Abstract
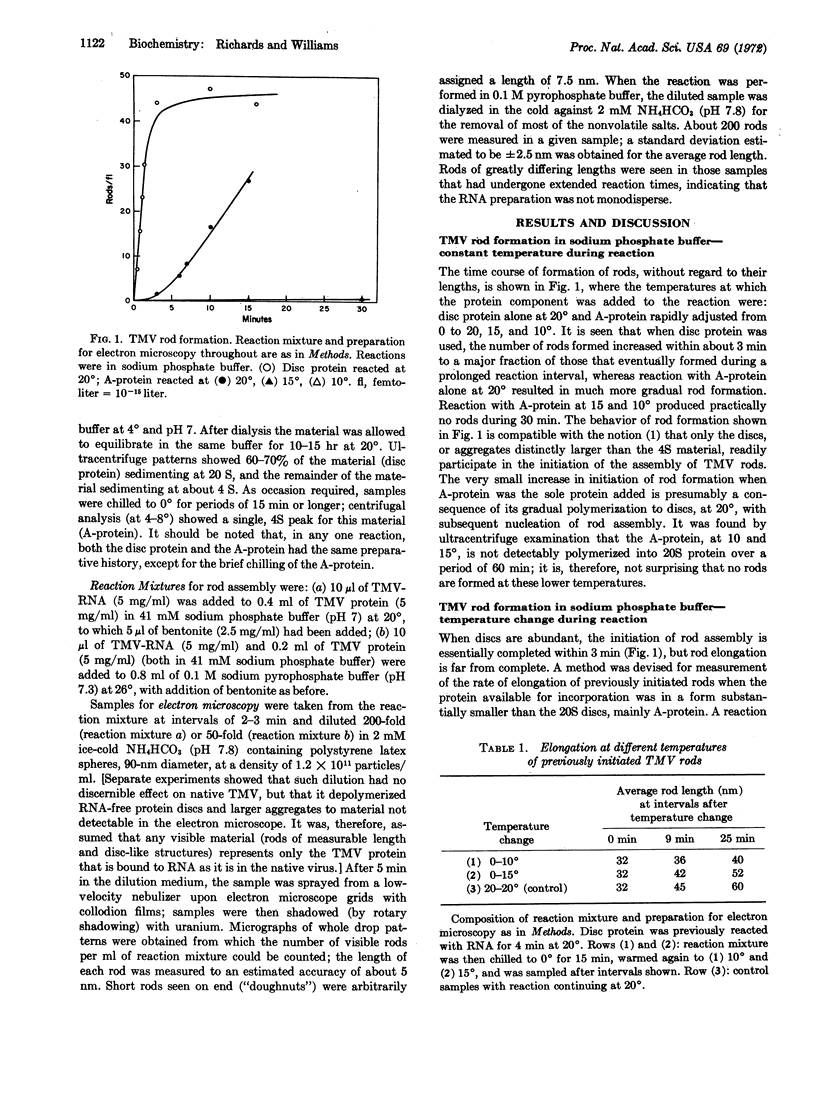
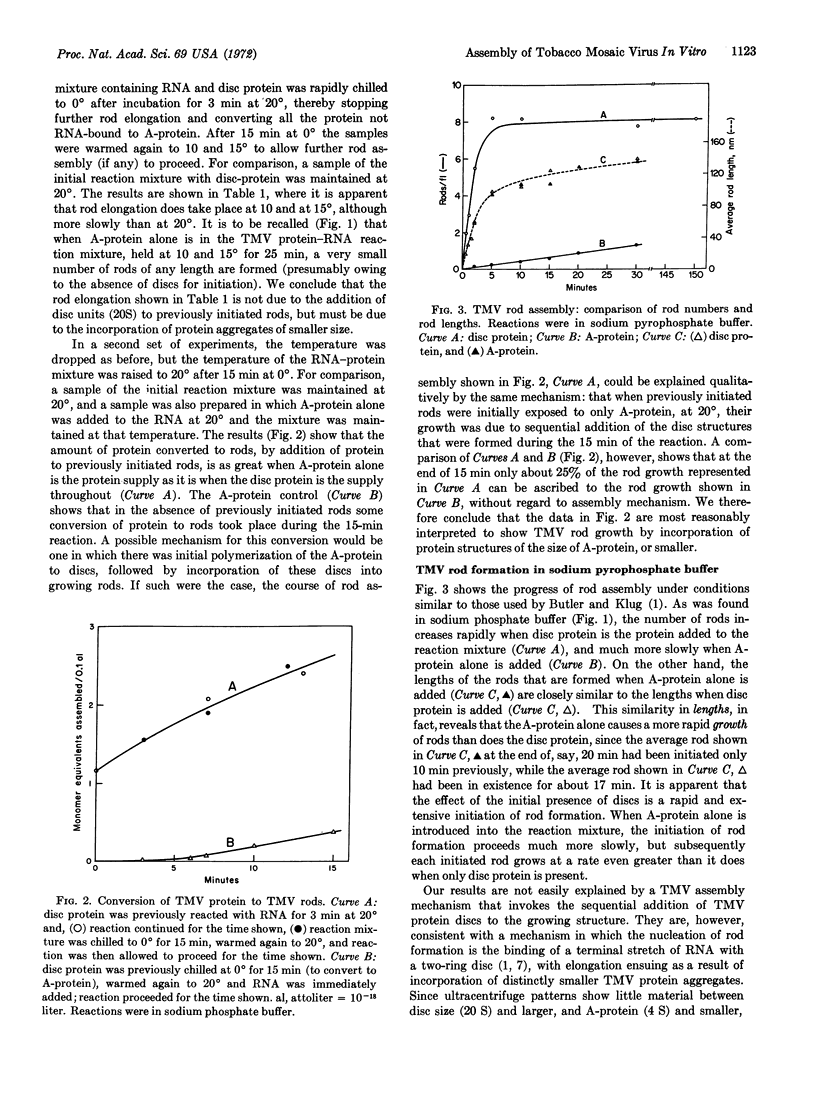
The in vitro assembly of tobacco mosaic virus from its constituent RNA and protein was followed by methods of electron microscopy. The effect of the state of polymerization of the protein upon the initiation of assembly of tobacco mosaic virus rods, and the subsequent rod elongation, was investigated. Protein in two identifiable states of polymerization was used: the 20S “disc”, consisting of 34 monomers arrayed as a two-ring structure, and the 4S “A-protein”, consisting of polymers in the trimer range of size. It is concluded, in confirmation of results of others, that rod assembly is initiated by the attachment of one end of the RNA chain of tobacco mosaic virus to one (or possibly a few) disc structure. Rod elongation, on the other hand, is found to take place by the sequential addition of structures of the size of A-protein, or smaller, to the previously initiated rods.
Keywords: quantitative electron microscopy, RNA-protein interactions
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler P. J., Klug A. Assembly of the particle of tobacco mosaic virus from RNA and disks of protein. Nat New Biol. 1971 Jan 13;229(2):47–50. doi: 10.1038/newbio229047a0. [DOI] [PubMed] [Google Scholar]
- Durham A. C., Finch J. T., Klug A. States of aggregation of tobacco mosaic virus protein. Nat New Biol. 1971 Jan 13;229(2):37–42. doi: 10.1038/newbio229037a0. [DOI] [PubMed] [Google Scholar]
- Durham A. C., Klug A. Polymerization of tobacco mosaic virus protein and its control. Nat New Biol. 1971 Jan 13;229(2):42–46. doi: 10.1038/newbio229042a0. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]


