Abstract
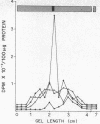
The hormonal induction of glutamine synthetase (EC 6.3.1.2) in embryonic neural retina tissue in vitro is blocked preferentially and reversibly by proflavine (3,6-diaminoacridine) in the absence of cell or DNA replication; cell viability is not affected, and the synthesis of total cellular proteins and RNA is only slightly reduced. In the induction of this enzyme, there is a rapid increase in the synthesis and accumulation of the enzyme selectively elicited by the steroid inducer; for this effect, transcription is essential. Radioimmunochemical measurements have shown that proflavine inhibits induction by prevention of de novo synthesis of catalytically active and immunologically reactive glutamine synthetase protein. It does not measurably affect the uptake of the steroid inducer by the retina cells. Since the translation of this enzyme by preformed RNA templates is not stopped by proflavine, the inhibitory effect of proflavine on induction is apparently due to interference with transcriptional or pretranslational processes required for the provision of active transcripts for enzyme synthesis. The finding that proflavine inhibits preferentially a tissue-specific, inducible differentiation in postmitotic embryonic neural cells offers new approaches to the study of regulation of gene expression in eukaryotes.
Keywords: acridines, enzyme induction, gene regulation, differentiation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott J., Holtzer H. The loss of phenotypic traits by differentiated cells, V. The effect of 5-bromodeoxyuridine on cloned chondrocytes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1144–1151. doi: 10.1073/pnas.59.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alescio T., Moscona A. A. Immunochemical evidence for enzyme synthesis in the hormonal induction of glutamine synthetase in embryonic retina in culture. Biochem Biophys Res Commun. 1969 Jan 27;34(2):176–182. doi: 10.1016/0006-291x(69)90628-7. [DOI] [PubMed] [Google Scholar]
- HURWITZ J., FURTH J. J., MALAMY M., ALEXANDER M. The role of deoxyribonucleic acid in ribonucleic acid synthesis. III. The inhibition of the enzymatic synthesis of ribonucleic acid and deoxyribonucleic acid by actinomycin D and proflavin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1222–1230. doi: 10.1073/pnas.48.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada M., Inouye M., Eda M., Tsugita A. Frameshift mutation in the lysozyme gene of bacteriophage T4: demonstration of the insertion offour bases and the preferential occurrence of base addition in acridine mutagenesis. J Mol Biol. 1970 Dec 14;54(2):199–217. doi: 10.1016/0022-2836(70)90427-4. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. A., Moscona M. H., Saenz N. Enzyme induction in embryonic retina: the role of transcription and translation. Proc Natl Acad Sci U S A. 1968 Sep;61(1):160–167. doi: 10.1073/pnas.61.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. A., Moscona M., Jones R. E. Induction of glutamine synthetase in embryonic neural retina in vitro by inhibitors of macromolecular synthesis. Biochem Biophys Res Commun. 1970 Jun 5;39(5):943–949. doi: 10.1016/0006-291x(70)90415-8. [DOI] [PubMed] [Google Scholar]
- Moscona A. A., Piddington R. Stimulation by hydrocortisone of premature changes in the developmental pattern of glutamine synthetase in embryonic retina. Biochim Biophys Acta. 1966 Jun 29;121(2):409–411. doi: 10.1016/0304-4165(66)90131-0. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Spelsberg T. C., Scharder W. T., Chytil F., Steggles A. W. Mechanisms of interaction of a hormone--receptor complex with the genome of a eukaryotic target cell. Nature. 1972 Jan 21;235(5334):141–144. doi: 10.1038/235141a0. [DOI] [PubMed] [Google Scholar]
- ORGEL A., BRENNER S. Mutagenesis of bacteriophage T4 by acridines. J Mol Biol. 1961 Dec;3:762–768. doi: 10.1016/s0022-2836(61)80081-8. [DOI] [PubMed] [Google Scholar]
- Piddington R. Distribution and development of glutamine synthetase in the embryonic cerebral hemisphere. J Exp Zool. 1971 Jun;177(2):219–227. doi: 10.1002/jez.1401770209. [DOI] [PubMed] [Google Scholar]
- Sarkar P. K., Moscona A. A. Changes in macromolecular synthesis associated with the induction of glutamine synthetase in embryonic retina. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2308–2311. doi: 10.1073/pnas.68.9.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]