Abstract
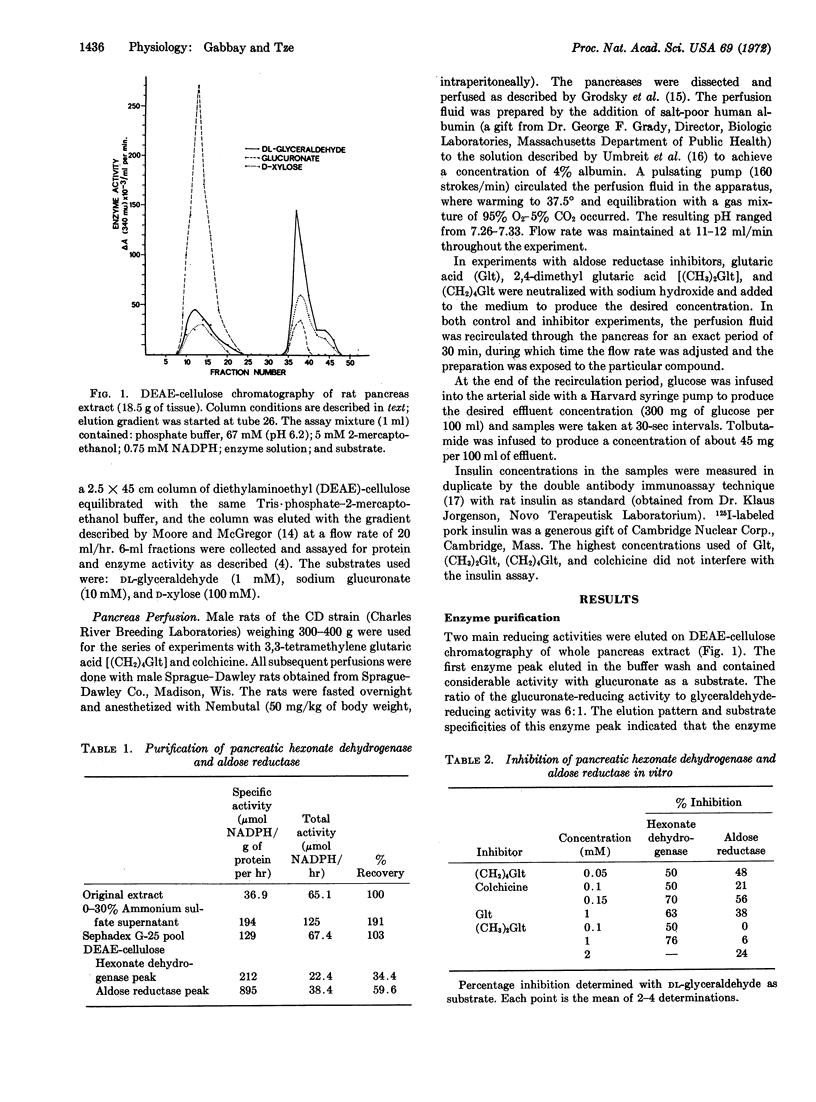
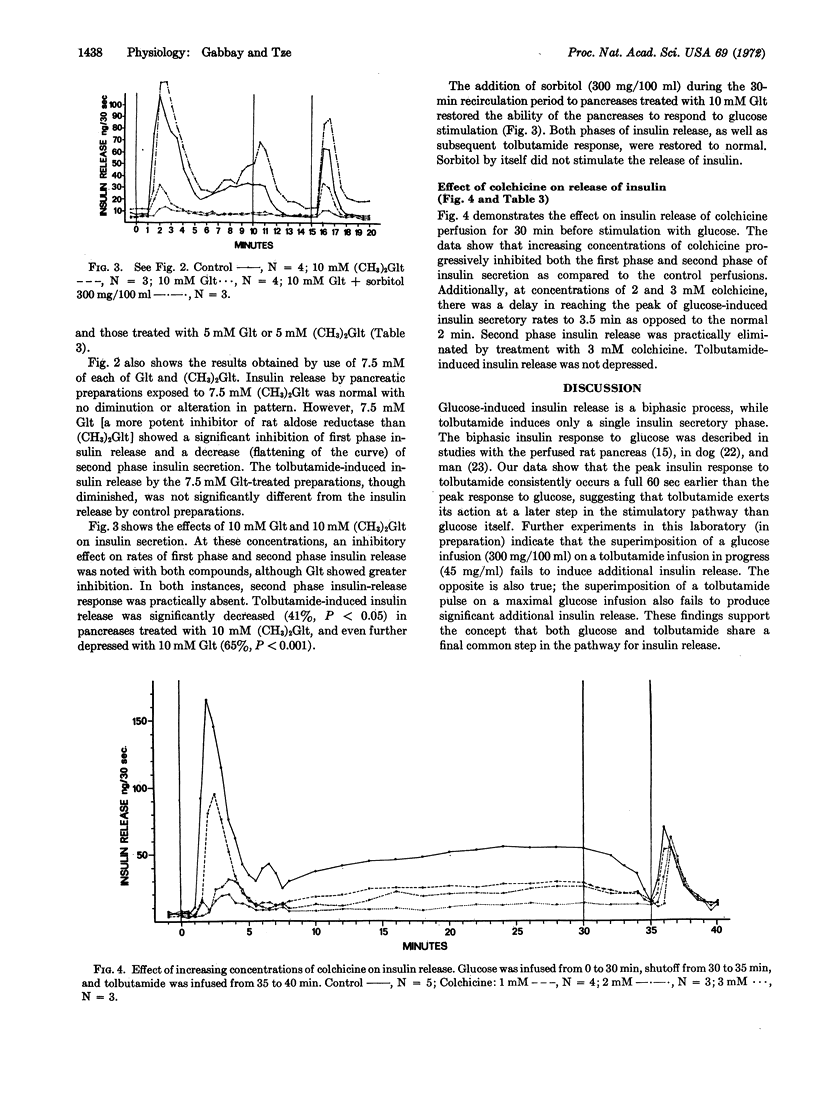
Aldose reductase (alditol: NADP oxidoreductase, EC 1.1.1.21) is the enzyme responsible for the conversion of glucose to its sugar alcohol, sorbitol. In this study, aldose reductase and a closely related enzyme, L-hexonate dehydrogenase (L-gulonate: NADP oxidoreductase, EC 1.1.1.19), were purified from rat pancreas. Glutaric acid, 2,4-dimethyl glutaric acid, 3,3-tetramethylene glutaric acid, and colchicine inhibited both enzymes, albeit with different potencies. These compounds also inhibited both phases of glucose-induced release of insulin by the perfused rat pancreas. The potencies of these inhibitors in depressing the release of insulin correlated with their effectiveness in inhibiting aldose reductase. At higher concentrations of inhibitors, tolbutamide-induced release of insulin was also depressed. The addition of exogenous sorbitol to pancreases treated with glutaric acid restored their ability to respond to glucose and tolbutamide. These findings suggest that the conversion of free intracellular glucose to sorbitol in the beta cell is an essential step in the glucose-induced release mechanism.
Keywords: sorbitol pathway, beta cell, glutaric acid, colchicine
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammon H. P., Steinke J. 6-Amnionicotinamide (6-AN) as a diabetogenic agent. In vitro and in vivo studies in the rat. Diabetes. 1972 Mar;21(3):143–148. doi: 10.2337/diab.21.3.143. [DOI] [PubMed] [Google Scholar]
- Cerasi E., Luft R. "What is inherited--what is added" hypothesis for the pathogenesis of diabetes mellitus. Diabetes. 1967 Sep;16(9):615–627. doi: 10.2337/diab.16.9.615. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Weaver J. P., Winegrad A. I. The distribution of polyol: NADP oxidoreductase in mammalian tissues. Biochem Biophys Res Commun. 1969 Oct 8;37(2):347–353. doi: 10.1016/0006-291x(69)90741-4. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968 Sep;83(3):572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., O'Sullivan J. B. The sorbitol pathway in diabetes and galactosemia: enzyme and sobstrate localization and changes in kidney. Diabetes. 1968;17:300–300. [PubMed] [Google Scholar]
- Gabbay K. H., O'Sullivan J. B. The sorbitol pathway. Enzyme localization and content in normal and diabetic nerve and cord. Diabetes. 1968 May;17(5):239–243. doi: 10.2337/diab.17.5.239. [DOI] [PubMed] [Google Scholar]
- HAYMAN S., KINOSHITA J. H. ISOLATION AND PROPERTIES OF LENS ALDOSE REDUCTASE. J Biol Chem. 1965 Feb;240:877–882. [PubMed] [Google Scholar]
- Jedziniak J. A., Kinoshita J. H. Activators and inhibitors of lens aldose reductase. Invest Ophthalmol. 1971 May;10(5):357–366. [PubMed] [Google Scholar]
- KINOSHITA J. H., FUTTERMAN S., SATOH K., MEROLA L. O. FACTORS AFFECTING THE FORMATION OF SUGAR ALCOHOLS IN OCULAR LENS. Biochim Biophys Acta. 1963 Aug 13;74:340–350. doi: 10.1016/0006-3002(63)91377-5. [DOI] [PubMed] [Google Scholar]
- Kanazawa Y., Kuzuya T., Ide T., Kosaka K. Plasma insulin responses to glucose in femoral, hepatic, and pancreatic veins in dogs. Am J Physiol. 1966 Aug;211(2):442–448. doi: 10.1152/ajplegacy.1966.211.2.442. [DOI] [PubMed] [Google Scholar]
- Kinoshita J. H. Cataracts in galactosemia. The Jonas S. Friedenwald Memorial Lecture. Invest Ophthalmol. 1965 Oct;4(5):786–799. [PubMed] [Google Scholar]
- Kinoshita J. H., Dvornik D., Kraml M., Gabbay K. H. The effect of an aldose reductase inhibitor on the galactose-exposed rabbit lens. Biochim Biophys Acta. 1968 Jun 24;158(3):472–475. doi: 10.1016/0304-4165(68)90305-x. [DOI] [PubMed] [Google Scholar]
- MANO Y., SUZUKI K., YAMADA K., SHIMAZONO N. Enzymic studies on TPN L-hexonate dehydrogenase from rat liver. J Biochem. 1961 Jun;49:618–634. doi: 10.1093/oxfordjournals.jbchem.a127352. [DOI] [PubMed] [Google Scholar]
- MOORE B. W., MCGREGOR D. CHROMATOGRAPHIC AND ELECTROPHORETIC FRACTIONATION OF SOLUBLE PROTEINS OF BRAIN AND LIVER. J Biol Chem. 1965 Apr;240:1647–1653. [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E., Krzanowski J., Kotler-Brajtburg J., Landgraf R., Fertel R. The dual function of glucose in islets of Langerhans. J Biol Chem. 1971 Feb 25;246(4):1007–1011. [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E. Metabolism of glucose in the islets of Langerhans. J Biol Chem. 1968 May 25;243(10):2730–2736. [PubMed] [Google Scholar]
- Moonsammy G. I., Stewart M. A. Purification and properties of brain aldose reductase and L-hexonate dehydrogenase. J Neurochem. 1967 Dec;14(12):1187–1193. doi: 10.1111/j.1471-4159.1967.tb06166.x. [DOI] [PubMed] [Google Scholar]
- Morrison A. D., Winegrad A. I., Fink C. J., Lacy P. E. Sorbitol synthesis in isolated rat pancreatic islets. Biochem Biophys Res Commun. 1970 Feb 6;38(3):491–495. doi: 10.1016/0006-291x(70)90740-0. [DOI] [PubMed] [Google Scholar]
- Renold A. E. Insulin biosynthesis and secretion--a still unsettled topic. N Engl J Med. 1970 Jan 22;282(4):173–182. doi: 10.1056/NEJM197001222820401. [DOI] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Spector A., Zorn M., Li L. K. Architecture of calf lens alpha-crystallin. Exp Eye Res. 1968 Jan;7(1):154–163. doi: 10.1016/s0014-4835(68)80040-5. [DOI] [PubMed] [Google Scholar]


