Abstract
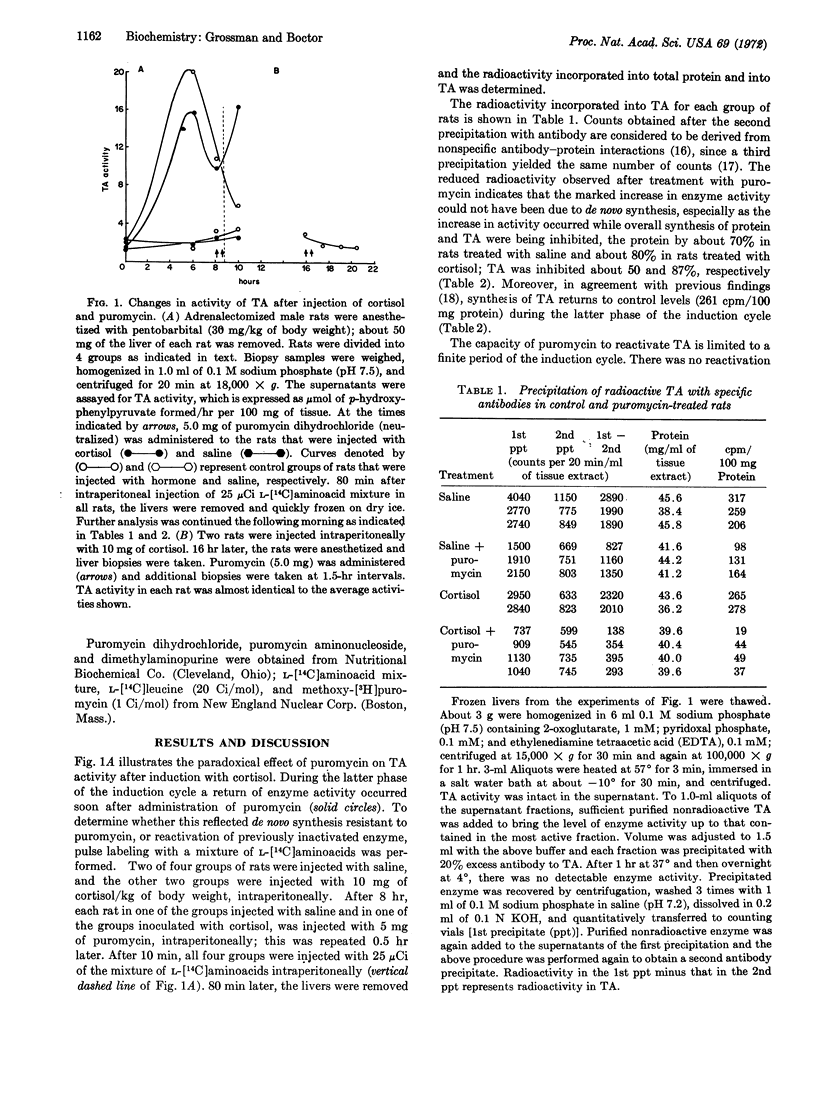
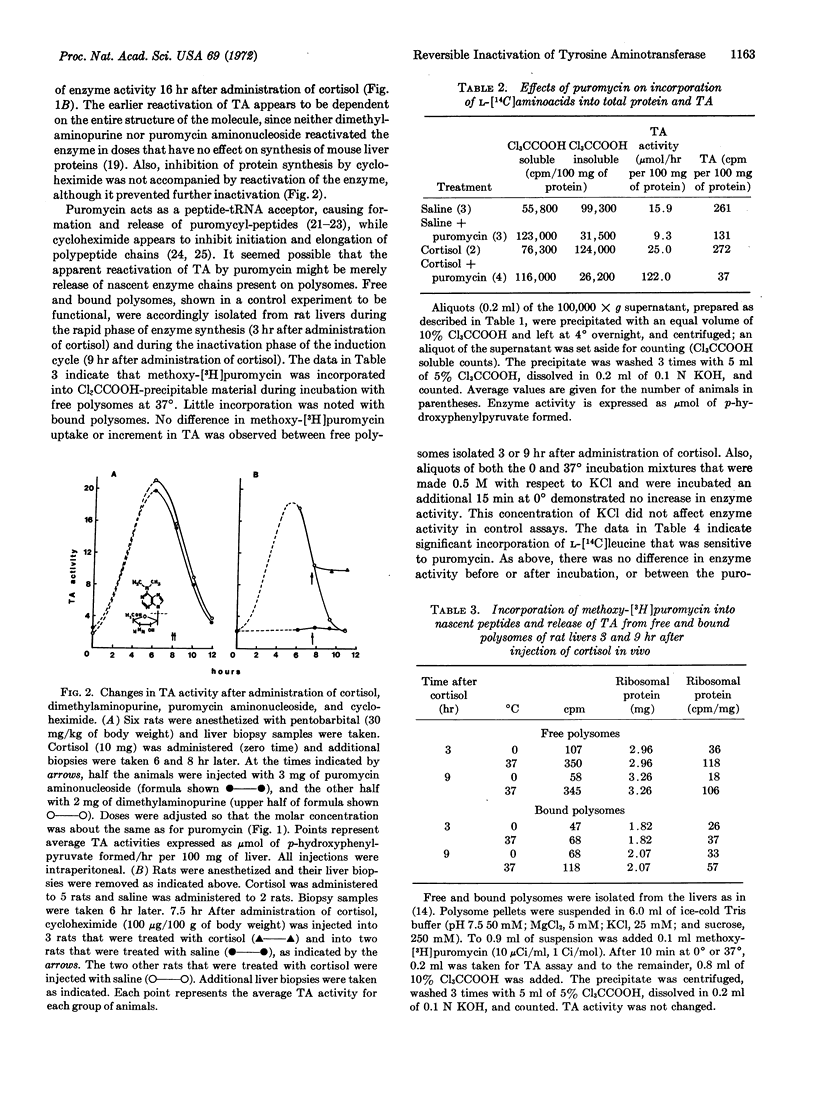
Induction of tyrosine aminotransferase (EC 2.6.1.5) with cortisol is followed by a rapid phase of inactivation of the enzyme, during which administration of puromycin causes a sharp increase in enzyme activity. The increase is not due to puromycin-resistant synthesis of protein, since precipitation of the enzyme with antibodies to tyrosine aminotransferase after pulse labeling inhibited incorporation of radioactive amino acids. Reactivation of the enzyme was specific, since the structurally similar dimethylaminopurine and purine aminonucleoside were ineffective. An action of puromycin other than its capacity to inhibit protein synthesis was required for reactivation, since cycloheximide did not demonstrate such an effect. In vitro studies with free and bound polysomes, isolated during the phase of inactivation, indicated that reactivation by puromycin was not due to release of nascent tyrosine aminotransferase peptides. We conclude that the enzyme is initially reversibly inactivated; therefore, the overall degradation of this intracellular protein is a multistep phenomenon.
Keywords: cortisol, puromycin, Sprague-Dawley rats
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Potter V. R. Ribosomes in rat liver: an estimate of the percentage of free and membrane-bound ribosomes interacting with messenger RNA in vivo. J Mol Biol. 1967 Sep 28;28(3):539–542. doi: 10.1016/s0022-2836(67)80103-7. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. II. Interaction of ribosomes and membranes. J Mol Biol. 1967 Jun 14;26(2):293–301. doi: 10.1016/0022-2836(67)90298-7. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boctor A., Grossman A. Alteration of tyrosine aminotransferase turnover in rat liver following glucocorticoid administration. J Biol Chem. 1970 Dec 10;245(23):6337–6345. [PubMed] [Google Scholar]
- Bucher N. L. Experimental aspects of hepatic regeneration. N Engl J Med. 1967 Sep 28;277(13):686–contd. doi: 10.1056/NEJM196709282771306. [DOI] [PubMed] [Google Scholar]
- COLOMBO B., FELICETTI L., BAGLIONI C. INHIBITION OF PROTEIN SYNTHESIS BY CYCLOHEXIMIDE IN RABBIT RETICULOCYTES. Biochem Biophys Res Commun. 1965 Feb 3;18:389–395. doi: 10.1016/0006-291x(65)90719-9. [DOI] [PubMed] [Google Scholar]
- EAGLE H. METABOLIC CONTROLS IN CULTURED MAMMALIAN CELLS. Science. 1965 Apr 2;148(3666):42–51. doi: 10.1126/science.148.3666.42. [DOI] [PubMed] [Google Scholar]
- Granner D. K., Hayashi S., Thompson E. B., Tomkins G. M. Stimulation of tyrosine aminotransferase synthesis by dexamethasone phosphate in cell culture. J Mol Biol. 1968 Jul 28;35(2):291–301. doi: 10.1016/s0022-2836(68)80025-7. [DOI] [PubMed] [Google Scholar]
- Grossman A., Mavrides C. Studies on the regulation of tyrosine aminotransferase in rats. J Biol Chem. 1967 Apr 10;242(7):1398–1405. [PubMed] [Google Scholar]
- HOFERT J. F., BOUTWELL R. K. PUROMYCIN-INDUCED GLYCOGENOLYSIS AS AN EVEN INDEPENDENT FROM INHIBITED PROTEIN SYNTHESIS IN MOUSE LIVER; EFFECTS OF PUROMYCIN ANALOGS. Arch Biochem Biophys. 1963 Dec;103:338–344. doi: 10.1016/0003-9861(63)90423-5. [DOI] [PubMed] [Google Scholar]
- Kenney F. T. Turnover of rat liver tyrosine transaminase: stabilization after inhibition of protein synthesis. Science. 1967 Apr 28;156(3774):525–528. doi: 10.1126/science.156.3774.525. [DOI] [PubMed] [Google Scholar]
- LIN E. C., PITT B. M., CIVEN M., KNOX W. E. The assay of aromatic amino acid transaminations and keto acid oxidation by the enol borate-tautomerase method. J Biol Chem. 1958 Sep;233(3):668–673. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitan I. B., Webb T. E. Hydrocortisone-mediated changes in the concentration of tyrosine transaminase in rat liver: an immunochemical study. J Mol Biol. 1970 Mar 14;48(2):339–348. doi: 10.1016/0022-2836(70)90165-8. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Mosteller R. D., Hardesty B. The mechanism of sodium fluoride and cycloheximide inhibition of hemoglobin biosynthesis in the cell-free reticulocyte system. J Mol Biol. 1966 Oct 28;21(1):51–69. doi: 10.1016/0022-2836(66)90079-9. [DOI] [PubMed] [Google Scholar]
- NATHANS D. PUROMYCIN INHIBITION OF PROTEIN SYNTHESIS: INCORPORATION OF PUROMYCIN INTO PEPTIDE CHAINS. Proc Natl Acad Sci U S A. 1964 Apr;51:585–592. doi: 10.1073/pnas.51.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969 May;5(3):253–262. [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- SCHIMKE R. T. THE IMPORTANCE OF BOTH SYNTHESIS AND DEGRADATION IN THE CONTROL OF ARGINASE LEVELS IN RAT LIVER. J Biol Chem. 1964 Nov;239:3808–3817. [PubMed] [Google Scholar]
- SWICK R. W. Measurement of protein turnover in rat liver. J Biol Chem. 1958 Apr;231(2):751–764. [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON A. R., SCHWEET R. ROLE OF THE GENETIC MESSAGE IN INITIATION AND RELEASE OF THE POLYPEPTIDE CHAIN. Nature. 1964 May 2;202:435–437. doi: 10.1038/202435a0. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Haba G. L. INHIBITION BY PUROMYCIN OF AMINO ACID INCORPORATION INTO PROTEIN. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1721–1729. doi: 10.1073/pnas.45.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]


