Abstract
Inhibitors of apoptosis (IAPs) limit the effectiveness of radiation in non-small cell lung cancer (NSCLC). Debio 1143 (D1143) is an antagonist of IAPs. The purpose of this study was to investigate the potential of D1143 as a radiosensitizer in NSCLC. MTS assays were performed in two NSCLC cell lines: HCC193 and H460. Extent of apoptotic cell death was characterized by Annexin V assay and Western blot for cleaved caspase-3, -8, and IAPs. TNF-α release was determined by ELISA. Radiosensitivities were compared with dose enhancement ratios (DERs). HCC193 cells D1143 IC50 was 1 μM. HCC193 cells demonstrated noticeable cleaved caspase-3, -8, and a decrease in IAP levels with 2.5 μM D1143; H460 cells, with 10 μM; both in a time-dependent manner. Additionally, HCC193 cells exhibited an increase in TNF-α. D1143 radiosensitized cells: HCC193, 2.5 μM D1143, 24 h incubation, DER of 2.19, p = 0.001; H460 cells, 10 μM D1143, 48 h incubation, DER of 1.29, p = 0.082. Treatment of H460 cells with radiation therapy, TNF-α, and D1143 further radiosensitized the cells (DER of 1.92, p = 0.026). D1143 significantly enhanced the radiosensitization of HCC193 and H460 cells in vitro. TNF-α contributed to the sensitization in the more sensitive cell line (HCC193). More research is warranted to test the mechanism of D1143, and to assess its potential in vivo in the clinical setting.
Keywords: D1143, non-small cell lung cancer, radiotherapy
Introduction
Lung cancer is the leading cause of cancer-related mortality in the United States, with an estimated annual mortality of 160,000 [1]. Non-small cell lung cancer (NSCLC) represents 85% of all lung cancers and typically presents at an advanced stage, where multimodal therapy (including systemic therapy, radiation therapy [RT], and surgery) is necessary. Despite recent advances in all treatment techniques, the 5-year overall survival for NSCLC remains at 15%.
Certain NSCLCs have been shown to be resistant to current therapeutic approaches due to defects in apoptotic mechanisms. Therapies that promote apoptosis alone or in combination with existing modalities are promising. Two distinct apoptotic pathways have been identified: (1) the intrinsic pathway, where stress triggers the mitochondria to release Apaf-1/cytochrome c complex, which leads to the activation of procaspase-9; and (2) the extrinsic pathway, where cell surface death receptors are activated by specific ligands (e.g. tumor necrosis factor [TNF]-α; TNF-related apoptosis-inducing ligand [TRAIL]), resulting in the activation of procaspase-8 [2]. Both the intrinsic and extrinsic pathways converge by activation of procaspases-3, -6, and -7, which ultimately cause DNA fragmentation and cell death. Moreover, both pathways are activated in RT-induced apoptosis.
Inhibitors of apoptosis proteins, including cellular IAP 1 (cIAP1), cIAP2, and X-linked apoptosis protein (XIAP), inhibit effector caspases directly or by ubiquitination [2-4]. In particular, cIAP1 and cIAP2 modulate NF-κB signaling and inhibit TNF-α-mediated cancer cell apoptosis [3,5]. IAPs are upregulated in a variety of cancers, and overexpression of IAPs is associated with resistance to conventional therapies (e.g. RT) [6] and poor patient outcomes [7].
Human cells naturally possess an IAP antagonist: second mitochondria-derived activator of caspases (Smac). Smac has been found to be downregulated in lung cancers, and underexpression of Smac is associated with a worse prognosis [8]. Smac mimetics are a novel line of targeted therapy for multiple lung cancer subtypes. Debio 1143 (D1143, also called AT-406 or SM-406) is a novel orally active Smac mimetic that antagonizes cIAP1, cIAP2, and XIAP [9]. The goal of this study is to investigate the ability of D1143 to sensitize NSCLC to RT.
Materials and methods
Cell culture and reagents
Cell culture methods have been described [10]. Briefly, the human NSCLC cell line NCI-H460 (H460) was obtained from the American Type Culture Collection (Manassas, VA). The HCC193 cell line was provided by Dr. David Carbone (Vanderbilt University, Nashville, TN). Cells were irradiated using a 137Cs irradiator (J.L. Shepherd and Associated) at room temperature with 1.8 Gy/min. D1143 (Debiopharm, SA, Lausanne, Switzerland) was dissolved in DMSO.
Cell viability/MTS assay
MTS assays were performed using tetrazolium based CellTiter 96® AQueous One Solution Cell Proliferation assay (Promega; Fitchburg, WI). Control groups were exposed to the same concentration of DMSO. MTS assay was performed at 24-96 hours after treatment. At 37°C in humidified 5% CO2 plates were read at the absorbance of 490-nm on a microplate reader (SpectraMax M5, Phoenix, AZ). Half maximal inhibitory concentration (IC50) values were calculated using Prism 5.01 (GraphPad, La Jolla, CA). For the TNF-α neutralizing antibody assay, cells were exposed to 2.5 and 10 μM of D1143 with or without 10 μg/mL infliximab, and the assay was performed 24, 48, 72, or 92 hours later. Plates were read at the absorbance of 490-nm on a microplate reader. The concentrations used in the in vitro experiments are within a clinically relevant range and, importantly, not toxic for both humans and mice at these doses [20].
Western blot analysis
Western blot analysis methods have been previously described [10]. Briefly, separated proteins were incubated overnight at 4°C with antibodies against total caspase-3, cleaved caspase-3, caspase-8, cleaved caspase-8, cIAP1 and XIAP. Immune complexes were detected with chemiluminescence reagents (Perkin-Elmer; Waltham, MA Life Science). The gels were then quantitatively analyzed comparing the expression levels of the experimental group with a control of actin.
Clonogenic survival assay
Exponentially growing cells in a 100 mm dish were trypsinized and counted. After incubation for 1 hour, the cells were irradiated at room temperature at 1.8 Gy/min and dose range was 0 to 6 Gy. After correcting for drug toxicity, the dose enhancement ratio (DER) was calculated as the radiation dose that yielded a surviving fraction of 0.3 for vehicle-treated cells divided by that for D1143-treated cells.
Annexin V analysis
Annexin V analysis was conducted by Annexin V and fluorescein isothiocyanate (FITC) staining using a commercial Annexin V–FITC kit (BD Biosciences, Franklin Lakes, NJ, USA). Cells were harvested by trypsinization and collected by centrifugation, and then washed once with phosphate-buffered saline (PBS). 10,000 Cells in 500uL binding buffer received. 5 uL Annexin V–FITC and 5 uL propidium iodide (PI 50 ug/mL), and they were then incubated for 15 min at room temperature. The stained cells were analyzed using a Cytomics FC 500 flow cytometer (Beckman Coulter, Brea, CA, USA).
Statistical analysis
For the clonogenic assay, a paired t-test was used; for the MTS assay, apoptosis assay, ELISA assay, a student’s t-test was used. Experiments were conducted in triplicate. Data were expressed as mean ± standard deviation (SD) or standard error (SE), with p < 0.05 was considered significant.
Results
D1143 decreases cell viability by inhibiting cIAP1 and XIAP in HCC193 and H460 cells
Based on MTS assays (Figure 1), HCC193 cells were sensitive to D1143 (IC50 of 1 μM) and demonstrated noticeable cleaved caspase-3 and -8 levels when D1143 was increased from 0 to 2.5 μM D1143 (Figure 2A), while H460 cells exhibited cleaved caspase at 10 μM of D1143 (Figure 2B).
Figure 1.
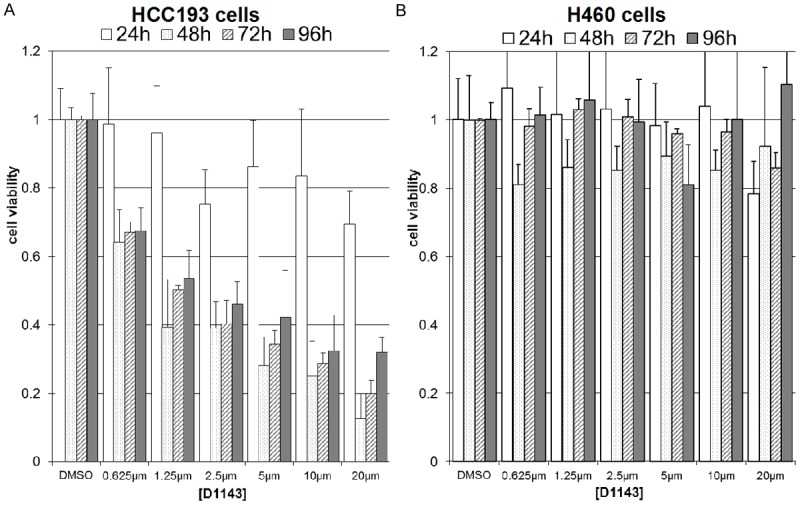
D1143 inhibits HCC193 cell growth in a dose-dependent manner and inhibits H460 growth to a lesser extent. HCC193 (A) and H460 (B) lung cancer cells were exposed to various concentrations of D1143. MTS assay was performed after 24 hours, 48 hours and 72 hours.
Figure 2.

D1143 induces apoptosis by inhibiting cIAP1 and XIAP in a time- and dose-dependent manner in HCC193 and H460 cells Cell lysates were analyzed by Western blot analysis following incubation with increasing concentrations of D1143 for 24 h. HCC193 cells demonstrated noticeable caspase-3 and caspase-8 levels when D1143 was increased from 0 to 2.5 μM (A, left; emphasized with dashed box). H460 cells showed cleaved caspase-8 expression with 10 μM of D1143 (B, right; emphasized with dashed box). Administration of D1143 to both cell lines induced depletion of IAPs in a dose dependent manner (C and D, left). Administration of 2.5 μM of D1143 to HCC193 cells induced near-complete depletion of XIAP and cIAP levels at 1 to 2 hours post treatment in both cell lines (C and D, right).
Consistent with previous reports showing that Smac mimetics are able to induce a rapid autoubiquitination and proteasomal degradation of cIAPs in cells [5], administration of D1143 to HCC193 and H460 cells induced depletion of cIAP1 in a dose dependent manner: cIAP1 was completely depleted at 5 μM and 20 μM of D1143 in HCC193 and H460 cells, respectively (Figure 2C, 2D, left). Interestingly, D1143 was able to partially deplete XIAP at 5 μM in HCC193 (Figure 2C, left) and attenuated XIAP at 20 μM in H460 cell line in this study (Figure 2D, left).
Based on the relative sensitivities of the cell lines (Figure 1) and effect on IAPs at 24 hours (Figure 2, dashed lines), 2.5 and 10 μM of D1143 were selected for other experiments in HCC193 and H460 cells, respectively. Administration of 2.5 μM of D1143 to HCC193 cells induced partial depletion of XIAP and cIAP levels at 2 hours post treatment in both cell lines (Figure 2C, 2D, right). In D1143-treated HCC193 cells, trace amounts of XIAP remained at 1-24 hours without an observed rebound; meanwhile, levels of cIAP1 were low at 1-4 hours and slowly began to increase by 24 hours. In D1143-treated H460 cells, trace amounts of cIAP1 remained at 1-24 hours without an observed rebound; however, trace levels of XIAP remained at 1 hour and slowly began to increase over 24 hours. These data suggest that the specific ability of D1143 to inhibit individual IAPs varies among D1143-resistant (H460) and -sensitive (HCC193) cell lines.
D1143-induced apoptosis of HCC193 cells occurs through activation of the extrinsic apoptotic pathway
Sensitivity to D1143 has previously been shown to be mediated by TNF-α release, an activator of the extrinsic apoptotic pathway [4,5]. To further investigate if sensitivity in both cell lines was mediated by TNF-α release, cells were treated in the same manner as in Figure 2A and 2B. HCC193 cells exhibited an increase in concentrations of TNF-α in the media (Figure 3A, p < 0.05, compared to control) at both 2.5 and 10 μM D1143, while H460 cells did not.
Figure 3.
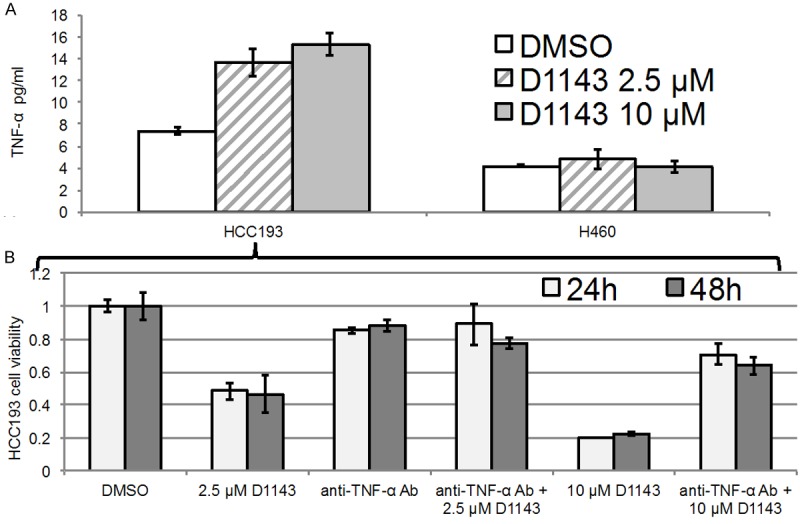
Radiosensitization of HCC193 cells occurs through activation of the extrinsic apoptotic pathway. A. Culture media were analyzed by ELISA following 12 hours treatment with DMSO (control); 2.5 μM, or 10 μM D1143, in HCC193 and H460 cells. HCC193 cells secreted TNF-α in a dose-dependent manner, while H460 cells did not. B. MTS assays were repeated with DMSO (control); 2.5 μM D1143, with or without infliximab; or 10 μM D1143, with or without infliximab. Tests were performed after a 24 (light gray bars) or 48 (dark gray bars) hours incubation period. In the absence of infliximab, HCC193 cells demonstrated 48% and 20% cell viability at 24 hours with 2.5 and 10 μM D1143, respectively. In the presence of infliximab, cell survival rates were 88% and 71%, respectively.
In the absence of infliximab, HCC193 cells demonstrated 48% and 20% cell viability at 24 hours with 2.5 and 10 μM D1143, respectively; whereas, in the presence of infliximab, cell survival rates were 88% and 71%, respectively (Figure 3B, Figure S1). MTS assays were not performed in H460 cells because they showed no increase in concentrations of TNF-α. This suggests that TNF-α-related apoptosis in HCC193 cells could be partially prevented if an anti-TNF-α antibody is co-administered with the drug.
D1143 significantly enhances the radiosensitivity of both HCC193 and H460 lung cancer cell lines
In vitro clonogenic assays were used to determine the effect of D1143 administration on the radiosensitivity of HCC193 and H460 cells (Figure 4A, 4B, solid lines). Using sensitivity data from experiments in Figures 1, 2 and 3, we administered drug doses and incubation times to ensure D1143-induced apoptosis in both cell lines (HCC193 cells: 2.5 μM D1143 for 24 hours; H460 cells: 10 μM of D1143 for 24 hours). Treatment of HCC193 cells with 2.5 μM D1143 for 24 hours caused a significant survival curve shift relative to DMSO-treated cells (DER of 2.19, p = 0.001). In contrast, no change was observed in the H460 cells at 10 μM of D1143 for 24 hours (data not shown). To determine whether additional treatment exposure time and dose affected H460 cells, 10 μM of D1143 was administered for 48 hours and found that these parameters increased the radiosensitivity the cell line (DER of 1.29, p = 0.082).
Figure 4.

D1143 significantly enhances the radiosensitivity of both HCC193 and H460 lung cancer cell lines. A, B. In clonogenic assays, a significant dose enhancement ratio (DER) was evident for HCC193 cells treated with D1143, but not H460 cells (solid black lines; DERs = 2.19 [p = 0.001] vs. 1.29 [p = 0.082], respectively). B. H460 cells were also treated with RT and TNF-α (diamond points, dashed black lines); and RT, TNF-α, and D1143 (square points, dashed black lines). Treatment of H460 cells with RT and TNF-α did not significantly affect the DER; however, treatment with all three caused a significant survival curve shift with a DER of 192 (p = 0.026).
To determine if H460 cell apoptosis could be promoted by stimulating the extrinsic apoptotic pathway, H460 cells were also treated with (1) RT and TNF-α; and (2) RT, TNF-α, and D1143 (Figure 4B, dashed lines). Treatment of H460 cells with RT and TNF-α did not significantly affect the DER; however, treatment with all three caused a significant survival curve shift with a DER of 1.92 (p = 0.026).
Discussion
In this study we examined the effect of D1143 on the radiosensitivity of NSCLC cells. D1143 induced depletion of IAPs (particularly cIAP1 and, to a lesser extent, XIAP) in two NSCLC cell lines (HCC193 and H460) in vitro. The radiosensitization was mediated by autocrine TNF-α production in the more sensitive cell line (HCC193), suggesting that apoptosis favored the extrinsic apoptotic pathway. Radiosensitivity could be potentiated in H460 cells by increasing drug concentration, incubation time, and stimulation of TNF receptors (TNFRs).
Overexpression of IAPs has been linked to radioresistance in cervical cancer [6] and leukemia [11]. Smac mimetics have been shown to be promising treatment for lung [10] and brain cancers [12]. A survey of 50 NSCLC cell lines found that 22% were sensitive to Smac mimetics alone [4]. Smac mimetics also restore sensitivity to chemotherapy in a variety of cell lines [13,14] and radiosensitize colorectal [15] and lung cancers [10].
D1143 is a novel orally active Smac mimetic. The pharmacological properties of D1143 have been described [9]. However, its effects on different cancers, specificity for individual IAPs, and synergy with chemotherapy or radiation are poorly understood. To examine these effects, two NSCLC (sensitive-HCC193 and resistant-H460) cell lines were chosen and showed that D1143 inhibited cell viability in HCC193 cells, with less of an effect in H460 cells (Figure 1). To evaluate the mechanisms of D1143, we increased dose in the cells and measured levels of intact and cleaved caspase-8 and caspase-3 (Figure 2B vs. 2A).
Next, we investigated the mechanism by which D1143 induced apoptosis in these cells. Monovalent Smac mimetics (e.g. D1143, MV1) have been shown to be 100-1000 times less potent than their bivalent counterparts (e.g. BV6). Moreover, monovalent Smac mimetics have a decreased ability to inhibit XIAP compared to cIAP1 [5]. Despite these challenges, the specificity of Smac mimetics for individual IAPs is biologically and clinically important. XIAP is a ubiquitous IAP family member, and it inhibits both intrinsic and extrinsic apoptotic pathways by binding directly to caspases 3, 7, and 9. In contrast, cIAPs have a larger effect on TNF-α signaling, tumor necrosis receptor-associated factor (TRAF2), NF-κβ inducing kinase (NIK), and the potentiation of the oncogene Myc by promoting ubiquitination and proteasomal degradation of the Myc inhibitory protein, Mad1 [2].
We examined how the monovalent D1143 affected activation of XIAP and cIAP1. Consistent with previous work [9], D1143 was more effective in depleting cIAP1 than XIAP in both the sensitive and resistant cell lines (Figure 2C, 2D). cIAP1 was depleted in HCC193 cells with lower concentrations of D1143 than in H460 cells. In contrast, XIAP was not depleted in either cell line at the highest concentrations tested or with prolonged periods of time. H460 cells have been shown to overexpress XIAP (but not cIAP1) [16] and overexpression of XIAP contributes to their radioresistance. These effects could potentially explain the limited effects of D1143 on the H460 cell line.
Previous studies have shown that in the extrinsic pathway cells release TNF-α, which binds to TNFRs, stimulates the Fas-associating protein with a death domain (FADD)/ receptor interactor protein 1 (RIP1)/RIP3, and subsequently activates caspase-8 and caspase-3 [2]. When exposed to D1143, HCC193 cells secreted increased levels of TNF-α, while H460 cells did not (Figure 3A). The TNF-α-related apoptosis could be partially prevented if an anti-TNF-α antibody (i.e. infliximab) was co-administered with the D1143; however, the ability of infliximab to prevent apoptosis was limited, as increasing D1143 concentration further decreased cell viability compared to control (Figure 3B). Over 80% of NSCLC cell lines are resistant to single-agent Smac mimetic therapy [4]. However, the radiosensitization of H460 cells could be potentiated with increased drug concentration, incubation, RT, and exogenous/paracrine TNF-α (Figure 4A, 4B).
The apoptotic pathways involving RT, Smacs, and IAPs are complex and under investigation (Figure 5). Smac mimetics were initially thought to potentiate apoptosis by promoting activation of caspases 3, 8, and 9. Reports published since 2005, however, show that the anti-apoptotic effects of Smac mimetics as single agents are instead largely due to auto-ubiquitination and degradation of cIAPs, their disinhibition of RIPK3, and induction of TNF-α secretion [2,19]. One report indicates that “IAP antagonist-induced activation of both the canonical and noncanonical NF-κB pathways leads to increased expression of the NF-κB responsive gene TNF-α, which in the absence of c-IAP proteins promotes apoptosis by binding to TNF-R1 [2]”. The culmination of this experiments data and previous reports of the nature of IAPs can help explain the role of TNF-α in radiosensitizing HCC193 and H460 cell lines.
Figure 5.
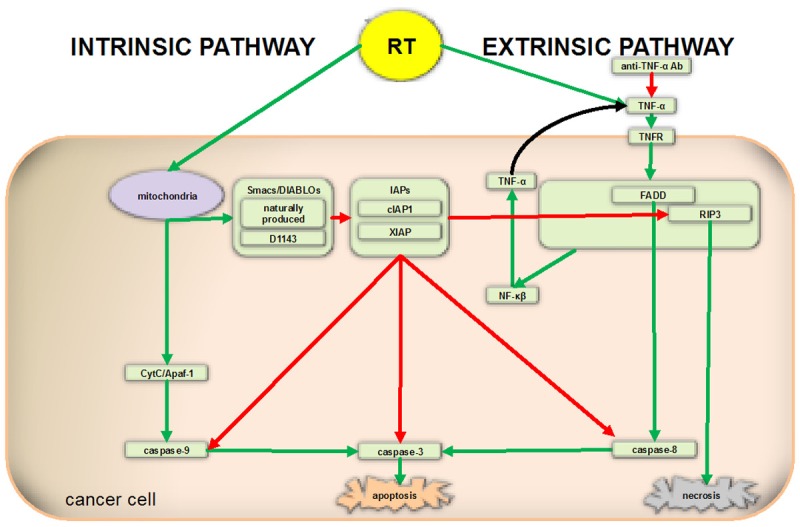
Schematic representation of molecular pathways involved in apoptosis. Smac mimetics were initially thought to potentiate apoptosis by promoting activation of caspases 3, 8, and 9. Reports published since 2005 show that the anti-apoptotic effects of Smac mimetics are largely due to auto-ubiquitination and degradation of cIAPs, their disinhibition of RIPK3, and increased TNF-α production. Certain cancer cells may be resistant to Smac mimetics, in part because of upregulation of FLIP, which downregulates caspase-8 activation, preventing apoptosis.
Our goal was to provide a translational research background that supports the use of D1143 for lung cancers in animal models and the clinical setting. Future projects should explore the effect of D1143 on the intrinsic apoptotic pathway, which may be the primary mechanism of apoptosis in certain cancer cell lines. Additionally, further work is necessary to elucidate the effect of D1143 on extrinsic apoptotic pathway, including the identification of cancers that are resistant to D1143. These results would personalize D1143 to specific cancers and minimize the chance of toxicity. Finally, further studies using broader multimodal approaches (e.g. RT, chemotherapy, immunotherapy) are necessary in animals to elucidate the role of D1143 in vivo.
Disclosure of conflict of interest
None.
Abbreviations
- cIAP
cellular inhibitor of apoptosis protein
- DIABLO
direct IAP-binding protein with low pI
- FADD
Fas-Associated protein with Death Domain
- FLIP
FLICE-inhibitory protein
- NF-κβ
nuclear factor κβ
- NIK
NF-κβ inducing kinase
- TNF-α
tumor necrosis factor-α
- TNFR
TNF receptor
- RANK
Receptor activator of NF-κβ ligand
- RIP
receptor interactor protein
- Smac
second mitochondria-derived activator of caspase
- XIAP
X-linked inhibitor of apoptosis protein.
Supporting Information
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Dynek JN, Vucic D. Antagonists of IAP proteins as cancer therapeutics. Cancer Lett. 2013;332:206–14. doi: 10.1016/j.canlet.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliot LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Imoto I, Tsuda H, Hirasawa A, Miura M, Sakamoto M, Hirohashi S, Inazawa J. Expression of cIAP1, a target for 11q22 amplification, correlates with resistance of cervical cancers to radiotherapy. Cancer Res. 2002;62:4860–4866. [PubMed] [Google Scholar]
- 7.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 8.Sekimura A, Konishi A, Mizuno K, Kobayashi Y, Sasaki H, Yano M, Fukai I, Fujii Y. Expression of Smac/DIABLO is a novel prognostic marker in lung cancer. Oncol Rep. 2004;11:797–802. [PubMed] [Google Scholar]
- 9.Cai Q, Sun H, Peng Y, Lu J, Nikolovska-Coleska Z, McEachern D, Liu L, Qiu S, Yang CY, Miller R, Yi H, Zhang T, Sun D, Kang S, Guo M, Leopold L, Yang , Wang S. A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment. J Med Chem. 2011;54:2714–2726. doi: 10.1021/jm101505d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Li B, Giacalone NJ, Torossian A, Sun Y, Niu K, Lin-Tsai O, Lu B. BV6, an IAP antagonist, activates apoptosis and enhances radiosensitization of non-small cell lung carcinoma in vitro. J Thorac Oncol. 2011;6:1801–1809. doi: 10.1097/JTO.0b013e318226b4a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weston VJ, Austen B, Wei W, Marston E, Alvi A, Lawson S, Darbyshire PJ, Grittiths M, Hill F, Mann JR, Moss PA, Taylor AM, Stankovic T. Apoptotic resistance to ionizing radiation in pediatric B-precursor acute lymphoblastic leukemia frequently involves increased NF-kappaB survival pathway signaling. Blood. 2004;104:1465–1473. doi: 10.1182/blood-2003-11-4039. [DOI] [PubMed] [Google Scholar]
- 12.Vellanki SH, Grabrucker A, Liebau S, Proepper C, Eramo A, Braun V, Boeckers T, Debatin KM, Fulda S. Small-molecule XIAP inhibitors enhance gamma-irradiation-induced apoptosis in glioblastoma. Neoplasia. 2009;11:743–752. doi: 10.1593/neo.09436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 14.Dineen SP, Roland CL, Greer R, Carbon JG, Toombs JE, Gupta P, Bardeesy N, Sun H, Williams N, Minna JD, Brekken RA. Smac mimetic increases chemotherapy response and improves survival in mice with pancreatic cancer. Cancer Res. 2010;70:2852–2861. doi: 10.1158/0008-5472.CAN-09-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huerta S, Gao X, Livingston EH, Kapur P, Sun H, Anthony T. In vitro and in vivo radiosensitization of colorectal cancer HT-29 cells by the smac mimetic JP-1201. Surgery. 2010;148:346–353. doi: 10.1016/j.surg.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Mashima T, Sato S, Mochizuki M, Sakamoto H, Yamori T, Oh-Hara T, Tsuruo T. Predominant suppression of apoptosome by inhibitor of apoptosis protein in non-small cell lung cancer H460 cells: therapeutic effect of a novel polyarginine-conjugated Smac peptide. Cancer Res. 2003;63:831–837. [PubMed] [Google Scholar]
- 17.Brunckhorst MK, Lerner D, Wang S, Yu Q. AT-406, an orally active antagonist of multiple inhibitor of apoptosis proteins, inhibits progression of human ovarian cancer. Cancer Biol Ther. 2012;13:804–811. doi: 10.4161/cbt.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C, Davis JL, Zeng R, Vora P, Su X, Collins Ll, Vangveravong S, Mach RH, Piwnica-Worms D, Weilbaecher KN, Faccio R, Novack DV. Antagonism of Inhibitor of Apoptosis Proteins Increases Bone Metastasis via Unexpected Osteoclast Activation. Cancer Discov. 2013;3:212–223. doi: 10.1158/2159-8290.CD-12-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H, Tschopp J, Lin SC. Smac mimetics and TNFalpha: a dangerous liaison? Cell. 2007;131:655–658. doi: 10.1016/j.cell.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai Q, Sun H, Peng Y, Lu J, Nikolovska-Coleska Z, McEachern D, Liu L, Qiu S, Yang CY, Miller R, Yi H, Zhang T, Sun D, Kang S, Guo M, Leopold L, Yang D, Wang S. A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment. J Med Chem. 2011;54:2714–26. doi: 10.1021/jm101505d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


