Abstract
The yeast polysaccharide, β-glucan, has been shown to promote both anti-microbial and anti-tumor activities through its interaction with macrophages. Here we analyzed the effects of an insoluble whole glucan particle (WGP), a 1,3/1,6-β-glucan from Saccharomyces cerevisiae, and a soluble poly-1-6-β-d-glucopyranosyl-1-3-β-d-glucopyranose (PGG), a hydrolytic product of WGP, on the anti-microbial response of human macrophages against mycobacterial infection. Treatment of macrophages with WGP and PGG significantly decreased cell association and intracellular growth of Mycobacterium bovis BCG, but not Mycobacterium tuberculosis (M.tb) when compared to untreated controls. We characterized the influence of β-glucans on the generation of macrophage oxidative products and pro-inflammatory cytokines, two important anti-microbial defense mechanisms. WGP but not PGG treatment enhanced the oxidative response of macrophages as determined by the 2′,7′-dichlorofluorescin (DCF) assay. WGP treatment also induced macrophages to produce pro-inflammatory cytokines. The β-glucan receptor, Dectin-1, was found to be involved in the WGP-induced macrophage oxidative burst and intracellular growth inhibition of M. bovis BCG. This report indicates that although some forms of β-glucan are able to stimulate the respiratory burst and cytokine production in human macrophages, and exhibit antimicrobial properties against M. bovis BCG, the β-glucans tested here did not inhibit growth of M.tb within human macrophages.
Keywords: β-glucan, Mycobacterium tuberculosis, Mycobacterium bovis BCG, Macrophages, Dectin-1, Oxidative burst
1. Introduction
β-glucans, a group of carbohydrates that consist of (1,3)-linked β-d-glucopyranosyl residues, have been isolated from a variety of fungi, yeast, and grains. β-glucans have been tested for use as anti-tumor agents, and evidence is accumulating that they may have anti-infective and immune modulating properties [1–3]. Recent studies suggest that β-glucan treatment enhances the immune responses against various bacterial infections in animal models, including Streptococcus pneumoniae, Staphylococcus aureus, and Escherichia coli [4–6]. A study by Hetland and colleagues provided evidence that β-glucan may play a role in enhancing protection against Mycobacterium bovis BCG (the attenuated tuberculosis vaccine strain) infection, decreasing the bacterial burden in mice upon β-glucan treatment [7]. A subsequent study by the same group showed that treatment of BALB/c mouse peritoneal macrophages with β-glucan during infection of Mycobacterium tuberculosis H37Rv decreased the intracellular survival of the bacteria. These studies, although all done in animal models, give insight into the ability of various β-glucans to combat infections [8].
Several proposed mechanisms for the effects of β-glucans have been identified, including the increased generation of reactive oxygen intermediates (ROIs) [2]. The anti-tumor and microbicidal properties of β-glucans are thought to result in part from β-glucan binding to the non-opsonic domain of complement receptor 3 (CR3) on leukocytes, priming the cell for cytotoxic destruction of C3bi coated molecules [9]. However, most of β-glucan's proinflammatory and oxidative burst activities are thought to result from its binding to and activation of the β-glucan receptor Dectin-1, leading to downstream activation of NF-kB in immune cells [10]. Dectin-1 is a transmembrane pattern recognition receptor (PRR) that is expressed on macrophages, including alveolar macrophages, and dendritic cells [11]. β-glucan binding to Dectin-1 on macrophages results in increased production of pro-inflammatory cytokines and activation of phagocytosis [12–14].
M.tb is an intracellular pathogen of macrophages and has evolved strategies to evade host immune responses by entering and replicating within a unique phagosomal niche [15]. M.tb interacts with macrophages through a variety of PRRs located on the host immune cells and is able to bypass generation of an oxidative burst during phagocytosis [16]. The phagocytic receptors and PRRs important for the recognition of M.tb by human macrophages include CR3, the mannose receptor (MR) and Toll-like receptors (TLRs) [17,18]. M.tb is phagocytosed by CR3 in both a C3bi opsonic and non-opsonic dependent manner as well as by the MR [19]. Once phagocytosed by macrophages, most bacteria are degraded by acid hydrolases within the phagolysosome. However, M.tb evades phagolysosome fusion, in part, through use of specific PRRs during entry [20]. Therefore, inhibiting or altering entry of the bacteria into macrophages and allowing the clearance of M.tb through other mechanisms could potentially result in decreased survival of the organism.
Since some β-glucans are known to stimulate TNF-α production and other inflammatory mediators through binding to CR3 and Dectin-1, we hypothesized that use of this complex carbohydrate could augment the host defense against mycobacterial infection by engaging macrophage receptors that are important for mycobacterial recognition and/or augmenting innate host defense functions of the macrophage. Therefore, we investigated the role of β-glucans as potential therapeutics for mycobacterial infection by studying their effects using primary human macrophages as opposed to rodent models. We used an insoluble whole glucan particle (WGP) purified from Saccharomyces cerevisiae and a soluble hydrolytic product of WGP, poly-1-6-β-d-glucopyranosyl-1-3-β-d-glucopyranose (PGG). Our results indicate that β-glucans have a growth inhibitory effect on M. bovis BCG, but not on virulent M.tb, further supporting the notion that M.tb uses different mechanisms to evade host defenses within human macrophages.
2. Results
2.1. β-Glucan toxicity (cell death) of human macrophages
The toxicity of PGG and WGP on the monocyte-derived macrophage (MDM) monolayer was tested over 5 days to determine a tolerable concentration of β-glucan for experiments. MDM monolayers were exposed to different β-glucan concentrations (6.25, 12.5, 25, 50, or 100 μg/ml) and compared to the media control monolayer. Cell morphology and the integrity of the monolayer were analyzed daily using an inverted microscope and cell death was examined by Trypan Blue Exclusion staining on day 5. Results showed that the MDM monolayer remained intact at day 1 with the use of PGG and WGP as high as 100 μg/ml (Fig. 1), however, at day 3 the MDM monolayer treated with 100 μg/ml WGP showed partial lifting and progressive loss of cells through day 5. Thus WGP (100 μg/ml) proved to be either partially or completely toxic to the monolayers, while all other monolayers appeared intact throughout the 5-day period (Fig. 2). Trypan Blue Exclusion staining of cell monolayers on day 5 showed a similar pattern of results as observed by microscopy (Table 1). On day 5, 80% cell death was seen with 100 μg/ml WGP, while only 9% cell death occurred at 50 μg/ml. All other WGP concentrations exhibited no cellular death. PGG at 100 μg/ml showed 6% cell death only on day 5, while all other concentrations and incubation times for PGG showed no effect on the MDM monolayer. To confirm and complement data on cell death induced by WGP, we used a highly sensitive fluorescent death cell marker for analysis of the cells by confocal microscopy (Fig. 3). Use of WGP at the concentrations of 25, 50, and 100 μg/ml caused 8, 20, and 95% cell death, respectively (compared to ~3% cell death in the absence of β-glucan treatment). Overall, these results were used to determine an appropriate concentration of β-glucan preparation for bacterial intracellular survival and cell association assays.
Fig. 1.
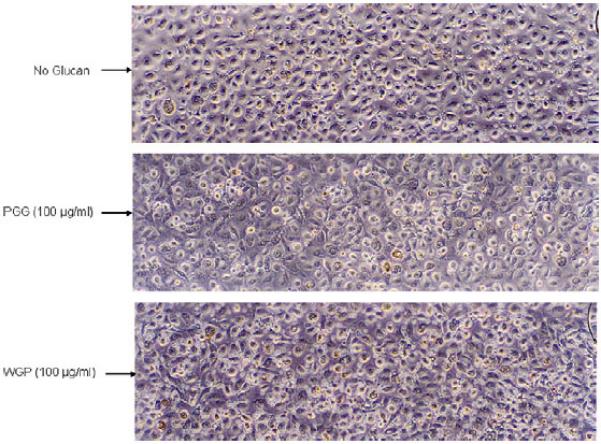
Photomicrographs of human macrophage monolayers at day 1 of exposure to β-glucan preparations. MDMs were grown in RPMI containing autologous serum in a 24-well tissue culture plate for 12 days when the cells were exposed to different concentrations of PGG or WGP. The morphology of the MDM monolayer was observed after 1 day under an inverted phase microscope. MDM monolayers appeared healthy and intact at all concentrations of PGG and WGP used. There was no noticeable change in morphology or intactness of the MDM monolayer between the no-glucan- and β-glucan-treated groups. Photomicrographs were taken at 100× magnification and are representative of two experiments.
Fig. 2.

Photomicrographs of human macrophage monolayers at day 5 of exposure to β-glucan preparations. MDMs were grown in RPMI containing autologous serum in a 24-well tissue culture plate for 12 days when the cells were exposed to different concentrations of PGG or WGP. The morphology of the MDM monolayer was observed daily over 5-day period under an inverted phase microscope. Following incubation for 5 days with β-glucans, only WGP at 100 mg/ml concentration proved partially or more completely toxic for the MDMs. All other concentrations of WGP had no significant effect on the monolayer. Photomicrographs were taken at 100× magnification and are representative of two experiments.
Table 1.
β-glucan toxicity for human macrophage monolayers.
| β-Glucan | Concentration (μg/ml) | Mean Cell Deatha (%) |
|---|---|---|
| PGG | 0.0 | 0 |
| 6.25 | 0 | |
| 12.5 | 0 | |
| 25.0 | 0 | |
| 50.0 | 0 | |
| 100.0 | 6 | |
| WGP | 0.0 | 0 |
| 6.25 | 0 | |
| 12.5 | 0 | |
| 25.0 | 0 | |
| 50.0 | 9 | |
| 100.0 | 80 |
Mean of duplicate wells (200 cells). 0.4% Trypan Blue Staining was used to assess cellular death in β-glucan pre-treated MDM monolayers at day 5. WGP (100 μg/ml) showed high toxicity for human macrophages with 80% cell death at Day 5. All other concentrations of PGG and WGP had little or no effect on macrophage death (n = 2, by duplicate).
Fig. 3.
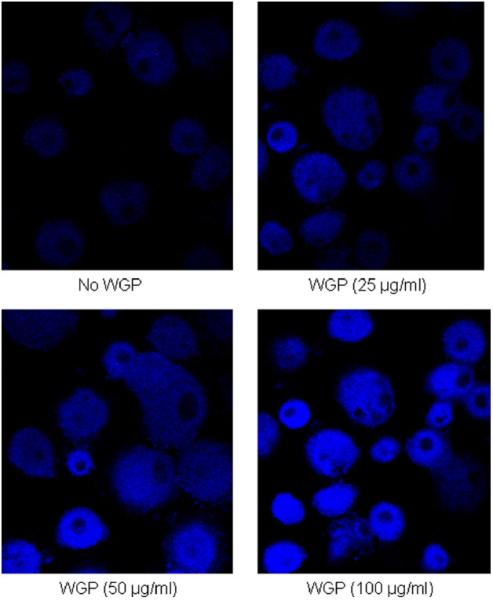
Fluorescence microscopy images of human macrophage monolayers at day 5 of exposure to WGP after staining with a fluorescent cell death marker. MDMs were grown in RPMI containing autologous serum on glass coverslips in a 24-well tissue culture plate for 12 days when the cells were exposed to different concentrations WGP. After washing away β-glucan, the cell monolayers were stained with aqua fluorescent reactive dye, then processed for and analyzed by confocal fluorescence microscopy (Olympus FV1000). Differential intensity of staining was used to count live (low intensity) and dead (high intensity) cells from at least 150 macrophages in each treatment sample in 2 independent experiments. High numbers of cells were scored as dead following treatment with 100 μg/ml WGP.
2.2. β-Glucan treatment prior to infection decreases the survival of M. bovis BCG within human macrophages through the Dectin-1 pathway
Previous studies with various derivatives of β-glucan have provided evidence for the anti-infective potential of these molecules [4–6]. In this study, we sought to determine the anti-mycobacterial activity of WGP (particulate) and PGG (soluble) in human macrophages. We pre-incubated MDMs with WGP and PGG for 30 min, then added M. bovis BCG [multiplicity of infection (MOI) = 1:1] to the β-glucan-treated MDMs and allowed for bacterial replication within macrophages over a 3-day period. Each day post-infection, the infected MDMs were lysed and plated for colony forming units (CFUs; expressed as CFU/ml; Fig. 4). At 24 h and 48 h post-infection, there was no significant difference in the number of CFUs of M. bovis BCG between the β-glucan-treated and non-treated groups (Fig. 4A). However, at 72 h post-infection, a significant decrease in CFUs was observed in the β-glucan-treated groups compared to the control group. The CFU results at 72 h were computed and plotted in percentage values for both β-glucan groups compared to the no-glucan control (Fig. 4B). The results of CFUs from WGP and PGG treatment show a significant decrease in M. bovis BCG survival in macrophages by 74 ± 5.9% (p < 0.0005) and 54 ± 4.8% (p < 0.005), respectively. These results provide evidence for a growth inhibitory effect of β-glucan-treated human macrophages for M. bovis BCG. To determine whether the β-glucan receptor, Dectin-1, is involved in M. bovis BCG intracellular growth inhibition, we pre-treated macrophages with anti-Dectin-1 antibody to block the receptor before adding WGP, followed by infection with M. bovis BCG. CFU analysis show that blocking the receptor Dectin-1 significantly increases the growth of M. bovis BCG at each time point (p < 0.05) compared to isotype control (Fig. 4C) which indicates that intracellular growth inhibition of M. bovis BCG by β-glucan treatment is Dectin-1 mediated.
Fig. 4.
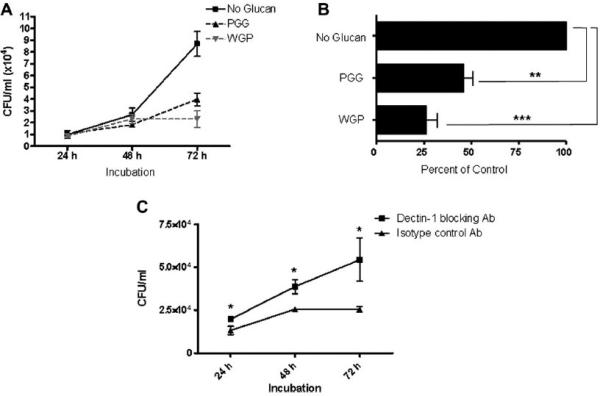
β-glucan inhibits intracellular growth of M. bovis BCG within macrophages through dectin-1. MDMs in each well (2 × 105 cells/well) of a 24-well plate were incubated at 37 °C/5% CO2 in the absence or presence of PGG (50–100 μg/ml) or WGP (25–50 μg/ml). MDMs were then infected with 2 × 105 M bovis BCG (MOI = 1:1) and incubated at 37 °C/5% CO2 for 2 h. The infection was stopped by washing and the cells were incubated in RPMI + 2.0% autologous serum for 24, 48 or 72 h. At each time period, cells were lysed and lysates plated on 7H11 agar for CFU analysis (expressed as CFUs/ml). A) Growth curve of M. bovis BCG showing inhibition of growth at 72 h in both PGG and WGP pre-treated macrophages. B) Results from A expressed in percentage values compared to no-glucan control which is set at 100%. Compared to the control, both PGG and WGP treatment showed a significant decrease in bacterial growth in MDMs by 54 ± 4.8% (**p < 0.005) and 74 ± 5.9% (***p < 0.0005), respectively. C) Growth curve of M. bovis BCG in macrophages pre-treated with anti-Dectin-1 or isotype control antibody, followed by the addition of WGP. Blocking the receptor increased the survival of M. bovis BCG following the addition of WGP. Data shown in A, B and C are from representative experiments (A and B, n = 3; C n = 2; each performed in triplicate).
2.3. Treatment of human macrophages with PGG and WGP prior to infection does not decrease intracellular growth of M.tb
We next analyzed the effects of β-glucans on virulent M.tb infection of macrophages. MDMs were pre-treated with WGP and PGG for 30 min, and then infected with virulent M.tb Erdman (MOI = 1:1). The MDMs were lysed every 24 h for 3 days and CFUs generated. The growth curve of M.tb Erdman shows no inhibition of growth during the 3-day incubation period in WGP and PGG pre-treated macrophages compared to the no-glucan control (Fig. 5A). Thus, despite showing growth inhibitory activity against M. bovis BCG, these data indicate that a single pre-infection dose of β-glucans has no effect on virulent M.tb growth within macrophages.
Fig. 5.
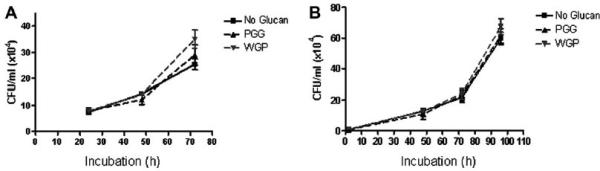
Treatment of macrophages with WGP or PGG prior to and after infection does not inhibit intracellular growth of M.tb Erdman. MDMs in each well (2 × 105 cells) of a 24-well plate were incubated at 37 °C/5% CO2 in the absence or presence of PGG (50–100 μg/ml) or WGP (25–50 μg/ml). MDMs were then infected with 2 × 105 M.tb Erdman (MOI = 1:1) and incubated at 37 °C/5% CO2 for 2 h. The infection was stopped by washing and the cells were incubated in RPMI + 2.0% autologous serum for 24, 48 or 72 h. At each time point, cells were lysed and lysates plated on 7H11 agar for CFU analysis. A) Growth curve of M.tb Erdman shows no significant inhibition of growth during the 3-day incubation period in PGG and WGP pre-treated macrophages compared to the no-glucan control. Shown is a representative experiment (n = 2, each performed in triplicate). B) β-glucan treatment was given before and 24 and 72 h after infection. At each time point (2, 48, 72, 96 h) cells were lysed and plated on 7H11 agar for CFU analysis. The growth curve of M.tb Erdman shows that MDMs treated both before and 24 and 72 h after infection did not affect bacterial growth. Shown is a representative experiment (n = 2, each performed in triplicate).
2.4. WGP and PGG treatment of human macrophages before and after infection does not affect the intracellular growth of virulent M.tb
Since treatment of MDMs prior to infection with WGP or PGG did not inhibit the growth of M.tb within macrophages, we next tested whether subsequent treatment of MDMs with β-glucans following infection would affect the intracellular growth of M.tb Erdman. MDMs were treated with WGP and PGG for 30 min prior to infection with M.tb as was done in the previous experiment. Then, at 24 h and again at 72 h post-infection, MDMs were treated with an additional dose of WGP or PGG. As shown in Fig. 5B, the growth curve of M.tb shows no inhibition of bacterial growth at any time point after infection.
2.5. WGP and PGG decrease M. bovis BCG association with human macrophages
We next wanted to determine the mechanism(s) whereby WGP and PGG inhibit the growth of M. bovis BCG within macrophages. Both M. bovis BCG and M.tb use phagocytic receptors and PRRs on human macrophages for opsonic and non-opsonic phagocytosis [17]. CR3 on macrophages is one major phagocytic receptor for these mycobacteria. Studies have shown that β-glucans bind to the non-opsonic region of CR3 [2]. In this experiment we determined whether β-glucans could inhibit M. bovis BCG association with macrophages. Cell association experiments were performed where MDMs were pre-treated with WGP or PGG and then infected with M. bovis BCG for 2 h. There was a significant decrease in bacterial association with PGG-treated (35.0 ± 1.8%; p < 0.0005) and WGP-treated (37.8 ± 2.5%; p < 0.0005) MDMs compared to the control group (Fig. 6). Thus, these data provide evidence that both WGP and PGG inhibit cell association of M. bovis BCG with human macrophages, providing another explanation for the decreased bacterial CFUs (Fig. 4) seen in macrophages following phagocytosis.
Fig. 6.
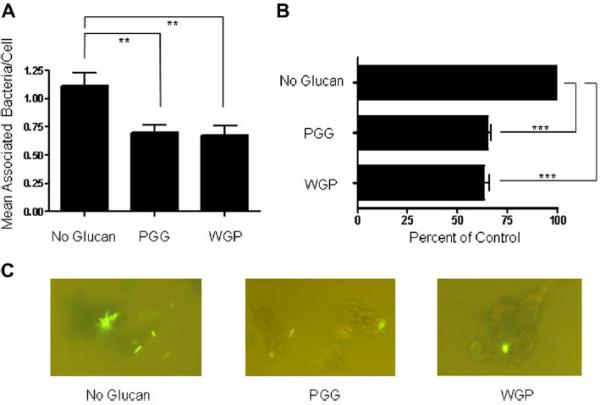
PGG and WGP inhibit cellular association of M. bovis BCG with human macrophages. MDMs were adhered to coverslips in a 24-well tissue culture plate (2 × 105 cells) and incubated at 37 °C/5% CO2 in the absence or presence of Imprime PGG™ (50 μg/ml) or WGP (50 μg/ml). MDMs were then infected with 2 × 106 M bovis BCG (MOI = 10:1) at 37 °C/5% CO2 for 2 h. The infection was stopped, and the cells were fixed and bacteria stained with Auramine-Rhodamine. Cell-associated bacteria were counted under a fluorescence microscope. A) Mean Associated Bacteria/Cell: β-glucan stimulation caused significantly less cellular association of M. bovis BCG with human macrophages compared to the no-glucan control (**p < 0.005). B) Results from A expressed in percentage values with no-glucan control set at 100%. Compared to the no-glucan control, PGG and WGP significantly reduced bacterial association with MDMs (***p < 0.0005). C) Representative fluorescence microscopy images showing macrophage-associated (both adhered and internalized) M. bovis BCG bacteria. Data shown in A and B represent cumulative values from three experiments, each performed in triplicate.
2.6. WGP, but not PGG, generates a macrophage respiratory burst and augments the respiratory burst to PMA and opsonized zymosan
We next determined whether β-glucans regulate the respiratory burst of human macrophages. We used the 2′,7′-dichloro-fluorescein diacetate (DCF) assay in order to measure the production of H202 -dependent ROIs [21]. Fluorescence, indicative of ROI production, was measured every 2 min for 90 min. As shown in Fig. 7A, WGP addition to MDMs alone induced ROI production. At 60 min (a median time point), WGP-induced significantly higher relative fluorescence units (RFUs) in MDMs compared to untreated control (761.6 ± 31.4 with WGP versus 507.2 ± 20.4 without glucan). Phorbol 12-myristate 13-acetate (PMA) was used as a positive control (Fig. 7B).
Fig. 7.
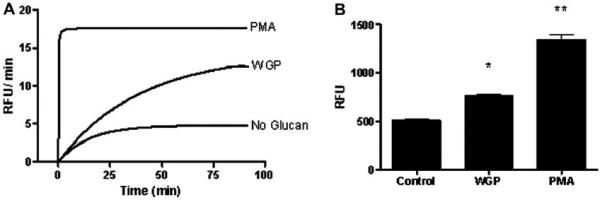
WGP induces a respiratory burst from human macrophages. MDMs were adhered to 96-well plates (~6 × 104 MDMs/well) in 10% autologous serum and repleted with DPBS + 32 mM DCF for 30 min at 37 °C, 5% CO2. Cells were then stimulated with WGP (25 μg/ml), PMA (200 nM or 0.123 μg/ml), or medium control only. Relative fluorescence, indicative of ROI production, was measured every 2 min for 90 min. A) Rate of ROI production over time in mean relative fluorescence units (RFUs) per minute, from triplicate wells. Shown is a representative experiment (n = 3). B) ROI values at 60 min (triplicate wells in each test group) in response to the indicated stimuli, expressed in RFUs. PMA- and WGP-stimulated cells produced significantly higher amounts of ROIs compared to unstimulated cell controls (*p < 0.05; **p < 0.005).
Next, we determined if WGP could prime macrophages for an enhanced response to known stimuli of the NADPH oxidase, specifically opsonized zymosan, a ligand for CR3 and Dectin-1, and PMA. Opsonized zymosan is a β-glucan-containing compound which uses a receptor-dependent phagocytic process to induce ROI production, while PMA uses a receptor-independent pathway through activation of Protein Kinase C (PKC). ROI production from MDMs treated with WGP and then stimulated with opsonized zymosan showed no difference in RFU compared to WGP pre-treatment alone (Fig. 8A). In contrast, treatment of macrophages with WGP and then stimulation with PMA did increase ROI production compared to PMA alone at early time points (Fig. 8B). WGP treatment alone induced ROI production at early time points that was greater than PMA alone. At later time points, WGP pre-treatment followed by PMA, and PMA alone generated high ROI production that was greater than WGP alone (Fig. 8B).
Fig. 8.
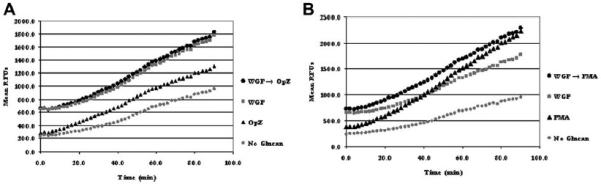
WGP pre-treatment does not further increase ROI production with opsonized zymosan, but does further increase ROI production upon PMA stimulation. MDMs were adhered to 96-well plates (~6 × 104 MDMs/well) in 10% autologous serum. MDM monolayers were repleted with RHH medium containing WGP (25 μg/ml) and incubated at 37 °C/5% CO2 for 30 min. WGP was washed away with DPBS, and the monolayer repleted with DPBS + 32 mM DCF, and incubated for 30 min at 37 °C, 5% CO2. MDMs were then stimulated with opsonized zymosan particles (OpZ; MOI 12:1) or PMA (200 nM or 0.123 μg/ml), or no treatment (control). Relative fluorescence, indicative of ROI production, was measured every 2 min for 90 min. A) RFU profile obtained from MDMs stimulated with either opsonized zymosan or WGP alone as well as from WGP pre-treated MDMs stimulated with opsonized zymosan. B). RFU profile obtained from MDMs stimulated with either PMA or WGP alone as well as from WGP pre-treated MDMs stimulated with PMA. Graphs show the mean values of RFUs from triplicate wells from a representative experiment (n = 2).
In similar experiments described above, PGG alone did not stimulate an increased respiratory burst compared to the untreated control group (Fig. 9A). In addition, PGG pre-treatment did not significantly alter the respiratory burst of MDMs stimulated with opsonized zymosan (Fig. 9A) or PMA (Fig. 9B).
Fig. 9.
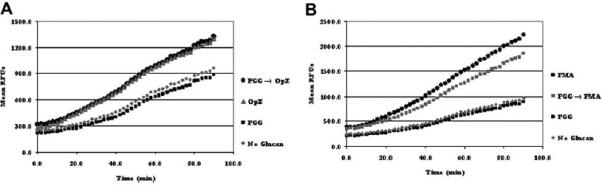
PGG pre-treatment does not induce a respiratory burst in human macrophages. The experiment was performed as described in the legend of Fig. 7, except that MDM monolayers were repleted with RHH medium containing PGG (50 μg/ml). Graphs show the mean values of RFUs from triplicate wells from a representative experiment (n = 3). A) RFU profile obtained from MDMs stimulated with either opsonized zymosan or PGG alone as well as from PGG pre-treated MDMs stimulated with opsonized zymosan. B) RFU profile obtained from MDMs stimulated with either PMA or PGG alone as well as from PGG pre-treated MDMs stimulated with PMA.
2.7. Generation of the macrophage respiratory burst by WGP is mediated through the Dectin-1 pathway
In order to determine whether Dectin-1 is involved in the WGP-induced respiratory burst by macrophages, we blocked Dectin-1 on MDMs by pre-incubating them with receptor-specific antibody and measured ROI production over a 90 min period (Fig. 10A). The respiratory burst, expressed in RFUs, increased over time with PMA (positive control) but not media control. The end-point data at 90 min (Fig. 10B) shows that prior blocking of Dectin-1 with specific antibody significantly reduces the respiratory burst by MDMs upon exposure to WGP compared to controls (media only and isotypic control antibody, p < 0.05 and <0.005, respectively). These results indicate that WGP induces a respiratory burst in macrophages through its binding to and activation of Dectin-1.
Fig. 10.
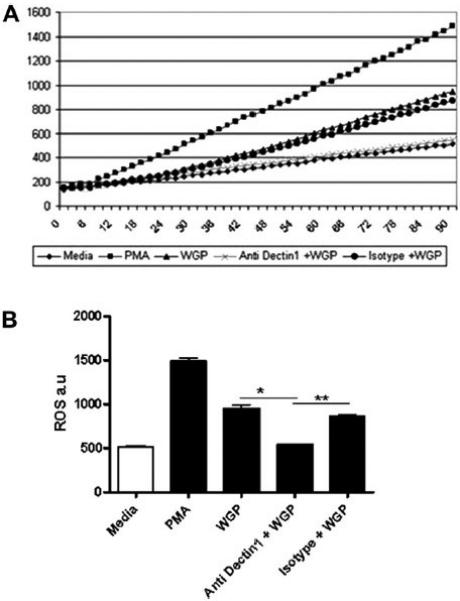
Induction of the macrophage respiratory burst by WGP is mediated by Dectin-1. The experiment was performed as described in the legend of Fig. 7, except that MDM monolayers were pre-treated with anti-Dectin-1 or isotype control antibody before the addition of DCF followed by WGP. Graphs show the mean values of RFUs from triplicate wells from a representative experiment (n = 2). A) RFU profile over 90 min period obtained from MDMs pre-treated with anti-dectin-1 or isotype control antibody and stimulated with WGP or media control. B) Bar graph shows the end-point ROI values at 90 min plotted from mean RFUs in graph A. Antibody blocking of Dectin-1 significantly reduced ROI production in response to WGP (*p < 0.05; **p < 0.005).
2.8. β-Glucan (WGP) induces the production of pro-inflammatory cytokines in human macrophages
As another possible mechanism of mycobacterial growth inhibition within macrophages, we sought to determine the levels of pro-inflammatory cytokines such as IL-1β, TNF-α and IL-6 from MDMs when stimulated by WGP. Both TNF-α and IL-6 were produced by WGP-stimulated cells to a significantly higher level than unstimulated or resting cells (*p < 0.05 and **p < 0.005, respectively) (Fig. 11). Levels of TNF-α (Fig. 11A) and IL-6 (Fig. 11B) were increased both in a time (6 and 24 h)- and dose (25 and 50 mg WGP/ml)-dependent fashion. Production of IL-1β was negligible and inconsistent (data not shown).
Fig. 11.
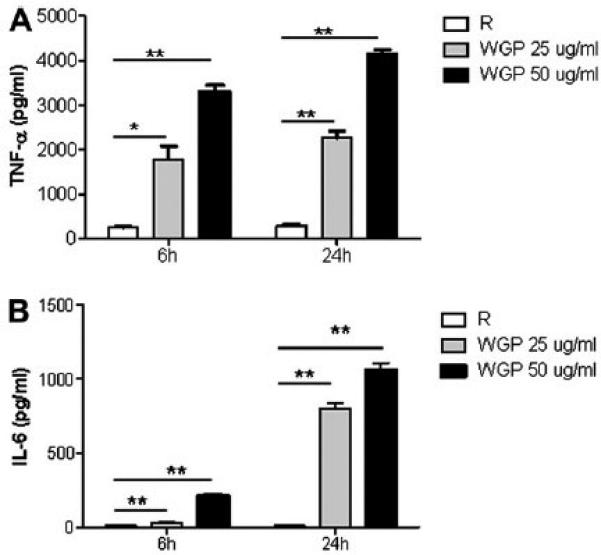
WGP induces pro-inflammatory cytokine production in human macrophages. MDMs were adhered to 24-well tissue culture plates (4 × 105 cells/well) and left overnight at 37 °C in RPMI containing human serum. MDM monolayers were then stimulated with either 25 ug or 50 ug/ml of WGP for the designated time periods. Culture supernatants were analyzed for cytokines by ELISA. Bar graphs show the levels of production of TNF-α (A) and IL-6 (B) by MDMs upon exposure to WGP. MDMs without WGP treatment were used as the resting control (R). Both cytokines were produced in significant amounts by β-glucan-treated cells compared to resting control. Shown is a representative experiment (n = 2, each performed in triplicate).*p < 0.05; **p < 0.005.
3. Discussion and conclusions
Recent studies with 1,3/1,6-β-d-glucan compounds have shown promising results to indicate that they may be able to combat various microbial infections [5,22–25], yet nearly all of these studies have been performed in animal models. Here, we studied the effects of both soluble and particulate forms of yeast-derived bglucans, PGG and WGP, on mycobacterial infection of human macrophages. To our knowledge this study is the first to test the effects of β-glucans on mycobacterial survival in human macrophages, and the use of human macrophages is crucial in the testing and validation of β-glucans as possible therapeutic agents. Our results show that treatment of macrophages with both PGG and WGP prior to infection significantly decreases the intracellular survival of M. bovis BCG when compared to controls (Fig. 4A and B) and also decreases cellular association of the bacteria with macrophages (Fig. 6). The decreased survival of M. bovis BCG in macrophages in response to β-glucan treatment appears to be mediated by Dectin-1 (Fig. 4C).
In contrast to the results with M. bovis BCG, neither WGP nor PGG affects the intracellular growth of virulent M.tb (Fig. 5). Thus, the differential effects of the β-glucans on M. bovis BCG and M.tb growth in human macrophages suggest fundamentally different pathogenic properties for these two slow-growing mycobacterial species.
Our results are consistent with the only other study that has analyzed the effects of β-glucans on M. bovis BCG infection. Hetland and colleagues showed in in vivo experiments with BALB/c mice that β-glucan treatment before and after infection with M. bovis BCG decreased the bacterial burden weeks after infection [7]. They also investigated whether particulate or soluble β-glucan had a protective effect against M.tb infection in murine peritoneal macrophages. They found that treatment of macrophages with the particulate, but not the soluble form of β-glucan during infection decreased intracellular survival of M.tb H37Rv in murine macrophages. Despite our different results with M.tb in human macrophages, our studies agree that the particulate β-glucan, WGP, induces greater protection against mycobacterial infection than the soluble β-glucan, PGG. Because both β-glucans in our study were able to produce a protective effect against M. bovis BCG, but were unable to provide an anti-microbial response against M.tb, our model indicates a unique mechanism or intracellular niche that allows virulent mycobacteria to survive within human macrophages.
The known β-glucan receptors on leukocytes are CR3 and Dectin-1. Previous studies have shown that β-glucans bind to CR3 in a non-opsonic manner and stimulate cytotoxic activation of C3bi-opsonized tumor cells [9], while production of inflammatory mediators, including cytokines and ROIs, is dependent on interaction and signaling through the surface receptor Dectin-1 [26,27]. Other studies indicate that WGP induces TNF-α and radical production in macrophages, while PGG likely interacts with the same receptors as WGP, but is unable to elicit either ROIs or proinflammatory cytokines [27,28]. Our data from several experiments support these results (Figs. 8,9 and 11A). Furthermore, priming with WGP and stimulation with opsonized zymosan did not further increase ROI production (Fig. 8A) which suggests that WGP utilizes a similar receptor-mediated process used by the zymosan particle (either CR3 or most probably Dectin-1) to induce ROIs. In fact, we found that WGP induces ROI production by using Dectin-1 pathway (Fig. 10). PGG itself did not generate an oxidative burst and pre-treatment did not alter the level of ROI production by opsonized zymosan (Fig. 9A). These results suggest a decreased ability of PGG to bind to Dectin-1 on human macrophages or the possibility that PGG, as a soluble ligand, enters the cell through pinocytosis rather than phagocytosis, thus engaging fewer receptors in the process. Our data are consistent with results from two recent studies. One study showed that yeast-derived soluble β-glucan binds to dendritic cells and macrophages independently of Dectin-1 [29]. Another study showed that, despite its ability to bind to both soluble and particulate β-glucans, Dectin-1 signaling is only activated by the particulate form, which is capable of clustering the receptor [30].
Our intra-macrophage survival studies indicate that WGP induces relatively greater protection and anti-microbial effect than PGG against M. bovis BCG infection (Fig. 4A and B), while both WGP and PGG inhibited M. bovis BCG cell association with MDMs equally (Fig. 6). We observed that WGP-induced growth inhibition of M. bovis BCG was, at least partially, mediated by Dectin-1 (Fig. 4C). Therefore, we believe that the intracellular growth inhibition of M. bovis BCG by WGP may have been due to the macrophage production of ROIs (Figs. 7 and 8) and pro-inflammatory cytokines (Fig. 11) through Dectin-1, in combination with competitive inhibition of phagocytosis (Fig. 6), through CR3. In contrast, PGG, which lacks the ability to induce ROI production, inhibited infection of M. bovis BCG mainly through the competitive blocking of CR3; however, we cannot rule out its ability to regulate other inflammatory mediators.
The study by Yadav and Schorey indicates that a ligand for macrophage Dectin-1 is present on M. bovis BCG, but not on virulent M.tb species [18]. The same study also demonstrated that immune responses to macrophage infection with M. bovis BCG are partially dependent on Dectin-1 activation, while responses to infection with virulent strains of M.tb are not. These findings support our results that M.tb survival is not affected by β-glucan compounds (Fig. 5) and that M.tb is not as susceptible as M. bovis BCG to macrophage microbicidal mechanisms induced through Dectin-1 (Fig. 4). In contrast to its effects on macrophages, more recent studies indicate an anti-mycobacterial effect of Dectin-1 present on dendritic cells through inducing protective immune responses [31,32].
β-glucan signaling through Dectin-1, which is at least partially dependent on TLR2, is known to induce spleen tyrosine kinase (Syk) and PKC leading to induction of NF-kB and a proinflammatory response [33]. In our model, Dectin-1 activation through this pathway is the most likely mechanism for the production of ROIs and pro-inflammatory cytokines in response to WGP (Figs. 10 and 11, respectively). Despite pre-treatment of macrophages with β-glucan and proposed activation of the Dectin-1 signaling pathway, M.tb is able to evade the host immune response and survive within macrophages. This variation between M. bovis BCG and M.tb pathogenesis suggests that M.tb infection is able to inhibit signaling downstream of Dectin-1, potentially by preventing induction of NF-kB and pro-inflammatory responses.
In summary, the results of this study provide evidence for anti-mycobacterial effects of specific β-glucans on M. bovis BCG infection of human macrophages, likely due to the ability of WGP to induce ROIs and pro-inflammatory cytokines through Dectin-1 combined with competitive inhibition of CR3, while PGG's effects are mediated mostly through CR3 inhibition. These biological processes did not prove sufficient to inhibit the growth of virulent M.tb in macrophages indicating unique mechanisms for immune evasion by M.tb.
4. Materials and methods
4.1. Reagents
β-glucan compounds, insoluble whole glucan particle (WGP), a 1,3/1,6-β-glucan purified from S. cerevisiae and soluble poly-1-6-β-d-glucopyranosyl-1-3-β-d-glucopyranose (PGG), formed by acid hydrolysis of WGP, were supplied by Biothera (Eagan, MN). Zymosan A particles from S. cerevisiae (Molecular Probes, Eugene, OR) were opsonized in 50% human serum for 30 min at 37 °C and then washed at 4 °C prior to use (34)].
4.2. Cell preparation
Human peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood from healthy donors (using a protocol approved by the OSU IRB) on Ficoll-Hypaque (Amersham, Piscataway, NJ) gradient and cultured in Teflon wells (Savillex, Minnetonka, MN) in RPMI 1640 + 20% autologous serum for 5 days at 37 °C, 5% CO2 [35]. Monocytes differentiated into macrophages (MDMs) by day 5. MDMs were isolated from cultured PBMCs by adhering the MDMs to tissue culture plates (BD Falcon, Bedford, MA) for 2–3 h at 37 °C/5% CO2 in 10% autologous serum. Lymphocytes were then washed away and MDM monolayers were repleted with either DPBS-HHG (Dulbecco's PBS + 10 mM HEPES + 1 mg/ml human serum albumin + 0.1% glucose) or RHH (RPMI + 10 mM HEPES + 1 mg/ml human serum albumin) medium as needed for the specific experiment.
4.3. β-Glucan toxicity (macrophage cell death) assays
MDM monolayers in a 24-well tissue culture plate at Day 5 were repleted with 20% serum-containing RPMI and incubated for 7 more days to ensure stable adherence of cells [36]. At Day 12, the macrophage monolayers in triplicate were exposed to different concentrations of PGG or WGP in RPMI containing 1–2% serum. The morphology of the MDM monolayer was checked daily for a period of five days under an inverted microscope and pictures taken to examine the intactness of the monolayer in the presence of the β-glucan compound. MDM viability was assessed by using 0.4%
Trypan Blue Exclusion Staining (Sigma) at the end of the 5-day period. MDM cell death induced by WGP was also evaluated by using the LIVE/DEAD Fixable Dead Cell Stain Kit from Invitrogen. After exposure to WGP, MDM monolayers were stained with aqua fluorescent reactive dye according to the manufacturer's instructions. Cell death was scored by counting ≥ 150 cells by fluorescence microscopy.
4.4. Mycobacterial strains and media
M. bovis BCG or M.tb (Erdman strain, ATCC #25618) was plated onto 7H11 agar (Becton, Dickinson and Company) plates. The plates were incubated at 37 °C/5% CO2 for 9–14 days to allow for bacterial lawn formation. These bacterial lawns were used to prepare bacterial single cell suspensions for MDM infections asdescribed [19]. Dilutions were made of the final suspension and bacterial cells were counted by using a Petroff-Hausser counting chamber (Hausser Scientific, Hor-sham, PA). The bacterial suspension was adjusted in RHH to a concentration of 4 × 107 bacteria/ml for the cell association assay and 4 × 106 bacteria/ml for the bacterial survival assay.
4.5. Cell association assay
A bacterial cell association assay was performed as described [19]. Monolayers of 5 day-old MDMs on coverslips in a 24-well tissue (2 × 105 MDMs/well) culture plate were washed and repleted with RHH containing 50 μg/ml of PGG or 25 μg/ml of WGP and incubated for 30 min at 37 °C/5% CO2. A M. bovis BCG single cell suspension (2 × 106 bacteria/well) was added to each well (MOI = 10:1 bacteria per cell) and incubated with shaking for 30 min at 37 °C/5% CO2, followed by an additional incubation without shaking for 90 min at 37 °C/5% CO2. The infected mono-layer was washed and fixed with 10% formalin (Sigma, St. Louis, MO) in PBS buffer. The monolayer was then washed and stained with Auramine-Rhodamine (Becton, Dickinson and Company, Sparks, MD). The coverslips containing MDM monolayers pre-treated with either PGG or WGP or the no-β-glucan control were used in triplicate for each test group. In each group, the MDM-associated M. bovis BCG bacterial cells were counted (≥300 MDMs) under a fluorescence microscope (Olympus BX51) and reported as average number of bacteria/macrophage.
4.6. M. bovis BCG and M.tb survival assays within human macrophages treated with β-glucan before and after infection
Triplicate monolayers of 12 day-old MDMs in a 24-well tissue culture plate were washed, repleted with RHH containing 50–100 μg/ml of PGG or 25–50 μg/ml of WGP and incubated for 30 min at 37 °C/5% CO2. Then, a bacterial single cell suspension (2 × 105 bacteria/well) in RHH was added to each well (MOI = 1:1) and incubated with shaking for 30 min at 37 °C/5% CO2, followed by an additional incubation without shaking for 90 min at 37 °C/5% CO2 (total 2 h) [37]. Monolayers were washed, repleted in RPMI containing 2.0% autologous serum and incubated at 37 °C/5% CO2. At 24 h and 72 h post-infection, PGG (50–100 μg/ml) or WGP (25–50 μg/ml) was added again to corresponding wells. In certain experiments, MDM monolayers were incubated with 10 μg/ml of either anti-Dectin-1 antibody (R & D Systems) or isotype control IgG2B antibody at 37 °C/5% CO2 before adding 25 μg/ml WGP to the monolayer. At different time periods (24, 48 and 72 h), cells were lysed and lysates plated on 7H11 agar for scoring CFUs at ~21 days as described [36]. CFUs were determined for each group and the mean data of the triplicate values were used to perform a two-tailed t-test to assess the significance of the results between test groups.
4.7. DCF assay for detecting the respiratory burst from human macrophages
The DCF assay was performed essentially as described previously [34]. Briefly, MDMs were adhered to 96-well plates at 3 × 106 PBMCs/ml (~6 × 104 MDMs/well) in 10% autologous serum. The monolayers were repleted with DPBS + 32 mM DCF and incubated for 30 min at 37 °C/5% CO2. Then, either PGG (50 μg/ml) or WGP (25 μg/ml) was added to the monolayers. Known stimuli, either opsonized zymosan particles (MOI = 12:1) or PMA (200 nM or 0.123 μg/ml) were used as positive controls, whereas, monolayers not receiving stimulant treatment were used as negative controls. Macrophage ROI production was also measured in response to bglucans with or without subsequent PMA or opsonized zymosan stimulation. In certain experiments, MDMs were pre-treated with either anti-Dectin-1 antibody or isotype control IgG2B antibody (10 μg/ml in each case) for 30 min before addition of DCF and stimuli. Relative fluorescence, indicative of ROI production, was measured every 2 min for 90 min using a SpectraMax plate reader (Molecular Devices). Test conditions were measured in triplicate wells, and RFUs calculated.
4.8. Cytokine assays
Five day-old MDM monolayers in 24-well tissue culture plates (4 × 105 cells/well) were incubated overnight at 37 °C in RPMI medium containing 10% human autologous serum. Monolayers were then washed with RPMI and re-suspended in RHH medium to which either 25 ug or 50 ug/ml of WGP was added and incubated at 37 °C for 6 and 24 h. Cell culture supernatants were harvested after designated time periods, and analyzed for TNF-α, IL-6 and IL-1β levels by ELISA (R &D Systems, Minneapolis, MN, USA).
4.9. Statistics
For all statistical analysis, two-tailed t-tests were performed using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA) to determine the significance of difference between test and control groups.
Acknowledgments
We would like to thank Jordi Torrelles, Ph.D. (The Ohio State University) for his help with cell association experiments and insight. Also, Heather Curry, Tracy Carlson, Michelle Brooks, and Hsiang-Ming Wang, Ph.D. for their help with work in the BSL-3 laboratory. We would also like to thank Biothera (Eagan, MN) for their financial support and for supplying β-glucan compounds for this study.
References
- 1.LeBlanc BW, Albina JE, Reichner JS. The effect of PGG-beta-glucan on neutrophil chemotaxis in vivo. J Leukoc Biol. 2006;79(4):667–75. doi: 10.1189/jlb.0305150. [DOI] [PubMed] [Google Scholar]
- 2.Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009;230(1):38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harada T, Kawaminami H, Miura NN, Adachi Y, Nakajima M, Yadomae T, et al. Mechanism of enhanced hematopoietic response by soluble beta-glucan SCG in cyclophosphamide-treated mice. Microbiol Immunol. 2006;50(9):687–700. doi: 10.1111/j.1348-0421.2006.tb03841.x. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Li DF, Xing JJ, Cheng ZB, Lai CH. Effects of beta-glucan extracted from Saccharomyces cerevisiae on growth performance, and immunological and somatotropic responses of pigs challenged with Escherichia coli lipopolysaccharide. J Anim Sci. 2006;84(9):2374–81. doi: 10.2527/jas.2004-541. [DOI] [PubMed] [Google Scholar]
- 5.Hetland G, Ohno N, Aaberge IS, Lovik M. Protective effect of beta-glucan against systemic Streptococcus pneumoniae infection in mice. FEMS Immunol Med Microbiol. 2000;27(2):111–6. doi: 10.1111/j.1574-695X.2000.tb01420.x. [DOI] [PubMed] [Google Scholar]
- 6.Liang J, Melican D, Cafro L, Palace G, Fisette L, Armstrong R, et al. Enhanced clearance of a multiple antibiotic resistant Staphylococcus aureus in rats treated with PGG-glucan is associated with increased leukocyte counts and increased neutrophil oxidative burst activity. Int J Immunopharmacol. 1998;20(11):595–614. doi: 10.1016/s0192-0561(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 7.Hetland G, Lovik M, Wiker HG. Protective effect of beta-glucan against mycobacterium bovis, BCG infection in BALB/c mice. Scand J Immunol. 1998;47(6):548–53. doi: 10.1046/j.1365-3083.1998.00350.x. [DOI] [PubMed] [Google Scholar]
- 8.Hetland G, Sandven P. beta-1,3-Glucan reduces growth of Mycobacterium tuberculosis in macrophage cultures. FEMS Immunol Med Microbiol. 2002;33(1):41–5. doi: 10.1111/j.1574-695X.2002.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 9.Xia Y, Vetvicka V, Yan J, Hanikyrova M, Mayadas T, Ross GD. The β-glucanginding lectin site of mouse cr3 (cd11b/cd18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to ic3b-opsonized target cells1. J.Immunol. 1999;162:2281–90. [PubMed] [Google Scholar]
- 10.Tsoni SV, Brown GD. beta-Glucans and dectin-1. Ann N Y Acad Sci. 2008;1143:45–60. doi: 10.1196/annals.1443.019. [DOI] [PubMed] [Google Scholar]
- 11.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, et al. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169(7):3876–82. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 12.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197(9):1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid DM, Gow NA, Brown DG. Pattern recognition: recent insights from Dectin-1. Curr Opin Microbiol. 2009;21:30–7. doi: 10.1016/j.coi.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kankkunen P, Teirila L, Rintahaka J, Alenius H, Wolff H, Matikainen S. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J Immunol. 2010;184(11):6335–42. doi: 10.4049/jimmunol.0903019. [DOI] [PubMed] [Google Scholar]
- 15.Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7(5):355–66. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson JS, Martin JL, Azad AK, McCarthy TR, Kang PB, Voelker DR, et al. Surfactant protein D increases fusion of Mycobacterium tuberculosis-containing phagosomes with lysosomes in human macrophages. Infect Immun. 2006;74(12):7005–9. doi: 10.1128/IAI.01402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenton MJ, Riley LW, Schlesinger LS. Receptor-Mediated recognition of Mycobacterium tuberculosis by host cells. In: Cole ST, Eisenach KD, McMurray DN, Jacobs WR Jr, editors. Tuberculosis and the Tubercle Bacillus. ASM Press; New York: 2005. pp. 405–26. [Google Scholar]
- 18.Yadav M, Schorey JS. The {beta}-glucan receptor Dectin-1 fucntions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–75. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–30. [PubMed] [Google Scholar]
- 20.Kang BK, Azad AK, Torrelles JB, Kaufman TM, Beharka AA, Tibesar E, et al. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202(7):987–99. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hempel SL, Buettner GR, O'Malley YQ, Wessels DA, Flaherty DM. Dihydro-fluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2’,7’-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2’,7’-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med. 1999;27(1–2):146–59. doi: 10.1016/s0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 22.Harnack U, Eckert K, Fichtner I, Pecher G. Oral administration of a soluble 1-3, 1-6 beta-glucan during prophylactic survivin peptide vaccination diminishes growth of a B cell lymphoma in mice. Int Immunopharmacol. 2009;9(11):1298–303. doi: 10.1016/j.intimp.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Kimura Y, Sumiyoshi M, Suzuki T, Sakanaka M. Antitumor and antimetastatic activity of a novel water-soluble low molecular weight beta-1, 3-D-glucan (branch beta-1,6) isolated from Aureobasidium pullulans 1A1 strain black yeast. Anticancer Res. 2006;26(6B):4131–41. [PubMed] [Google Scholar]
- 24.Yan J, Allendorf DJ, Brandley B. Yeast whole glucan particle (WGP) beta-glucan in conjunction with antitumour monoclonal antibodies to treat cancer. Expert Opin Biol Ther. 2005;5(5):691–702. doi: 10.1517/14712598.5.5.691. [DOI] [PubMed] [Google Scholar]
- 25.Stuyven E, Cox E, Vancaeneghem S, Arnouts S, Deprez P, Goddeeris BM. Effect of beta-glucans on an ETEC infection in piglets. Vet Immunol Immunopathol. 2009;128(1–3):60–6. doi: 10.1016/j.vetimm.2008.10.311. [DOI] [PubMed] [Google Scholar]
- 26.Shah VB, Huang Y, Keshwara R, Ozment-Skelton T, Williams DL, Keshvara L. Beta-glucan activates microglia without inducing cytokine production in Dectin-1-dependent manner. J Immunol. 2008;180(5):2777–85. doi: 10.4049/jimmunol.180.5.2777. [DOI] [PubMed] [Google Scholar]
- 27.Li B, Cramer D, Wagner S, Hansen R, King C, Kakar S, et al. Yeast glucan particles activate murine resident macrophages to secrete proinflammatory cytokines via MyD88- and Syk kinase-dependent pathways. Clin Immunol. 2007;124(2):170–81. doi: 10.1016/j.clim.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalek M, Melican D, Brunke-Reese D, Langevin M, Lemerise K, Galbraith W, et al. Activation of rat macrophages by Betafectin PGG-glucan requires cross-linking of membrane receptors distinct from complement receptor three (CR3). J Leukoc Biol. 1998;64(3):337–44. doi: 10.1002/jlb.64.3.337. [DOI] [PubMed] [Google Scholar]
- 29.Qi C, Cai Y, Gunn L, Ding C, Li B, Kloecker G, et al. Differential pathways regulating innate and adaptive anti-tumor immune responses by particulate and soluble yeast-derived {beta}-glucans. Blood. 2011 doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472(7344):471–5. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ni L, Gayet I, Zurawski S, Duluc D, Flamar AL, Li XH, et al. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J Immunol. 2010;185(6):3504–13. doi: 10.4049/jimmunol.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zenaro E, Donini M, Dusi S. Induction of Th1/Th17 immune response by Mycobacterium tuberculosis: role of dectin-1, Mannose Receptor, and DC-SIGN. J Leukoc Biol. 2009;86(6):1393–401. doi: 10.1189/jlb.0409242. [DOI] [PubMed] [Google Scholar]
- 33.Schorey JS, Lawrence C. The pattern recognition receptor Dectin-1: from fungi to mycobacteria. Curr Drug Targets. 2008;9(2):123–9. doi: 10.2174/138945008783502430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowther JE, Kutala VK, Kuppusamy P, Ferguson JS, Beharka AA, Zweier JL, et al. Pulmonary surfactant protein a inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J Immunol. 2004;172(11):6866–74. doi: 10.4049/jimmunol.172.11.6866. [DOI] [PubMed] [Google Scholar]
- 35.Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol. 2002;169:3565–73. doi: 10.4049/jimmunol.169.7.3565. [DOI] [PubMed] [Google Scholar]
- 36.Olakanmi O, Britigan BE, Schlesinger LS. Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect Immun. 2000;68(10):5619–27. doi: 10.1128/iai.68.10.5619-5627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–80. [PubMed] [Google Scholar]


