density in neuroleptic-naive and in neuroleptic-free schizophrenic patients: an 123I-IBZM-SPECT study. Psychopharmacology (Berl). 2004;172:165–169. [DOI] [PubMed] [Google Scholar]
- 98. Tuppurainen H, Kuikka JT, Laakso MP, Viinamäki H, Husso M, Tiihonen J. Midbrain dopamine D2/3 receptor binding in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2006;256:382–387. [DOI] [PubMed] [Google Scholar]
- 99. Nozaki S, Kato M, Takano H, et al. Regional dopamine synthesis in patients with schizophrenia using L-[beta-11C]DOPA PET. Schizophr Res. 2009;108:78–84. [DOI] [PubMed] [Google Scholar]
- 100. Hietala J, Någren K, Lehikoinen P, Ruotsalainen U, Syvälahti E. Measurement of striatal D2 dopamine receptor density and affinity with [11C]-raclopride in vivo: a test-retest analysis. J Cereb Blood Flow Metab. 1999;19:210–217. [DOI] [PubMed] [Google Scholar]
- 101. Lindström LH, Gefvert O, Hagberg G, et al. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biol Psychiatry. 1999;46:681–688. [DOI] [PubMed] [Google Scholar]
- 102. Kumakura Y, Cumming P, Vernaleken I, et al. Elevated [18F]fluorodopamine turnover in brain of patients with schizophrenia: an [18F]fluorodopa/positron emission tomography study. J Neurosci. 2007;27:8080–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dao-Castellana MH, Paillère-Martinot ML, Hantraye P, et al. Presynaptic dopaminergic function in the striatum of schizophrenic patients. Schizophr Res. 1997;23:167–174. [DOI] [PubMed] [Google Scholar]
- 104. Howes OD, Bose SK, Turkheimer F, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry. 2011;168:1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fusar-Poli P, Howes OD, Allen P, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75. [DOI] [PubMed] [Google Scholar]
- 106. Hietala J, Syvälahti E, Vuorio K, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346:1130–1131. [DOI] [PubMed] [Google Scholar]
- 107. Ellinwood EH, Jr, Sudilovsky A, Nelson LM. Evolving behavior in the clinical and experimental amphetamine (model) psychosis. Am J Psychiatry. 1973;130:1088–1093. [DOI] [PubMed] [Google Scholar]
- 108. Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93:9235–9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. [DOI] [PubMed] [Google Scholar]
- 110. Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naïve schizophrenic subjects. Biol Psychiatry. 2009;65:1091–1093. [DOI] [PubMed] [Google Scholar]
- 111. Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. [DOI] [PubMed] [Google Scholar]
- 112. Buchsbaum MS, Christian BT, Lehrer DS, et al. D2/D3 dopamine receptor binding with [F-18]fallypride in thalamus and cortex of patients with schizophrenia. Schizophr Res. 2006;85:232–244. [DOI] [PubMed] [Google Scholar]
- 113. Talvik M, Nordström AL, Olsson H, Halldin C, Farde L. Decreased thalamic D2/D3 receptor binding in drug-naive patients with schizophrenia: a PET study with [11C]FLB 457. Int J Neuropsychopharmacol. 2003;6:361–370. [DOI] [PubMed] [Google Scholar]
- 114. Yasuno F, Suhara T, Okubo Y, et al. Low dopamine d(2) receptor binding in subregions of the thalamus in schizophrenia. Am J Psychiatry. 2004;161:1016–1022. [DOI] [PubMed] [Google Scholar]
- 115. Suhara T, Okubo Y, Yasuno F, et al. Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Arch Gen Psychiatry. 2002;59:25–30. [DOI] [PubMed] [Google Scholar]
- 116. Glenthoj BY, Mackeprang T, Svarer C, et al. Frontal dopamine D(2/3) receptor binding in drug-naive first-episode schizophrenic patients correlates with positive psychotic symptoms and gender. Biol Psychiatry. 2006;60:621–629. [DOI] [PubMed] [Google Scholar]
- 117. Benes FM, Todtenkopf MS, Logiotatos P, Williams M. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat. 2000;20:259–269. [DOI] [PubMed] [Google Scholar]
- 118. Goto N, Yoshimura R, Moriya J, et al. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophr Res. 2009;112:192–193. [DOI] [PubMed] [Google Scholar]
- 119. Yoon JH, Maddock RJ, Rokem A, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tayoshi S, Nakataki M, Sumitani S, et al. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res. 2010;117:83–91. [DOI] [PubMed] [Google Scholar]
- 121. Ongür D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010;68:667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Rowland LM, Kontson K, West J, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. [DOI] [PubMed] [Google Scholar]
- 124. Rothstein JD. Excitotoxic mechanisms in the pathogenesis of amyotrophic lateral sclerosis. Adv Neurol. 1995;68:7–20; discussion 21. [PubMed] [Google Scholar]
- 125. Daikhin Y, Yudkoff M. Compartmentation of brain glutamate metabolism in neurons and glia. J Nutr. 2000;130:1026S–1031S. [DOI] [PubMed] [Google Scholar]
- 126. Expósito I, Del Arco A, Segovia G, Mora F. Endogenous dopamine increases extracellular concentrations of glutamate and GABA in striatum of the freely moving rat: involvement of D1 and D2 dopamine receptors. Neurochem Res. 1999;24:849–856. [DOI] [PubMed] [Google Scholar]
- 127. Segovia G, Del Arco A, Mora F. Endogenous glutamate increases extracellular concentrations of dopamine, GABA, and taurine through NMDA and AMPA/kainate receptors in striatum of the freely moving rat: a microdialysis study. J Neurochem. 1997;69:1476–1483. [DOI] [PubMed] [Google Scholar]
- 128. Menzies L, Ooi C, Kamath S, et al. Effects of gamma-aminobutyric acid-modulating drugs on working memory and brain function in patients with schizophrenia. Arch Gen Psychiatry. 2007;64:156–167. [DOI] [PubMed] [Google Scholar]
- 129. Benes FM, Sorensen I, Vincent SL, Bird ED, Sathi M. Increased density of glutamate-immunoreactive vertical processes in superficial laminae in cingulate cortex of schizophrenic brain. Cereb Cortex. 1992;2:503–512. [DOI] [PubMed] [Google Scholar]
- 130. Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chase TN, Oh JD. Striatal dopamine- and glutamate-mediated dysregulation in experimental parkinsonism. Trends Neurosci. 2000;23:S86–S91. [DOI] [PubMed] [Google Scholar]
- 132. Monte-Silva K, Liebetanz D, Grundey J, Paulus W, Nitsche MA. Dosage-dependent non-linear effect of L-dopa on human motor cortex plasticity. J Physiol (Lond). 2010;588:3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. [DOI] [PubMed] [Google Scholar]
- 134. Wang M, Pei L, Fletcher PJ, Kapur S, Seeman P, Liu F. Schizophrenia, amphetamine-induced sensitized state and acute amphetamine exposure all show a common alteration: increased dopamine D2 receptor dimerization. Mol Brain. 2010;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Seeman P, Weinshenker D, Quirion R, et al. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci USA. 2005;102:3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Matsuda Y, Marzo A, Otani S. The presence of background dopamine signal converts long-term synaptic depression to potentiation in rat prefrontal cortex. J Neurosci. 2006;26:4803–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wigström H, Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983;301:603–604. [DOI] [PubMed] [Google Scholar]
- 138. Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27: 11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hasan A, Nitsche MA, Rein B, et al. Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behav Brain Res. 2011;224:15–22. [DOI] [PubMed] [Google Scholar]
- 140. Frantseva MV, Fitzgerald PB, Chen R, Möller B, Daigle M, Daskalakis ZJ. Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill learning. Cereb Cortex. 2008;18:990–996. [DOI] [PubMed] [Google Scholar]
- 141. Rajji T. Theta-gamma coupling abnormalities in the dorsolateral prefrontal cortex of patients with Schizophrenia Society of Biological Psychiatry. New York City. 2014;75:384S. [Google Scholar]
- 142. Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Dysfunctional neural plasticity in patients with schizophrenia. Arch Gen Psychiatry. 2008;65:378–385. [DOI] [PubMed] [Google Scholar]
- 143. Fitzgerald PB, Brown TL, Marston NA, et al. Reduced plastic brain responses in schizophrenia: a transcranial magnetic stimulation study. Schizophr Res. 2004;71:17–26. [DOI] [PubMed] [Google Scholar]
- 144. Cavus I, Reinhart RM, Roach BJ, et al. Impaired visual cortical plasticity in schizophrenia. Biol Psychiatry. 2012;71:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Stagg CJ, Bachtiar V, Johansen-Berg H. What are we measuring with GABA magnetic resonance spectroscopy? Commun Integr Biol. 2011;4:573–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr Biol. 2011;21:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Frost JJ, Smith AC, Kuhar MJ, Dannals RF, Wagner HN., Jr In vivo binding of 3H-N-methylspiperone to dopamine and serotonin receptors. Life Sci. 1987;40:987–995. [DOI] [PubMed] [Google Scholar]
- 148. Rowland LM, Bustillo JR, Mullins PG, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162:394–396. [DOI] [PubMed] [Google Scholar]
- 149. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. [DOI] [PubMed] [Google Scholar]
- 150. Pearlson GD, Tune LE, Wong DF, et al. Quantitative D2 dopamine receptor PET and structural MRI changes in late-onset schizophrenia. Schizophrenia bulletin 1993;19(4):783–795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.