Abstract
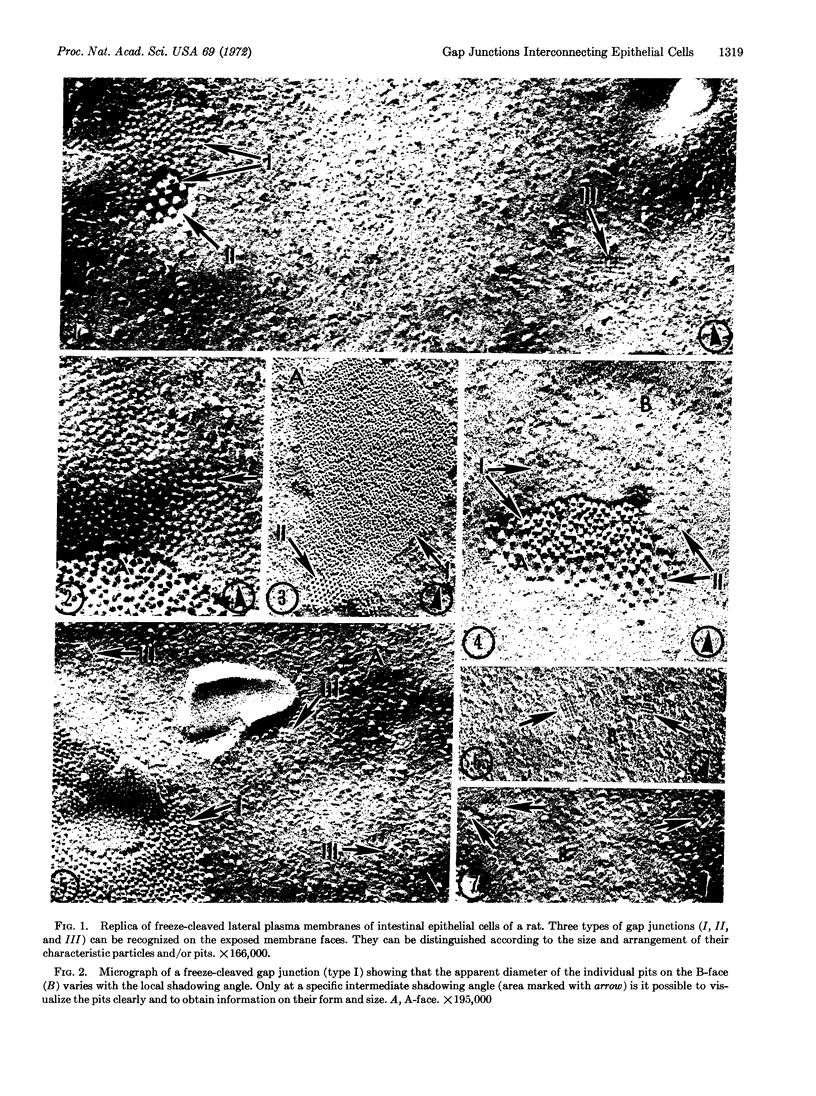
Gap junctions are specialized membrane regions that seem to mediate intercellular communication. They appear to contain closely packed arrays of equally sized particles all of which, upon freeze-cleaving, remain attached to one membrane leaflet and not to the other. One gap junction cleavage face, therefore, always exhibits a closely packed array of particles, while the other features a corresponding array of pits. By using these morphological criteria, we have been able to distinguish three different types of gap junctions interlinking adjacent epithelial cells of the small intestine. All three types may be found in close proximity to each other, and in all cases, the particles remain attached to the cleavage face of the cytoplasmic membrane leaflet (A-face). The most frequently encountered type-I gap junctions, which have already been observed in many other tissues, possess 8- to 9-nm (80- to 90-Å) particles with a center-to-center spacing of 9-10 nm (90-100 Å) when packed in a hexagonal lattice. Type-II gap junctions are always found in close association with type-I junctions. They can be distinguished from the type-I junctions by the greater size [10-11 nm (100-110 Å) in diameter] and the greater spacing (190-200 Å) of their hexagonally arrayed particles. In contrast, the particles of the type-III gap junctions are arranged in very small rectilinear arrays with a spacing of only 6-8 nm (60-80 Å). Gap junctions may be involved in the control of intercellular flow of different types of regulatory molecules.
Keywords: membranes, rat
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr L., Berger W., Dewey M. M. Electrical transmission at the nexus between smooth muscle cells. J Gen Physiol. 1968 Mar;51(3):347–368. doi: 10.1085/jgp.51.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti E. L., Emmelot P. Hexagonal array of subunits in tight junctions separated from isolated rat liver plasma membranes. J Cell Biol. 1968 Jul;38(1):15–24. doi: 10.1083/jcb.38.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalcroft J. P., Bullivant S. An interpretation of liver cell membrane and junction structure based on observation of freeze-fracture replicas of both sides of the fracture. J Cell Biol. 1970 Oct;47(1):49–60. doi: 10.1083/jcb.47.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey M. M., Barr L. Intercellular Connection between Smooth Muscle Cells: the Nexus. Science. 1962 Aug 31;137(3531):670–672. doi: 10.1126/science.137.3531.670-a. [DOI] [PubMed] [Google Scholar]
- Flower N. E. Septate and gap junctions between the epithelial cells of an invertebrate, the mollusc Cominella maculosa. J Ultrastruct Res. 1971 Nov;37(3):259–268. doi: 10.1016/s0022-5320(71)80123-5. [DOI] [PubMed] [Google Scholar]
- Gilula N. B., Satir P. Septate and gap junctions in molluscan gill epithelium. J Cell Biol. 1971 Dec;51(3):869–872. doi: 10.1083/jcb.51.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Revel J. P. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970 May;45(2):272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J., Revel J. P. Coexistence of gap and sseptate junctions in an invertebrate epithelium. J Cell Biol. 1971 Jul;50(1):92–101. doi: 10.1083/jcb.50.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. G., Sheridan J. D. Junctions between cancer cells in culture: ultrastructure and permeability. Science. 1971 Nov 12;174(4010):717–719. doi: 10.1126/science.174.4010.717. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Intercellular communication. Sci Am. 1970 May;222(5):79–86. [PubMed] [Google Scholar]
- Loewenstein W. R., Nakas M., Socolar S. J. Junctional membrane uncoupling. Permeability transformations at a cell membrane junction. J Gen Physiol. 1967 Aug;50(7):1865–1891. doi: 10.1085/jgp.50.7.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeability of membrane junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):441–472. doi: 10.1111/j.1749-6632.1966.tb50175.x. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Hershberg R. A., Weinstein R. S. Further observations on the occurrence of nexuses in benign and malignant human cervical epithelium. J Cell Biol. 1971 Dec;51(3):805–825. doi: 10.1083/jcb.51.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Weinstein R. S. The ultrastructure of the nexus. A correlated thin-section and freeze-cleave study. J Cell Biol. 1970 Dec;47(3):666–688. doi: 10.1083/jcb.47.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor H., Mühlethaler K. FINE STRUCTURE IN FROZEN-ETCHED YEAST CELLS. J Cell Biol. 1963 Jun 1;17(3):609–628. doi: 10.1083/jcb.17.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas G. D., Asada Y., Bennett M. V. Morphological correlates of increased coupling resistance at an electrotonic synapse. J Cell Biol. 1971 Apr;49(1):173–188. doi: 10.1083/jcb.49.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton B. W., Bennett M. V., Pappas G. D. Permeability and structure of junctional membranes at an electrotonic synapse. Science. 1969 Dec 26;166(3913):1641–1643. doi: 10.1126/science.166.3913.1641. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. D. THE OCCURRENCE OF A SUBUNIT PATTERN IN THE UNIT MEMBRANES OF CLUB ENDINGS IN MAUTHNER CELL SYNAPSES IN GOLDFISH BRAINS. J Cell Biol. 1963 Oct;19:201–221. doi: 10.1083/jcb.19.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Karnovsky M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Yee A. G., Hudspeth A. J. Gap junctions between electrotonically coupled cells in tissue culture and in brown fat. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2924–2927. doi: 10.1073/pnas.68.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Depression of junctional membrane permeability by substitution of lithium for extracellular sodium. Biochim Biophys Acta. 1969 Jan 28;173(1):146–148. doi: 10.1016/0005-2736(69)90046-7. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Bertaud W. S. Temperature and contamination dependent freeze-etch images of frozen water and glycerol solutions. J Ultrastruct Res. 1971 Oct;37(1):146–168. doi: 10.1016/s0022-5320(71)80047-3. [DOI] [PubMed] [Google Scholar]