Abstract
Phosphatidylcholine vesicles are permeable to tempotartrate, a spin-label derivative of tartaric acid. The inside-outside distribution of tempotartrate is coupled to the inside-outside distribution of H+, so it must be a measure of the transmembrane electrical potential difference in vesicles permeable to H+. This prediction is borne out by the finding that the inside-outside distribution of tempotartrate is the reciprocal of the inside-outside distribution of K+ in vesicles prepared in the presence of valinomycin.
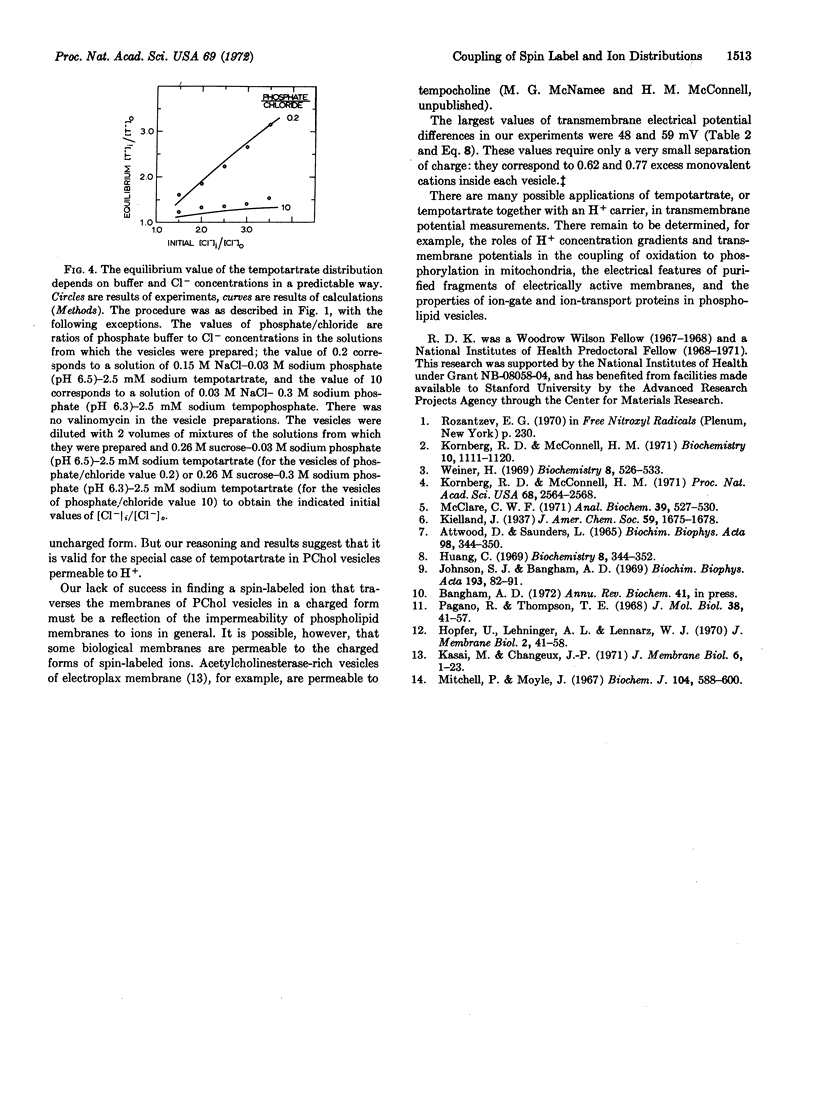
The inside-outside distribution of tempotartrate is, by contrast, equal to the inside-outside distribution of Cl- in vesicles without valinomycin. This is evidence that an inside-outside Cl- concentration gradient induces an H+ gradient, which must be due to HCl permeation.
Keywords: spin labels, valinomycin
Full text
PDF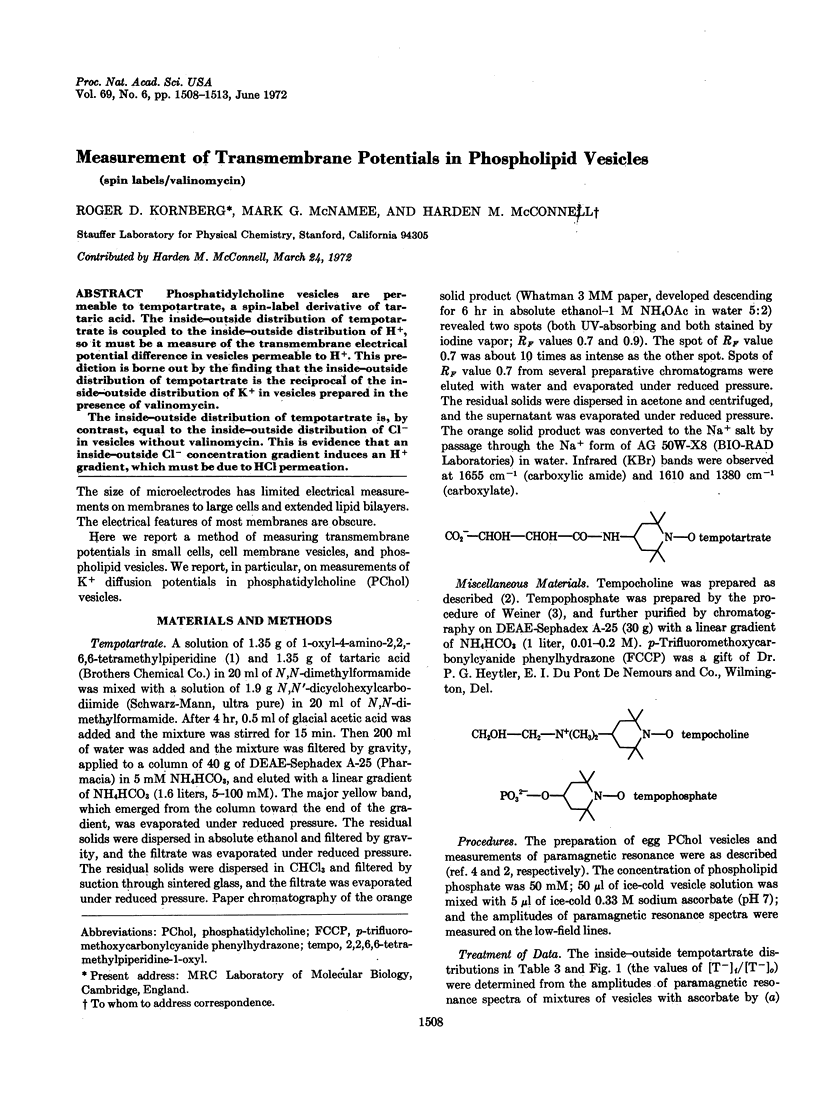




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATTWOOD D., SAUNDEES L. A LIGHT-SCATTERING STUDY OF ULTRASONICALLY IRRADIATED LECITHIN SOLS. Biochim Biophys Acta. 1965 Apr 5;98:344–350. doi: 10.1016/0005-2760(65)90126-8. [DOI] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Bangham A. D. Potassium permeability of single compartment liposomes with and without valinomycin. Biochim Biophys Acta. 1969 Oct 14;193(1):82–91. doi: 10.1016/0005-2736(69)90061-3. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McConnell H. M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 1971 Mar 30;10(7):1111–1120. doi: 10.1021/bi00783a003. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McConnell H. M. Lateral diffusion of phospholipids in a vesicle membrane. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2564–2568. doi: 10.1073/pnas.68.10.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClare C. W. An accurate and convenient organic phosphorus assay. Anal Biochem. 1971 Feb;39(2):527–530. doi: 10.1016/0003-2697(71)90443-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R., Thompson T. E. Spherical lipid bilayer membranes: electrical and isotopic studies of ion permeability. J Mol Biol. 1968 Nov 28;38(1):41–57. doi: 10.1016/0022-2836(68)90127-7. [DOI] [PubMed] [Google Scholar]
- Weiner H. Interaction of a spin-labeled analog of nicotinamide-adenine dinucleotide with alcohol dehydrogenase. I. Synthesis, kinetics, and electron paramagnetic resonance studies. Biochemistry. 1969 Feb;8(2):526–533. doi: 10.1021/bi00830a011. [DOI] [PubMed] [Google Scholar]


