Abstract
Peroxisome proliferator-activated receptor (PPAR) δ is implicated in the carcinogenesis of several types of cancer. However, the therapeutic efficacy of PPARδ ligands against cancer progression is unclear. Here, we showed that PPARδ modulates the migration and invasion of melanoma cells by up-regulating Snail expression. Activation of PPARδ by GW501516, a specific ligand for PPARδ, significantly increased the migration and invasion of highly metastatic A375SM cells, but not that of low metastatic A375P cells. The migration- and invasion-promoting effects of PPARδ on A375SM cells was associated with increased Snail expression, which was accompanied by a decrease in E-cadherin expression. Furthermore, a significant concentration- and time-dependent increase in the levels of Snail mRNA and protein was observed in A375SM cells (but not A375P cells) treated with GW501516. The effects of GW501516 were almost completely abrogated by a small interfering RNA against PPARδ, suggesting that PPARδ mediates the effects of GW501516. Activation of PPARδ in SK-MEL-2 and SK-MEL-5 (but not SK-MEL-3) melanoma cell lines also led to significant increases in the expression of Snail mRNA and protein, which mirrored the invasive and migratory potential of these cell lines. These results suggest that PPARδ promotes the aggressive phenotype observed in highly metastatic melanoma cells by up-regulating Snail.
Keywords: PPARδ, Snail, melanoma, migration, invasion
Introduction
Melanoma, a highly aggressive and frequently chemoresistant type of skin cancer caused by the malignant transformation of melanocytes, is characterized by a high capacity for metastasis and invasion [1]. Invasive melanomas are clinically aggressive and prone to metastasis; malignant cancer cells spread from the primary site to distant tissues, resulting in disseminated disease [2,3]. Epithelial-mesenchymal transition (EMT), which plays a role in the metastasis and invasion of cancer cells, is a common phenotypic transformation that causes loss of cell-cell adhesion and the acquisition of cell motility, thereby increasing metastatic potential [4-7]. Snail, along with other transcription factors such as Slug and TWIST, regulates EMT [6,7]. These transcription factors are upregulated in a variety of cancers by various EMT inducers, leading to increased motility and invasiveness [8,9]. Indeed, Snail, Slug, and TWIST are overexpressed in human cancer specimens and are useful prognostic factors [10-12].
Peroxisome proliferator-activated receptor (PP-AR)δ, a member of the ligand-dependent transcription factor PPAR family, modulates multiple biological processes including skin homeostasis and tumorigenesis [13]. PPARδ is ubiquitously expressed in multiple cell lineages, including skin and skin-derived tumor cells, and is implicated in skin carcinogenesis [14-16]. The major barrier to the development of PPARδ as a therapeutic target is that the efficacy of PPARδ ligand(s) during tumorigenesis is unclear and highly controversial [17,18]. Several studies show that the activation of PPARδ increases the proliferation of liver, intestinal adenoma, breast, and prostate cancer cell lines [19-21]. However, other studies show that PPARδ agonists induce terminal differentiation and show anti-tumorigenic effects in keratinocytes, intestinal epithelium, osteoblasts, and oligodendrocytes [22-25]. In addition, a retrospective study shows that low expression of PPARδ by colorectal cancer patients is associated with increased mortality [26]. A recent study shows that activation of PPARδ by its ligand(s) mediates anti-tumorigenic effects in the skin by regulating differentiation and proliferation [24]. A study in a model of DMBA/TPA-induced carcinogenesis showed that PPARδ-deficient mice develop more severe tumors than wild-type mice, suggesting that PPARδ may attenuate the development of chemically-induced skin cancers [15].
Very little is known about the activity of PPARs, particularly PPARδ in melanocytes and melanoma. Three PPARs have been identified in mammals (PPARα [NR1C1], PPARδ [NR1C2], and PPARγ [NR1C3]), and all are expressed in cultured human melanocytes [27] and melanoma cells [28]. Ligand-mediated activation of PPARα and PPARγ inhibits cell proliferation and stimulates melanin production in melanocyte cultures [27,29]. PPARδ agonists also inhibit the proliferation of human and murine melanoma cells by repressing the Wilms’ tumor suppressor, WT1 [30]. Although it is postulated that PPARδ is involved in suppressing melanoma cell growth, much remains to be explored with respect to the putative roles of PPARs in melanoma metastasis and invasion. The present study explored the role of PPARδ in the migration and invasion of melanoma cells. The results show that Snail is a direct response gene for ligand-activated PPARδ, and that Snail expression induces an aggressive phenotype of highly metastatic melanoma cell by accelerating EMT. These findings suggest that PPARδ participates in the modulation of melanoma invasiveness by regulating Snail expression.
Materials and methods
Cell culture
The human melanoma cell lines A375P and A375SM, established by sequential selection for metastatic tumor formation in nude mice [31], were obtained from Dr I. J. Fidler (MD Anderson Cancer Center, Houston, TX). Human melanoma cell lines SK-MEL-2, SK-MEL-3, and SK-MEL-5 were obtained from the Korean Cell Line Bank. A375P and A375SM were maintained in Dulbecco’s modified Eagle’s medium (DMEM), and SK-MEL-2, SK-MEL-3, and SK-MEL-5 were maintained in Roswell Park Memorial Institute (RPMI)-1640, containing 100 U/ml penicillin and 100 µg/ml streptomycin supplemented with 10% heat-inactivated fetal bovine serum at 37°C in an atmosphere of 95% air and 5% CO2.
Matrigel invasion and migration assay
Transwells with polycarbonate membranes (8 µm pores) (BD Biosciences) were coated with collagen type I (1 mg/ml; BD Biosciences) and incubated for 1 h at 37°C. After washing once with culture medium, cells were seeded into the upper compartment of the Transwell insert (2 × 105 cells/ml) in culture medium containing 8 µg/ml mitomycin C (Sigma-Aldrich) to prevent proliferation. The cells were incubated for 2 h and washed with PBS. Medium containing DMSO (Sigma-Aldrich) or GW501516 (Enzo Life Science) was then added to the lower well as a chemo-attractant. After incubating for the indicated times, the cells were fixed and stained with 0.05% crystal violet solution (Sigma-Aldrich). The fixed cells were then observed under a microscope and the number of cells was counted. Cell migration was assayed using the same procedure, except that the Transwell membrane was not coated with type I collagen.
Gene silencing with small interfering (si)RNA
Cells were seeded into 60 mm culture dishes 18-24 h prior to transfection. Cells were transfected with control siRNA (Ambion) or human PPARδ siRNA (Ambion) in serum-free medium using Welfect-Q (WelGENE). Following incubation for 6 h, the transfection medium was replaced with fresh medium and cells were incubated for an additional 24 h, at which point they were transferred to Transwells.
Western blot analysis
Cells treated with the indicated reagents were washed in ice-cold PBS and lysed in PRO-PREP Protein Extraction Solution (iNtRON Biotechnology). Aliquots of cell lysates or conditioned media were subjected to SDS-polyacrylamide gel electrophoresis and transferred to a Hybond-P+ polyvinylidene difluoride membrane (GE Healthcare). Membranes were blocked overnight at 4°C with 5% non-fat milk in Tris-buffered saline (TBS) containing 0.1% Tween-20 and then reacted with the indicated specific antibodies (diluted 1:1,000) in TBS containing 0.05% Tween-20 overnight at 4°C. The membranes were then incubated with a peroxidase-conjugated anti-goat antibody (diluted 1:5,000) for 1 h at room temperature. After extensive washing in TBS containing 0.1% Tween-20, immunoreactive bands were detected using West-ZOL Plus (iNtRON Biotechnology). Polyclonal antibodies specific for MMP-1, Fibronectin, Collagen type I, and PPARδ were obtained from Santa Cruz Biotechnology (Santa Cruz). Monoclonal antibodies specific for Snail and E-cadherin were purchased from Cell Signaling Technology. Polyclonal rabbit anti-β-actin antibody and Ponceau S solution were purchased from Sigma-Aldrich.
Determination of MMP-1 secretion
Aliquots of conditioned culture medium from equal numbers of cells were used to determine the relative amounts of secreted MMP-1. Equal volumes of conditioned culture medium were mixed with 80% ice-cold acetone and incubated at -20°C for 2 h. After precipitation by centrifugation and washing with 80% acetone, the protein pellets were resuspended in SDS-PAGE sample buffer and subjected to Western blot analysis. Ponceau S staining was used to confirm equal loading.
Real-time PCR analysis
Total RNA was isolated from cells using TRIzol reagent (Invitrogen) and reverse transcribed into cDNA using the TOPscript RT DryMIX kit (Enzynomics). Equal amounts of cDNA were diluted and amplified in a 10 μl reaction volume containing 1 × SYBR PCR master mix (QIAGEN) and 10 μM primers. Real-time PCR was performed in a Rotor Gene RG-3000 (Corbett life Science). The PCR amplification conditions were as follows: initial denaturation for 5 min at 95°C, followed by 40 cycles of 10 s at 95°C, 10 s at 58.2°C, and 10 s at 72°C. The primers used were as follows: Snail forward, 5’-CCCAATCGGAAGCCTAACTA-3’; Snail reverse, 5’-GCTGGAAGGTAAACTCTGGA-3’; GAPDH forward, 5’-CATGGCCTTCCGTGTTCCTA3’; and GAPDH reverse, 5’-CCTGCTTCACCACCTTCTTGAT-3’. The levels of Snail mRNA in each sample were normalized to that of GAPDH in the same sample; the fold change in target gene cDNA relative to that of the GAPDH control was determined as described previously [32].
Northern blot analysis
Aliquots (5 µg) of total RNA were heat-denatured at 65°C for 15 min in gel-running buffer (40 mM MOPS, 10 mM sodium acetate, 1 mM EDTA, pH 7.0) containing 50% formamide and then subjected to electrophoresis on a 1% agarose gel containing 2.2 M formaldehyde. Size-fractionated RNA was transferred overnight onto a Hybond-N+ nylon membrane (GE Healthcare) by capillary action and then hybridized with a 32P-labeled Snail cDNA probe at 68°C in QuikHyb solution (Stratagene). The membrane was then washed and the radioactivity on the membrane was detected using a BAS-2500 Bioimaging Analyzer (Fujifilm). The blots were stripped and re-probed with a 32P-labeled GAPDH cDNA probe. The cDNA probes were generated by PCR using primers specific for nucleotides 81-455 and 280-563 of human Snail and human GAPDH, respectively.
Statistical analysis
Data are expressed as the mean ± SE. Statistical significance was determined by Student’s t-test. A value of P < 0.05 was considered statistically significant.
Results
Activation of PPARδ promotes the migration and invasion of A375SM cells
Because the migration and invasion of tumor cells are important events in cancer metastasis [33], we examined whether ligand-activated PPARδ affects the migration and invasion of two human melanoma cell lines, A375P and A375SM, which have different phenotypic potential in term of metastasis. When cells were treated with GW501516 for 72 h, we observed a significant increase in the migration and invasion of A375SM cells, but not A375P cells (Figure 1A, 1B).
Figure 1.
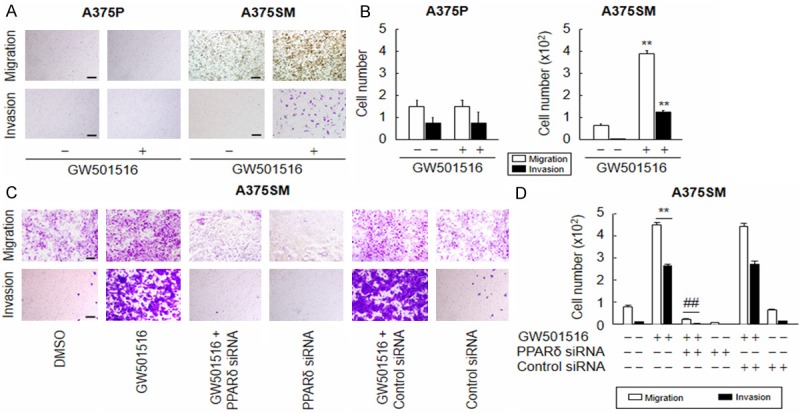
Ligand-activated PPARδ promotes the migration and invasion of A375SM, but not A375P, cells. (A and B) Cells treated with vehicle (DMSO) or 100 nM GW501516 were incubated for 72 h and then subjected to migration (A: upper panel) or invasion (A: lower panel) assays and quantitated (B). (C and D) A375SM cells transfected with or without siRNA for 24 h were treated with vehicle (DMSO) or 100 nM GW501516. After incubation for 72 h, cells were subjected to migration (C: upper panel) or invasion (C: lower panel) assays and quantitated (D). Representative images from four independent experiments are shown. The results are expressed as the mean ± SE (n = 4). Scale bars: 400 µm. **p < 0.01 versus the vehicle-treated group; ##P < 0.01 versus the GW501516-treated group.
To verify the role of PPARδ in the migration and invasion of A375SM cells, we next examined the effects of GW501516 in cells treated with a (si)RNA against PPARδ. The level of PPARδ in A375SM cells was markedly reduced upon transfection with PPARδ siRNA, whereas control siRNA (comprising a pool of nonspecific sequences) had no effect ( Supplementary Figure 1). As expected, PPARδ siRNA, but not control siRNA, significantly suppressed the GW501516-mediated induction of A375SM cell migration and invasion (Figure 1C, 1D).
Activation of PPARδ regulates the expression of EMT-related genes
To investigate the molecular mechanism underlying the PPARδ-mediated induction of A375SM cell migration and invasion, we examined the expression of various EMT-related genes. As shown in Figure 2, GW501516 significantly increased the expression of fibronectin and type I collagen in both A375SM and A375P cells. However, Snail expression increased only in A375SM cells, with a concomitant reduction in the expression of E-cadherin. These results suggest that the differential expression of EMT-related genes induced by GW501516 was attributable to distinct roles played by PPARδ in the regulation of migration and invasion in different melanoma cell lines.
Figure 2.
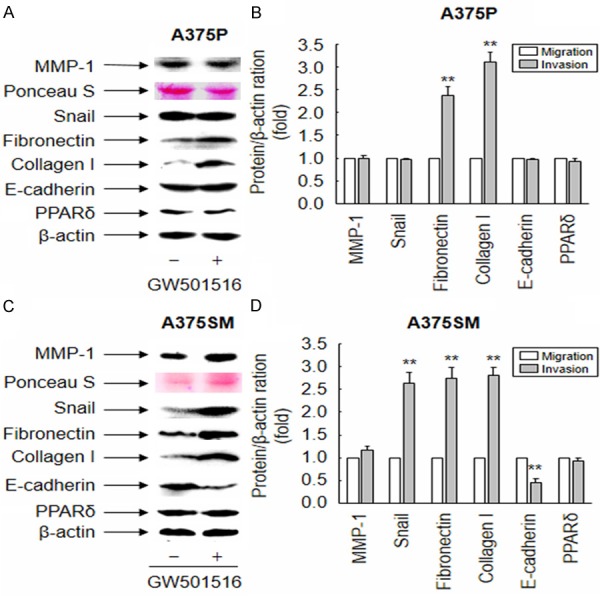
Ligand-activated PPARδ regulates expression of EMT-related genes in melanoma cells. (A-D) Cells treated with vehicle (DMSO) or 100 nM GW501516 were incubated for 38 h. Conditioned media or whole cell lysates were then subjected to Western blot analysis with the indicated antibodies. Ponceau S or β-actin was used as an internal control. Representative images from three independent experiments are shown (A and C). The intensity of each band was quantified using image analysis, and fold changes were plotted (B and D). The results are expressed as the mean ± SE (n = 3). **p < 0.01 versus the vehicle-treated group.
Activation of PPARδ induces expression of Snail mRNA and protein in A375SM cells
To examine the different roles of PPARδ in A375P and A375SM cells, we examined the basal level of PPARδ expression in both cell lines. As shown in Figure 3A, A375P and A375SM cells expressed similar levels of endogenous PPARδ. While GW501516 did not affect Snail expression in A375P cells (Figure 3B), it induced a significant time- and dose-dependent increase in the levels of Snail mRNA and protein in A375SM cells (Figure 3C, 3D, Supplementary Figure 2). To further characterize the role of PPARδ in the GW501516-mediated upregulation of Snail, we transfected A375SM cells with a siRNA against PPARδ. The siRNA-mediated down-regulation of PPARδ reversed the expression of Snail induced by the PPARδ agonist (Supplementary Figure 3). These data indicate that Snail expression in A375SM cells is regulated in a PPARδ-dependent manner.
Figure 3.
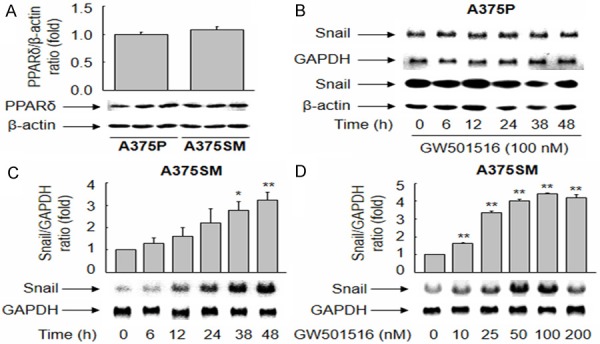
Ligand-activated PPARδ upregulates the expression of Snail in A375SM, but not A375P, cells. (A) Aliquots of proteins from whole cell lysates were subjected to Western blot analysis. (B-D) A375P (B) and A375SM (C and D) cells were treated with 100 nM GW501516 for the indicated times or incubated for 38 h with different concentrations of GW501516. Northern and Western blot analyses were performed using cDNA probes (B: upper panel, and C, D) and an anti-Snail antibody (B: lower panel), respectively. An image analyzer was used to quantify the band intensity, and changes in the ratio of PPARδ or Snail to GAPDH or β-actin are plotted. Representative images from three independent experiments are shown. The results are expressed as the mean ± SE (n = 3). **p < 0.01 and *p < 0.05 versus the vehicle-treated group.
Activation of PPARδ regulates the phenotype of human melanoma cells
To further confirm that PPARδ regulates the migration and invasion of melanoma cells, we examined Snail expression and the metastatic properties of three other human melanoma cell lines: SK-MEL-2, SK-MEL-3, and SK-MEL-5. Exposure to GW501516 increased the levels of Snail mRNA and protein in SK-MEL-2 and SK-MEL-5 cells, but not in SK-MEL-3 cells (Figure 4). Consistent with these results, GW501516 induced the migration and invasion of SK-MEL-2 and SK-MEL-5 cells, but not that of SK-MEL-3 cells (Figure 5), suggesting that invasiveness is regulated by the level of Snail expression, which in turn is regulated by ligand-activated PPARδ.
Figure 4.
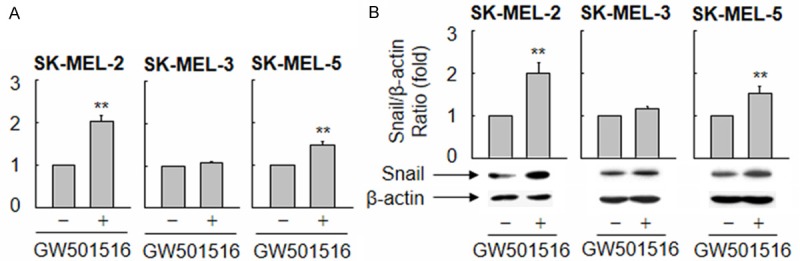
Ligand-activated PPARδ differentially regulates the expression of Snail mRNA and protein in melanoma cell lines. (A and B) Human melanoma cell lines (SK-MEL-2, SK-MEL-3, and SK-MEL-5) were treated with vehicle (DMSO) or 100 nM GW501516 for 38 h, and Snail expression was analyzed by real-time PCR (A) and Western blotting (B). The fold changes in Snail expression relative to those of GAPDH or β-actin were determined and plotted. The results are expressed as the mean ± SE (n = 3). **p < 0.01 versus the vehicle-treated group.
Figure 5.
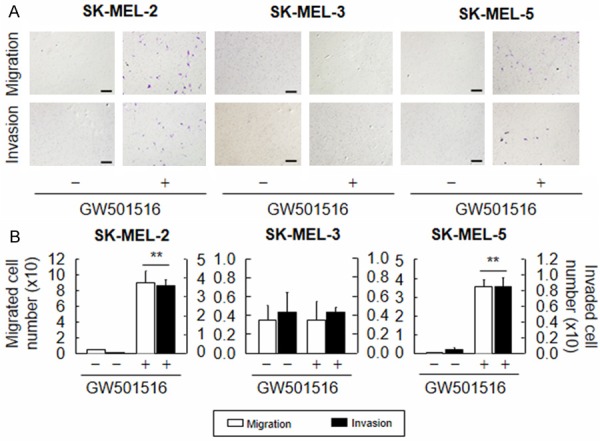
Ligand-activated PPARδ differentially regulates the migration and invasion of melanoma cell lines. (A and B) Human melanoma cell lines (SK-MEL-2, SK-MEL-3, and SK-MEL-5) were treated with vehicle (DMSO) or 100 nM GW501516 for 72 h. The cells were then subjected to migration (A: upper panel) or invasion (A: lower panel) and quantitated (B). Representative images from four independent experiments are shown. The results are expressed as the means ± SE (n = 4). Scale bars: 400 µm. **p < 0.01 versus the vehicle-treated group.
Discussion
Studies of the role(s) of the nuclear receptor PPARδ in carcinogenesis have yielded conflicting results [17,18,25]. While many factors associated with PPARδ activation are involved in cancer progression, little is known about the actual effector molecules that transmit the downstream signals. Here, we demonstrate that activation of PPARδ by a specific ligand, GW501516, promotes the migration and invasion of a highly metastatic human melanoma cell line, A375SM, but not that of the low metastatic line, A375P. The differential role of PPARδ in the invasiveness of A375P and A375SM cells correlated with the expression of Snail and E-cadherin, both of which are critical for EMT. The effects of PPARδ activation on the expression of Snail mRNA and protein in A375SM cells (but not A375P cells) were both time- and dose-dependent. siRNA-mediated inhibition of PPARδ expression antagonized the GW501516-mediated upregulation of Snail mRNA. Consistent with this, PPARδ activation differentially regulated Snail expression in SK-MEL-2, SK-MEL-3, and SK-MEL-5 melanoma cells, which correlated with the different levels of invasiveness shown by these lines.
Ligand-activated PPARδ led to a significant increase in the migration and invasion of highly metastatic A375SM human melanoma cells. The present findings are in line with those of a previous study showing that PPARδ activation by a specific ligand promotes the metastasis of gastric tumors in vivo [34]. In addition, the expression of both PPARδ and cyclooxygenase-2 in tissues may lead to liver metastasis in colorectal cancer patients, and is associated with a poor prognosis [35]. By contrast, a different study showed that PPARδ activation by a specific agonist negatively regulated the invasion and metastasis of human pancreatic cancer cells by down-regulating genes associated with invasion and metastasis [36]. The expression levels of PPARδ also correlate with tumor suppression in colorectal carcinogenesis [26]. Although the role of PPARδ in carcinogenesis is highly controversial, the present data clearly indicate that PPARδ activation promotes the migration and invasion of human metastatic A375SM melanoma cells. Since the expression of PPARδ has been detected in several primary cancer tissues and cell lines [19,21,26,35-37], it may be possible to control cancer progression by either activating or inhibiting PPARδ. Accordingly, the present findings provide new insights into the primary role of PPARδ as a promising new therapeutic target.
PPARδ-mediated induction of Snail expression in A375SM cells is a key event that promotes cell migration and invasion. Snail, a transcriptional repressor of E-cadherin and an inducer of EMT [9], modulates additional cellular processes, including cell proliferation, cell survival, and angiogenesis [38]. Previous reports indicate that the transcriptional regulation of Snail may be complex and involve hypoxia and transforming growth factor β-associated signaling networks in the tumor microenvironment [39,40]. However, the mechanism underlying the transcriptional regulation of Snail in cancer has not been fully elucidated. The results presented herein demonstrate that activating PPARδ with a specific agonist leads to a marked upregulation of Snail mRNA and protein expression in highly metastatic A375SM cells but not in low metastatic A375P cells. This finding is in line with the metastatic properties of these melanoma cells, in which the levels of Snail expression were differentially regulated by GW501516. In this context, the blockade of the PPARδ signaling cascades may serve as a target for preventing cancer metastasis.
The present findings show that PPARδ activation in melanoma cells regulates EMT by inducing Snail expression, thereby modulating their invasiveness. When viewed in this context, blocking PPARδ activity in highly metastatic melanoma cells may attenuate their invasiveness. Accordingly, the current data support the hypothesis that PPARδ is a key target for therapeutic intervention in the metastasis of melanoma cells.
Acknowledgements
This work was supported in part by a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (NRF-2014R1A2A2A01004847); and the Korean Health Technology R & D Project, Ministry of Health and Welfare (HI12C0802), Republic of Korea.
Disclosure of conflict of interest
The authors declare no conflict of interest.
Supporting Information
References
- 1.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann UB, Houben R, Bröcker EB, Becker JC. Role of matrix metalloproteinases in melanoma cell invasion. Biochimie. 2005;87:307–314. doi: 10.1016/j.biochi.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 5.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 6.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Höfler H, Becker KF. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002;161:1881–1891. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 10.Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- 11.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Yuen HF, Chua CW, Chan YP, Wong YC, Wang X, Chan KW. Significance of TWIST and E-cadherin expression in the metastatic progression of prostatic cancer. Histopathology. 2007;50:648–658. doi: 10.1111/j.1365-2559.2007.02665.x. [DOI] [PubMed] [Google Scholar]
- 13.Sertznig P, Seifert M, Tilgen W, Reichrath J. Peroxisome proliferator-activated receptors (PPARs) and the human skin: importance of PPARs in skin physiology and dermatologic diseases. Am J Clin Dermatol. 2008;9:15–31. doi: 10.2165/00128071-200809010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Michalik L, Wahli W. Peroxisome proliferator-activated receptors (PPARs) in skin health, repair and disease. Biochim Biophys Acta. 2007;1771:991–998. doi: 10.1016/j.bbalip.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Kim DJ, Akiyama TE, Harman FS, Burns AM, Shan W, Ward JM, Kennett MJ, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor beta (delta)-dependent regulation of ubiquitin C expression contributes to attenuation of skin carcinogenesis. J Biol Chem. 2004;279:23719–23727. doi: 10.1074/jbc.M312063200. [DOI] [PubMed] [Google Scholar]
- 16.Kim DJ, Murray IA, Burns AM, Gonzalez FJ, Perdew GH, Peters JM. Peroxisome proliferator-activated receptor-beta/delta inhibits epidermal cell proliferation by down-regulation of kinase activity. J Biol Chem. 2005;280:9519–9527. doi: 10.1074/jbc.M413808200. [DOI] [PubMed] [Google Scholar]
- 17.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 18.Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012;12:181–195. doi: 10.1038/nrc3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu L, Han C, Lim K, Wu T. Cross-talk between peroxisome proliferator-activated receptor delta and cytosolic phospholipase A(2)alpha/ cyclooxygenase-2/prostaglandin E(2) signaling pathways in human hepatocellular carcinoma cells. Cancer Res. 2006;66:11859–11868. doi: 10.1158/0008-5472.CAN-06-1445. [DOI] [PubMed] [Google Scholar]
- 20.Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-delta accelerates intestinal adenoma growth. Nat Med. 2004;10:245–247. doi: 10.1038/nm993. [DOI] [PubMed] [Google Scholar]
- 21.Stephen RL, Gustafsson MC, Jarvis M, Tatoud R, Marshall BR, Knight D, Ehrenborg E, Harris AL, Wolf CR, Palmer CN. Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 2004;64:3162–3170. doi: 10.1158/0008-5472.can-03-2760. [DOI] [PubMed] [Google Scholar]
- 22.Peters JM, Hollingshead HE, Gonzalez FJ. Role of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) in gastrointestinal tract function and disease. Clin Sci (Lond) 2008;115:107–127. doi: 10.1042/CS20080022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters JM, Gonzalez FJ. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in cell proliferation and cancer. Biochim Biophys Acta. 2009;1796:230–241. doi: 10.1016/j.bbcan.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bility MT, Zhu B, Kang BH, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-beta/delta and inhibition of cyclooxygenase-2 enhances inhibition of skin tumorigenesis. Toxicol Sci. 2010;113:27–36. doi: 10.1093/toxsci/kfp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters JM, Foreman JE, Gonzalez FJ. Dissecting the role of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in colon, breast and lung carcinogenesis. Cancer Metastasis Rev. 2011;30:619–640. doi: 10.1007/s10555-011-9320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Zhang H, Zhou ZG, Yan H, Adell G, Sun XF. Biological function and prognostic significance of peroxisome proliferator-activated receptor δ in rectal cancer. Clin Cancer Res. 2011;17:3760–3770. doi: 10.1158/1078-0432.CCR-10-2779. [DOI] [PubMed] [Google Scholar]
- 27.Kang HY, Chung E, Lee M, Cho Y, Kang WH. Expression and function of peroxisome proliferator-activated receptors in human melanocytes. Br J Dermatol. 2004;150:462–468. doi: 10.1111/j.1365-2133.2004.05844.x. [DOI] [PubMed] [Google Scholar]
- 28.Mossner R, Schulz U, Kruger U, Middel P, Schinner S, Fuzesi L, Neumann C, Reich K. Agonists of peroxisome proliferator-activated receptor gamma inhibit cell growth in malignant melanoma. J Invest Dermatol. 2002;119:576–582. doi: 10.1046/j.1523-1747.2002.01861.x. [DOI] [PubMed] [Google Scholar]
- 29.Kang HY, Lee JY, Lee JS, Choi YM. Peroxisome proliferator-activated receptors-gamma activator, ciglitazone, inhibits human melanocyte growth through induction of apoptosis. Arch Dermatol Res. 2006;297:472–476. doi: 10.1007/s00403-006-0646-4. [DOI] [PubMed] [Google Scholar]
- 30.Michiels JF, Perrin C, Leccia N, Massi D, Grimaldi P, Wagner N. PPARbeta activation inhibits melanoma cell proliferation involving repression of the Wilms’ tumour suppressor WT1. Pflugers Arch. 2010;459:689–703. doi: 10.1007/s00424-009-0776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozlowski JM, Hart IR, Fidler IJ, Hanna N. A human melanoma line heterogeneous with respect to metastatic capacity in athymic nude mice. J Natl Cancer Inst. 1984;72:913–917. [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci U S A. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollock CB, Rodriguez O, Martin PL, Albanese C, Li X, Kopelovich L, Glazer RI. Induction of metastatic gastric cancer by peroxisome proliferator activated receptor δ activation. PPAR Res. 2010;2010:571783. doi: 10.1155/2010/571783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshinaga M, Taki K, Somada S, Sakiyama Y, Kubo N, Kaku T, Tsuruta S, Kusumoto T, Sakai H, Nakamura K, Takayanagi R, Muto Y. The expression of both peroxisome proliferator-activated receptor delta and cyclooxygenase in tissues is associated with poor prognosis in colorectal cancer patients. Dig Dis Sci. 2011;56:1194–1200. doi: 10.1007/s10620-010-1389-9. [DOI] [PubMed] [Google Scholar]
- 36.Coleman JD, Thompson JT, Smith RW, Prokopczyk B, Vanden Heuvel JP. Role of Peroxisome Proliferator-Activated Receptor β/δ and B-Cell Lymphoma-6 in Regulation of Genes Involved in Metastasis and Migration in Pancreatic Cancer Cells. PPAR Res. 2013;2013:121956. doi: 10.1155/2013/121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Girroir EE, Hollingshead HE, Billin AN, Willson TM, Robertson GP, Sharma AK, Amin S, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) ligands inhibit growth of UACC903 and MCF7 human cancer cell lines. Toxicology. 2008;243:236–243. doi: 10.1016/j.tox.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 39.Imai T, Horiuchi A, Wang C, Oka K, Ohira S, Nikaido T, Konishi I. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol. 2003;163:1437–1447. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


