Abstract
The Mysore Parthenon Birth Cohort was established to examine the long-term effects of maternal glucose tolerance and nutritional status on cardiovascular disease risk factors in the offspring. During 1997–98, 830 of 1233 women recruited from the antenatal clinics of the Holdsworth Memorial Hospital (HMH), Mysore, India, underwent an oral glucose tolerance test. Of these, 667 women delivered live babies at HMH. Four babies with major congenital anomalies were excluded, and the remaining 663 were included for further follow-up. The babies had detailed anthropometry at birth and at 6–12-monthly intervals subsequently. Detailed cardiovascular investigations were done at ages 5, 9.5 and 13.5 years in the children, and in the parents at the 5-year and 9.5-year follow-ups. This ongoing study provides extensive data on serial anthropometry and body composition, physiological and biochemical measures, dietary intake, nutritional status, physical activity measures, stress reactivity measures and cognitive function, and socio-demographic parameters for the offspring. Data on anthropometry, cardiovascular risk factors and nutritional status are available for mothers during pregnancy. Anthropometry and risk factor measures are available for both parents at follow-up.
Keywords: Cohort profile, gestational diabetes, maternal nutrition, India, children
Key Messages.
The Mysore Parthenon study is one of few resources for long-term follow-up data in the offspring of diabetic mothers, especially in a developing country.
The study findings suggest that intrauterine exposure to maternal nutritional deficiencies as well as overnutrition may contribute to an increasing burden of cardiovascular disease in India, and that these two conditions may co-exist in the same mother, leading to dual insults to the offspring.
The study findings provide directions for initiating intervention studies aimed at optimizing maternal nutrition and promoting healthy lifestyle behaviours in the offspring in Indian settings.
Why was the cohort set up?
The prevalence of type 2 diabetes is rising rapidly in all parts of the world, but most rapidly in developing countries. Recent evidence suggests that impaired fetal and infant growth, coupled with rapid growth post infancy, leads to increased risk of adiposity, cardiovascular disease and impaired cognitive abilities later in life.1,2 Widespread maternal malnutrition leads to fetal growth restriction, and may confer the characteristic thin-fat phenotype in individuals of Indian origin.3 This may underlie their high susceptibility to cardiovascular disease, especially when exposed to an urban lifestyle. In a transitioning country like India, urban women are increasingly becoming adipose and glucose intolerant during pregnancy. This exposes their offspring not only to specific nutritional deficiencies common in Indian women, but also to intrauterine fuel surplus and long-term risk of adiposity and cardiovascular disease, thus adding significantly to the global non-communicable disease pool. Well-designed longitudinal studies are important to understanding the mechanisms underlying these associations, and to devise strategies to reduce the global burden of non-communicable disease. However, prospective studies exploring maternal nutrition and metabolism in relation to offspring outcomes are few. The Mysore Parthenon Cohort study was originally set up in 1997–98 to estimate the incidence of gestational diabetes mellitus (GDM) in one maternity hospital in India.4 Subsequently the offspring were followed up regularly, and detailed anthropometry and cardiovascular risk factor assessments were carried out during childhood and early adolescence to examine: (i) associations of maternal glucose and insulin concentrations during pregnancy with glucose tolerance and insulin concentrations, other cardiovascular risk factors and cognitive function in the children; (ii) associations of birth size and specific maternal nutrients with risk outcomes at these ages; and (iii) associations of the children’s current lifestyle factors such as diet, physical activity and stress responses with risk outcomes.
Who is in the cohort?
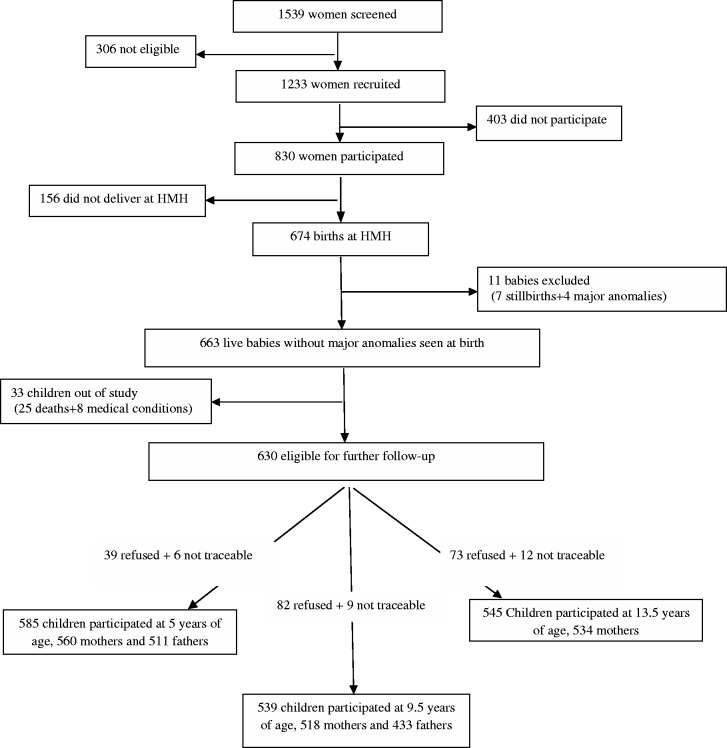
Figure 1 summarizes the number of participants at different stages of the Parthenon study. This cohort includes a group of children born in one maternity hospital in Mysore, India, and their parents. CSI Holdsworth Memorial Hospital (HMH), a 350-bed hospital situated at the centre of Mysore city, caters mainly to a middle and lower socioeconomic status population. Between June 1997 and August 1998, we screened 1539 women booking consecutively into the antenatal clinics of HMH. Of these, 1233 (80%) eligible women (<32 weeks of gestation at booking, no diabetes before pregnancy, planned to deliver at HMH, and with a singleton pregnancy) were invited to undergo detailed anthropometry and a 100-g, 3-h, oral glucose tolerance test (OGTT); 830 (67%) participated in the study, and 785 women completed the OGTT (49 of these with GDM).4
Figure 1.
Flow chart to illustrate the rate of participation and attrition at major follow-up stages of the Parthenon study. HMH: Holdsworth Memorial Hospital.
Because of the wide choice of maternity units in Mysore, and the local practice of going to the woman's maternal home for delivery, many women booking into the HMH antenatal clinic ultimately chose another hospital for the birth. Of the 830 women studied, 674 (81%) delivered at HMH (43 GDM mothers); 7 babies were stillborn and 4 were born with severe congenital anomalies. The remaining 663 offspring were included for further follow-up.5
How often have they been followed up?
The children were measured at birth, and were followed up annually until 5 years of age, and 6-monthly after that, for detailed anthropometry and pubertal stage assessment. Detailed cardiovascular investigations were carried out during three of these follow-up rounds at 5, 9.5 and 13.5 years of age. The children are currently between 15 and 16 years of age.
The mothers were examined for anthropometry and detailed cardiovascular risk factors at 5 and 9.5 years, and for cognitive function at 13.5 years following the index pregnancy. The fathers were measured for height and weight during the index pregnancy, and were examined for anthropometry and cardiovascular risk factors at 5 and 9.5 year follow-ups.
Attrition
Figure 1 illustrates the rate of attrition during different stages of the study.
Of the 1233 pregnant women eligible to take part and recruited, 403 (32.7%) did not participate further or have an OGTT. A further 156 (12.7%) women who had an OGTT did not deliver at HMH, and 11 babies who were either stillborn or had major congenital anomalies (0.9%) were excluded from the study. The general characteristics of the 1233 women who were initially recruited were similar to those of the 830 women who participated and had an OGTT, and the 663 who delivered at HMH and were included in the cohort (Table 1). However, the 403 women who did not participate after initial recruitment were younger than those who became part of the cohort [mean difference: 1.17 years; confidence interval (CI): 0.66–1.69 years] (Table 1). There were more rural women in the non-participants group (32.8% vs 26.1%), they were less likely to be employed compared with the women in the cohort, and were more likely to be Muslims (Table 1).
Table 1.
Baseline characteristics of the recruited women, women who participated in the pregnancy study and the women who were included in the cohort in comparison with the corresponding characteristics of ever-married 15–49-year-old urban women of Karnataka state, India
| Characteristics | Initially recruited | Had an OGTT in pregnancy | P1d | Initially recruited but did not participate further and did not have an OGTT | P2e | Delivered at HMH and included in the cohort | General populationa |
|---|---|---|---|---|---|---|---|
| (n = 1233) | (n = 830) | (n = 403) | (n = 663) | ||||
| Age (years) | 23.3 (4.2) | 23.6 (4.4) | 0.0009 | 22.8 (3.9) | 0.00001 | 23.9 (4.3) | Range: 15–49 |
| Range: 15–40 | Range:16–40 | Range: 17–40 | |||||
| Urban residence (n) | 854 (69.3%) | 583 (70.2%) | 0.29 | 271 (67.2%) | 0.02 | 490 (73.9%) | 41.5%b |
| Parity (n): | |||||||
| 0 | 634 (51.4%) | 433 (52.2%) | 0.75 | 201 (49.9%) | 0.97 | 335 (50.5%) | 30.2% |
| 1 | 414 (33.6%) | 275 (33.1%) | 139 (34.5%) | 230 (34.7%) | 11.7% | ||
| ≥2 | 185 (15.0%) | 122 (14.7%) | 63 (15.6%) | 98 (14.5%) | 58.1% | ||
| Religion (n): | |||||||
| Hindu | 701 (56.9%) | 493 (59.4%) | 0.0003 | 208 (51.6%) | 0.001 | 377 (56.9%) | 76.8% |
| Muslim | 456 (37.0%) | 276 (33.3%) | 180 (44.7%) | 230 (34.7%) | 16.8% | ||
| Christian and Other | 76 (6.1%) | 61 (7.3%) | 15 (3.7%) | 56 (8.4%) | 6.4% | ||
| Employed (n)c | 97 (7.9%) | 77 (9.3%) | 0.008 | 20 (5.0%) | 0.001 | 71 (10.7%) | 30.6% |
| Education (n): | |||||||
| Illiterate | – | 30 (3.6%) | – | – | – | 16 (2.4%) | 31.7% |
| <10 years | 175 (21.1%) | 129 (19.5%) | 30.5% | ||||
| 10 years | 327 (39.4%) | 262 (39.5%) | 19.2% | ||||
| >10 years | 298 (35.9%) | 256 (38.6%) | 18.6% |
Values given are mean (SD) or n (%).
aRepresentative data based on the National Family Health Survey-2, 1998–99, India8
bBased on the 2011 national population census report for all the population in Mysore district, Karnataka state, India30
cFor study women: values based on current employment status; for general population: values based on current as well as any employment within the past 12 months including self-employment and family business.
dP1, P-value for the difference between pregnant women who had an OGTT and those who were recruited but did not participate further.
eP2: P-value for the difference between women who were included in the cohort and those who were recruited but did not participate further.
Of the 663 offspring included in the cohort at birth, 25 (3.8%) children died and 8 (1.2%) had major medical conditions. Of the remaining cohort (630), the attrition rate was 8% at 5 years of follow-up (N = 585), and 14% at 9.5 (N = 539) and 13.5 years (N = 545). The children who were lost to follow-up at 13.5 years of age had higher scores for socioeconomic status and better educated parents (Table 2). Maternal weight, GDM status and the offspring birthweight and sex ratio were similar in both groups (Table 2).
Table 2.
Comparison of baseline characteristics between children who participated and those who were lost to follow-up at 13.5 years of age
| Characteristics | Followed up | Lost to follow-up | P-value |
|---|---|---|---|
| (n = 545) | (n = 118) | ||
| Gestational age (weeks) | 39.1 (1.7) | 38.8 (2.1) | 0.1 |
| Boys (n) | 259 (47.5%) | 63 (53.4%) | 0.2 |
| Birthweight (grams) | 2862 (450) | 2820 (569) | 0.4 |
| Socioeconomic status (score) | 14.7 (4.8) | 16.6 (5.9) | 0.001 |
| Maternal education >10 years (n) | 195 (35.8%) | 61 (51.7%) | 0.003 |
| Paternal education >10 years (n) | 241 (44.2%) | 71 (60.2%) | 0.00002 |
| Maternal GDM yes (n) | 32 (6.2%) | 9 (8.0%) | 0.5 |
Values given are mean (SD) or n (%). P-value for the difference has been derived using independent t test or chi2 test.
What has been measured?
Table 3 gives a catalogue of all the investigations carried out in the cohort since the index pregnancy. In the baseline study, the pregnant mothers had detailed anthropometry, measurement of systolic and diastolic blood pressure (BP) and OGTTs for measurement of glucose and insulin concentrations at 28–32 weeks of gestation. Maternal micronutrient status (vitamin B12, vitamin D, folate and homocysteine) was measured using stored plasma/serum samples. Education, occupation and socioeconomic status of the parents were recorded using the Kupuswamy score.6 At delivery, the babies were measured for birthweight, crown-rump and crown-heel length, head, chest, abdomen and mid-upper-arm circumferences and triceps and subscapular skinfold thickness. Placental weight and dimensions including length, breadth, thickness and cotyledon number were recorded, and a sample of cord blood plasma was stored. Weight and height were measured in 674 fathers (551 included in the cohort) during the index pregnancy.
Table 3.
List of the data collected in the Parthenon study
| Baseline | |
| Mother: 28–32 weeks pregnancy |
|
| Father: index pregnancy |
|
| Offspring: at birth |
|
| Placenta: at delivery |
|
| Annual follow-up: 1–4 years of age | |
| Offspring |
|
| 5-year follow-up | |
| Offspring |
|
| Parents |
|
| 6-monthly follow-up: 5.5–9 years of age | |
| Offspring |
|
| 9.5-year follow-up | |
| Offspring |
|
| Parents |
|
| 6-monthly follow-up: 10–13 years of age | |
| Offspring |
|
| 13.5-year follow-up | |
| Offspring |
|
| Mothers |
|
| 6-monthly follow-up: 14–15 years of age | |
| Offspring |
|
| Other data | |
| Offspring |
|
On further follow-up, detailed anthropometry and measurement of fat/lean body mass using bio-impedance have been carried out. Data on infant feeding (duration of breastfeeding and age at start of solids) was collected at 1, 2 and 3 years of age. From the age of 9 years, sexual maturity scoring (Tanner scales) has been incorporated into the 6-monthly assessments. In girls, external pelvic diameters have been measured from 12 years onwards. Cardiovascular risk markers, including plasma glucose and insulin concentrations, total and HDL-cholesterol and triglyceride concentrations, and systolic and diastolic BP were measured in the children at 5, 9.5 and 13.5 years of age. Plasma vitamin B12 and folate concentrations were measured at 5 and 9.5 years, and homocysteine concentration at 5 years only. A battery of cognitive function tests was performed at 9–10 years and 13.5 years of age. Physical activity was measured using 7-day Actigraph accelerometer recordings between 6 and 10 years and again between 11 and 13 years of age. Dietary intake, using quantified food frequency and 24-h recall questionnaires, was assessed at 9.5 years of age. Stored samples were used to measure plasma cortisol and corticosteroid binding globulin (CBG) concentrations at 9.5 years of age. Salivary cortisol and cardiovascular responses to a laboratory-induced stressor, the Trier Social Stress Test for Children/ TSST-C,7 were assessed in a subsample of 273 children at 13.5 years of age.
The family’s socioeconomic status was re-assessed using the Standard of Living Index questionnaire8 when the children were 9.5 years and 13.5 years old.
In both parents, detailed anthropometry was carried out, and cardiovascular risk markers were measured at 5 and 9.5 years following the index pregnancy. Mothers’ diabetes status was assessed using an OGTT and fathers’ diabetes status was determined using fasting blood glucose measured at 5-year follow-up. Maternal intelligence and adult temperament were assessed at the 13.5 year follow-up using questionnaires.
The offspring participants are currently between 15 and 16 years of age. The 6-monthly follow-up of the cohort is continuing for anthropometry and pubertal staging.
What has the Mysore Parthenon Cohort study found? Key findings and publications
In the baseline study, the incidence of GDM was 6.9% (N = 49) in 785 mothers who had a complete OGTT.4 The women with GDM had a higher incidence of diabetes and metabolic syndrome at the 5-year follow-up.9 Low maternal vitamin B12 and high folate concentrations were associated with a higher incidence of GDM, and type 2 diabetes 5 years after the index pregnancy.10
The newborn offspring were smaller, lighter but relatively more adipose compared with Caucasian children, confirming the ‘thin-fat’ phenotype described for Indian neonates in Pune; this phenotype persisted at 4 years.5,11 In all children, size at 5 and 9.5 years, especially subcutaneous adiposity, was a strong risk factor for higher BP, insulin resistance (HOMA-IR) and fasting triglyceride concentrations. Our study showed that lower birthweight, accelerated weight gain and also faster linear growth, during infancy as well as early childhood, are associated with higher cardiovascular risk factors during childhood.12 We also observed that smaller birth size, especially head circumference, was associated with lower cognitive abilities at 9–10 years of age in our cohort.13 Longer duration of breastfeeding was associated with lower risk of high BMI at 5 years of age;14 there was no association between breastfeeding duration and cognitive function at 9.5 years.15
Our purpose-designed study showed that, as expected, babies of GDM mothers were heavier, longer and more adipose than control babies at birth (offspring of non-GDM mothers and non-diabetic fathers).16 Cardiovascular risk markers, including body fat, glucose and insulin concentrations, HOMA-IR and BP were higher in offspring of diabetic mothers (ODM) than control offspring during childhood.16,17 The difference in subcutaneous adiposity between ODM and controls continued to increase as the children aged (Figure 2). Their cognitive ability was better than that of control children.18 Offspring of diabetic fathers (ODF) were smaller at birth. As with ODM, they were more adipose and had higher HOMA-IR than control children at 9.5 years of age, though not as large an effect as in ODM.17 Even in the control children, both maternal and paternal insulin concentrations were positively associated with offspring subscapular skinfold thickness and HOMA-IR.16,17
Figure 2.
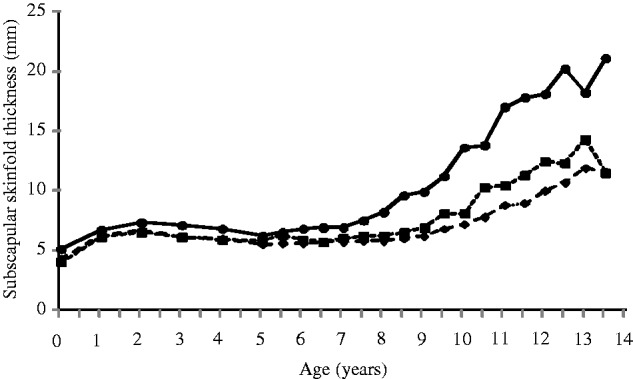
Median subscapular skinfold thickness (mm) for offspring of diabetic mothers (ODM), offspring of diabetic fathers (ODF) and controls, aged 0–13.5 years. Continuous lines with black circles, ODM; dashed lines with black squares, ODF; dashed lines with black diamonds, control offspring.
Our study also reported associations between specific maternal micronutrients and offspring outcomes. We found that lower maternal vitamin D during pregnancy was associated with lower arm-muscle area and higher insulin resistance (HOMA-IR) in the offspring at 9.5 years of age.19 A previous study from Pune, India, had shown that maternal folate was positively associated with offspring insulin resistance.20 We confirmed this association by showing that higher maternal folate was associated with higher HOMA-IR in the Mysore children at both 9.5 and 13.5 years of age.21 In contrast, higher maternal folate was related to better cognitive functions in the cohort children.22 Unlike the Pune study, we did not see any association between maternal B12 status and offspring HOMA-IR and cognitive function.
Our detailed measurement of placental dimensions showed that the size and shape of the placental surface is a predictor of childhood BP.23 We also showed that maternal current nutritional status, as well as early growth, are related to the shape and size of the placental surface and greater placental efficiency.24
Recently, it has been suggested that the link between fetal growth retardation and adult non-communicable disease may be explained by an abnormal hypothalamic-pituitary-adrenal (HPA) axis activity.25 We showed in the Parthenon children that smaller birth size was associated with higher corticosteroid binding globulin concentrations, but not cortisol concentrations, measured at 9.5 years.26 Higher cortisol was associated with higher values for cardiovascular risk factors. It is suggested that the impact of birthweight on HPA axis will be apparent only in a stressed state.25 Our recently completed investigations of neuro-endocrinal stress responses in a subgroup of the cohort will be able to provide more information on the associations of birth size, maternal GDM and specific micronutrients with stress physiology, and their relation to risk outcomes.
The findings of the Parthenon study have been reported in 33 peer-reviewed publications. A list of the publications can be found at the following web link: http://www.mrc.soton.ac.uk/developing-populations/mysore-parthenon-study/
What are the main strengths and weaknesses?
Strengths
The Mysore Parthenon Birth Cohort is a cohort of healthy children in urban India with an extensive dataset and a large number of repeat follow-ups. A major strength of the cohort has been the measurement of maternal glucose tolerance, insulin and circulating nutrients during pregnancy. Detailed neonatal and childhood anthropometry, and serial measurements of an array of cardiovascular risk factors, are other strengths. Availability of height, weight and socioeconomic status data during the index pregnancy, and data on anthropometry and cardiovascular risk factors at 5 and 9.5 years in a large number of fathers, add to the value of the cohort. Indeed, this is one of few prospective non-Pima Indian studies to examine the long-term risks of maternal diabetes in relation to fathers’ diabetes in children. Offspring outcomes were available at three time points during childhood and early adolescence, which allows longitudinal study of the observed associations. Lifestyle behaviours such as physical activity and dietary intake have been measured during childhood and/or early adolescence. A battery of cognitive function tests specifically adapted for, and validated in, a South Indian population has been used to assess cognitive abilities at two time points. This is also the first study in India to successfully adapt an internationally recognized stress test module for local children. Plasma and DNA samples stored for children as well as for both the parents facilitate exploration of other biological, genetic and epigenetic markers for cardiovascular risk factors. A range of potential confounding factors such as gestational age, parity, parents’ education and socioeconomic status has been assessed over the course of follow-up. Pubertal status has been measured serially in all children by trained investigators from 9 years of age.
Weaknesses
The main limitation is a relatively small number of ODMs, which reduces the power of association between GDM and offspring outcomes. However, the association between continuous measures of maternal glucose and insulin concentrations and offspring outcomes, uniquely, is possible in the whole cohort. Several important maternal characteristics that influence offspring cardiovascular risk, stress responses and cognitive abilities, such as dietary intake, lifestyle behaviours and psychosocial stress, were not measured during pregnancy. Only 54% of those who were initially recruited were included in the final cohort. As the women who were lost to follow-up were younger and more likely to live in a rural setting, the incidence of GDM may have been over-estimated. Our women and children may not be representative of the general population in this area. As HMH, a private hospital, mainly caters to the middle socioeconomic stratum of the Mysore population, the majority of our women were literate and were better educated than the representative urban population of the region (Table 1). However, the general characteristics of our mothers during pregnancy and of our children at birth and during childhood are similar to those in other urban cohorts in South India.27–29 This being a mainly urban cohort, many of our findings related to GDM and emerging childhood adiposity and risk factors may not be applicable to the vast rural population in India. However, considering the rapid urbanization of the villages in India and other developing countries, and escalating problem of obesity and related risk factors in urban India, our study findings provide a base for understanding some of the mechanisms leading to non-communicable disease risks in India. There was also a ∼14% loss to follow-up from birth to later childhood follow-up years, which might have reduced the power of some findings.
Can I get hold of the data? Where can I find out more?
The study's data are not freely available. However, the Parthenon Cohort team is open to data sharing with bona fide researchers, and subject to Government of India regulations. For further information contact the corresponding author: GV Krishnaveni at: (gv.krishnaveni@gmail.com).
Funding
The study was funded by the Parthenon Trust, Switzerland, the Wellcome Trust, UK (079877/Z/06/Z and 095147/Z/10/Z), the Department for International Development, UK and the Medical Research Council, UK [G0400519 (ID no.71108)].
Acknowledgements
We are grateful to the participating families, the director, and the obstetric and paediatric consultants of HMH. We thank the staff at the Epidemiology Research Unit, HMH, and the MRC Lifecourse Epidemiology Unit for their substantial contributions. We also thank Sneha-India for its support.
Conflict of interest: None declared.
References
- 1.Barker DJP. Mothers, Babies and Health in Later Life. 2nd edn Edinburgh, UK: Churchill Livingstone, 1998. [Google Scholar]
- 2.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr 2004;:–. [DOI] [PubMed] [Google Scholar]
- 4.Hill JC, Krishnaveni GV, Annamma I, Leary SD, Fall CHD. Glucose tolerance in pregnancy in South India: Relationships to neonatal anthropometry. Acta Obstet Gynecol Scand 2005;:–. [DOI] [PubMed] [Google Scholar]
- 5.Krishnaveni GV, Hill JC, Veena SR, et al. Truncal adiposity is present at birth and in early childhood in south Indian children. Indian Pediat 2005;:–. [PubMed] [Google Scholar]
- 6.Kuppuswamy B. Manual of Socioeconomic Status Scale. Delhi: Manasayan Publication, 1962. [Google Scholar]
- 7.Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med 1997;:–. [DOI] [PubMed] [Google Scholar]
- 8.International Institute for Population Sciences (IIPS) and Operations Research Centre (ORC) Macro 2001. National Family Health Survey (NFHS-2), India 1998–1999. Maharashtra, Mumbai: IIPS, 2001. [Google Scholar]
- 9.Krishnaveni GV, Hill JC, Veena SR, et al. Gestational diabetes and the incidence of diabetes in the 5 years following the index pregnancy in South Indian women. Diabetes Res Clin Pract 2007;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnaveni GV, Hill JC, Veena SR, et al. Low plasma vitamin b12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes. Diabetologia 2009;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yajnik CS, Fall CHD, Coyaji KJ, et al. Neonatal anthropometry: the thin-fat Indian baby; the Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord 2003;:–. [DOI] [PubMed] [Google Scholar]
- 12.Krishnaveni GV, Veena SR, Wills AK, Hill JC, Karat SC, Fall CHD. Adiposity, insulin resistance and cardiovascular risk factors in 9–10 year old Indian children: Relationships with birth size and postnatal growth. J Dev Orig Health Dis 2010;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veena SR, Krishnaveni GV, Wills AK, et al. Association of birthweight and head circumference at birth to cognitive performance in 9–10 year old children in South India: prospective birth cohort study. Pediatr Res 2010;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caleyachetty A, Krishnaveni GV, Veena SR, et al. Breast-feeding duration, age of starting solids, and high BMI risk and adiposity in Indian children. Matern Child Nutr 2013;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veena SR, Krishnaveni GV, Srinivasan K, et al. Infant feeding practice and childhood cognitive performance in south India. Arch Dis Child 2010;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnaveni GV, Hill JC, Leary SD, et al. Anthropometry, glucose tolerance and insulin concentrations in Indian children: relationships to maternal glucose and insulin concentrations during pregnancy. Diabetes Care 2005;:–. [DOI] [PubMed] [Google Scholar]
- 17.Krishnaveni GV, Veena SR, Hill JC, Kehoe S, Karat SC, Fall CH. Intra-uterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care 2010;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veena SR, Krishnaveni GV, Srinivasan K, et al. Childhood cognitive ability: relationship to gestational diabetes mellitus in India. Diabetologia 2010;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnaveni GV, Veena SR, Winder NR, et al. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: the Mysore Parthenon study. Am J Clin Nutr 2011;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yajnik CS, Deshpande SS, Jackson AA, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia 2008;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnaveni GV, Veena SR, Karat SC, Yajnik CS, Fall CH. Association between maternal folate concentrations during pregnancy and insulin resistance in Indian children. Diabetologia 2014;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veena SR, Krishnaveni GV, Srinivasan K, et al. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10- year-old children in South India. J Nutr 2010;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winder NR, Krishnaveni GV, Hill JC, et al. Placental programming of blood pressure in Indian children. Acta Paediatr 2011;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winder NR, Krishnaveni GV, Veena SR, et al. Mother’s lifetime nutrition and the size, shape and efficiency of the placenta. Placenta 2011;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips DI, Jones A, Goulden PA. Birthweight, stress and the metabolic syndrome in adult life. Ann N Y Acad Sci 2006;:–. [DOI] [PubMed] [Google Scholar]
- 26.Krishnaveni GV, Veena S, Dhube A, Karat S, Phillips D, Fall CHD. Size at birth, morning cortisol and cardiometabolic risk markers in healthy Indian children. Clin Endocrinol (Oxf) 2014;:–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthayya S, Dwarkanath P, Thomas T, et al. Anthropometry and body composition of south Indian babies at birth. Public Health Nutr 2006;:–. [DOI] [PubMed] [Google Scholar]
- 28.Bavdekar A, Yajnik CS, Fall CHD, et al. Insulin resistance syndrome in 8-year old Indian children: small at birth, big at 8 years, or both? Diabetes 1999;:–. [DOI] [PubMed] [Google Scholar]
- 29.Ranjani H, Sonya J, Anjana RM, Mohan V. Prevalence of glucose intolerance among children and adolescents in urban South India (ORANGE-2). Diabetes Technol Ther 2013;:–. [DOI] [PubMed] [Google Scholar]
- 30.Indian Population Census 2011. http://www.census2011.co.in/census/district/263-mysore.html (28 January 2014, date last accessed). [Google Scholar]