Significance
Aging is accompanied by disruptive alterations in large-scale brain systems, such as the default mode network (DMN) and the associated hippocampus (HC) subsystem, which support higher cognitive functions. However, the exact form of DMN–HC alterations and concomitant memory deficits is largely unknown. We identified age-related decrements in resting-state functional connectivity of the cortical DMN, whereas elevated connectivity between the bilateral HC was found along with attenuated HC–cortical connectivity. Critically, elevated HC at rest restricts the degree to which HC interacts with other brain regions during memory tasks, and thus results in memory deficits. This study provides empirical evidence of how the relationship between the DMN and HC breaks down in aging and how such alterations underlie deficient mnemonic processing.
Keywords: hippocampus, DMN, resting state, episodic memory, aging
Abstract
The brain is not idle during rest. Functional MRI (fMRI) studies have identified several resting-state networks, including the default mode network (DMN), which contains a set of cortical regions that interact with a hippocampus (HC) subsystem. Age-related alterations in the functional architecture of the DMN and HC may influence memory functions and possibly constitute a sensitive biomarker of forthcoming memory deficits. However, the exact form of DMN–HC alterations in aging and concomitant memory deficits is largely unknown. Here, using both task and resting data from 339 participants (25–80 y old), we have demonstrated age-related decrements in resting-state functional connectivity across most parts of the DMN, except for the HC network for which age-related elevation of connectivity between left and right HC was found along with attenuated HC–cortical connectivity. Elevated HC connectivity at rest, which was partly accounted for by age-related decline in white matter integrity of the fornix, was associated with lower cross-sectional episodic memory performance and declining longitudinal memory performance over 20 y. Additionally, elevated HC connectivity at rest was associated with reduced HC neural recruitment and HC–cortical connectivity during active memory encoding, which suggests that strong HC connectivity restricts the degree to which the HC interacts with other brain regions during active memory processing revealed by task fMRI. Collectively, our findings suggest a model in which age-related disruption in cortico–hippocampal functional connectivity leads to a more functionally isolated HC at rest, which translates into aberrant hippocampal decoupling and deficits during mnemonic processing.
The brain is not idle at rest (1). Rather, intrinsic neuronal signaling, which manifests as spontaneous fluctuations in the blood oxygen level-dependent (BOLD) functional MRI (fMRI) signal, is ubiquitous in the human brain and consumes a substantial portion of the brain’s energy (2). Coherent spontaneous activity has been revealed in a hierarchy of networks that span large-scale functional circuits in the brain (3–6). These resting-state networks (RSNs) show moderate-to-high test–retest reliability (7) and replicability (8), and some have been found in the monkey (9) and infant (10) brain. In the adult human brain, RSNs include sensory motor, visual, attention, and mnemonic networks, as well as the default mode network (DMN). There is evidence that the DMN entails interacting subsystems and hubs that are implicated in episodic memory (11–13). One major hub encompasses the posterior cingulate cortex and the retrosplenial cortex. Other hubs include the lateral parietal cortex and the medial prefrontal cortex. In addition, a hippocampus (HC) subsystem is distinct from, yet interrelated with, the major cortical DMN hubs (12, 14).
The functional architecture of the DMN and other RSNs is affected by different conditions, such as Alzheimer’s disease (AD), Parkinson’s disease, and head injury, suggesting that measurements of the brain’s intrinsic activity may be a sensitive biomarker and a putative diagnostic tool (for a review, see ref. 15). Alterations of the DMN have also been shown in age-comparative studies (16, 17), but the patterns of alterations are not homogeneous across different DMN components (18). Reduced functional connectivity among major cortical DMN nodes has been reported in aging (16, 17) and also in AD (19) and for asymptomatic APOE e4 carriers at increased risk of developing AD (20). Reduced cortical DMN connectivity has been linked to age-impaired performance on episodic memory (EM) tasks (21, 22). For instance, Wang and colleagues (21) showed that functional connectivity between cortical and HC hubs promoted performance on an EM task and was substantially weaker among low-performing elderly. This and other findings suggest that reductions in the DMN may be a basis for age-related EM impairment. However, elevated connectivity has been observed for the HC in individuals at genetic risk for AD (23, 24) and for elderly with memory complaints (25). Furthermore, a trend toward elevated functional connectivity for the medial temporal lobe (MTL) subsystem was observed in healthy older adults (26). Critically, higher subcortical RSN connectivity was found to correlate negatively with EM performance in an aging sample (27). Moreover, a recent combined fMRI/EEG study observed age increases in HC EEG beta power during rest (28).
Thus, the association of aging with components of the DMN is complex, and it has been argued that age-related increases in functional connectivity need further examination (18). Such increases could reflect a multitude of processes, including age-related degenerative effects on the brain’s gray and white matter (18). Additionally, increases in HC functional connectivity may reflect alterations in proteolytic processes, such as amyloid deposition (29). Amyloid deposition is most prominent in posterior cortical regions of the DMN (29). It has been argued that there is a topological relationship between high neural activity over a lifetime within the DMN and amyloid deposition (30). Increased amyloid β protein burden within the posterior cortical DMN may cause cortico–hippocampal functional connectivity disruption (31), leading to a more functionally isolated HC network, which translates into aberrant hippocampal decoupling (30, 32, 33). Correspondingly, a recent model hypothesized that progressively less inhibitory cortical input would cause HC hyperactivity in aging (34).
Elevated HC resting-state connectivity might thus be a sign of brain dysfunction, but the evidence remains inconclusive. Here, using data from a population-based sample covering the adult age span (n = 339, 25–80 y old), we tested the hypothesis that aging differentially affects distinct DMN components. A data-driven approach, independent component analysis (ICA), was used to identify DMN subsystems (4). We expected to observe age-related decreases in the connectivity of the cortical DMN. We also examined age-related alterations of HC RSN connectivity, and tested whether such alterations were related to HC volume and white matter integrity. We predicted that if increased HC connectivity was found, it would be accompanied by age-related decreases in internetwork connectivity of the HC RSN with cortical DMN regions. To constrain interpretations of age-related alterations, the DMN components were related to cognitive performance. Elevated HC RSN should negatively correlate with level and longitudinal change in EM performance. Such negative correlations could reflect an inability to flexibly recruit the HC and functionally associated areas during EM task performance due to aberrant hippocampal decoupling (23, 24). We tested this prediction by relating the HC RSN, within-person, to HC recruitment during an EM fMRI task (35, 36).
Results
Mapping Resting-State Networks.
Our primary results stem from ICA decomposition of resting-state fMRI data at an ICA dimensionality of 47. Twenty-four of the 47 components were considered to reflect RSNs (SI Text). All components exhibited high spatial correlations (r > 0.60, p < 0.0001) with established templates of RSNs (4, 5). In line with previous reports (3–5), these RSNs encompassed the basal ganglia, auditory cortex, and cerebellum, as well as several visual, sensorimotor, and attention networks (Fig. S1). Critically, three DMN components, including the posterior and anterior cortical components as well as the HC component, were part of the observed patterns (Fig. S1; see Fig. S2 after pre-ICA motion correction). It is also important to note that functional architecture of the DMN components remained relatively stable at different model orders (Fig. S3).
Two different ICA-driven connectivity measures were computed for each DMN per subject. Voxelwise functional connectivity indicates the local strength of connectivity for each voxel within each DMN, whereas global functional connectivity reflects a single composite measure of functional connectivity strength within each DMN component (see SI Text for details). In addition, we considered amplitude, a measure of BOLD signal fluctuation, as an index of level of activation within each DMN component.
To investigate the effect of age on cortical and HC DMN subsystems, we considered the different DMN measures (voxelwise/global connectivity, amplitude) as dependent variables. In addition to age and sex, a measure of head motion (framewise displacement) and a measure of data quality (temporal signal-to-noise ratio) were included. Moreover, parameters obtained by rigid-body head motion correction, their temporal derivatives, and the square of each parameter were regressed out from each DMN’s time course (post-ICA motion correction). In addition, in a separate analysis, motion correction [based on scrubbing (37) and the Friston model (38)] and global signal regression were conducted before the ICA (pre-ICA motion correction). Age differences across DMN measures [global connectivity and amplitude: r ≥ 0.20, p < 0.0001; voxelwise connectivity: p < 0.05 (familywise error-corrected), k > 20] are reported.
Age-Related Reductions in the Cortical DMN Components.
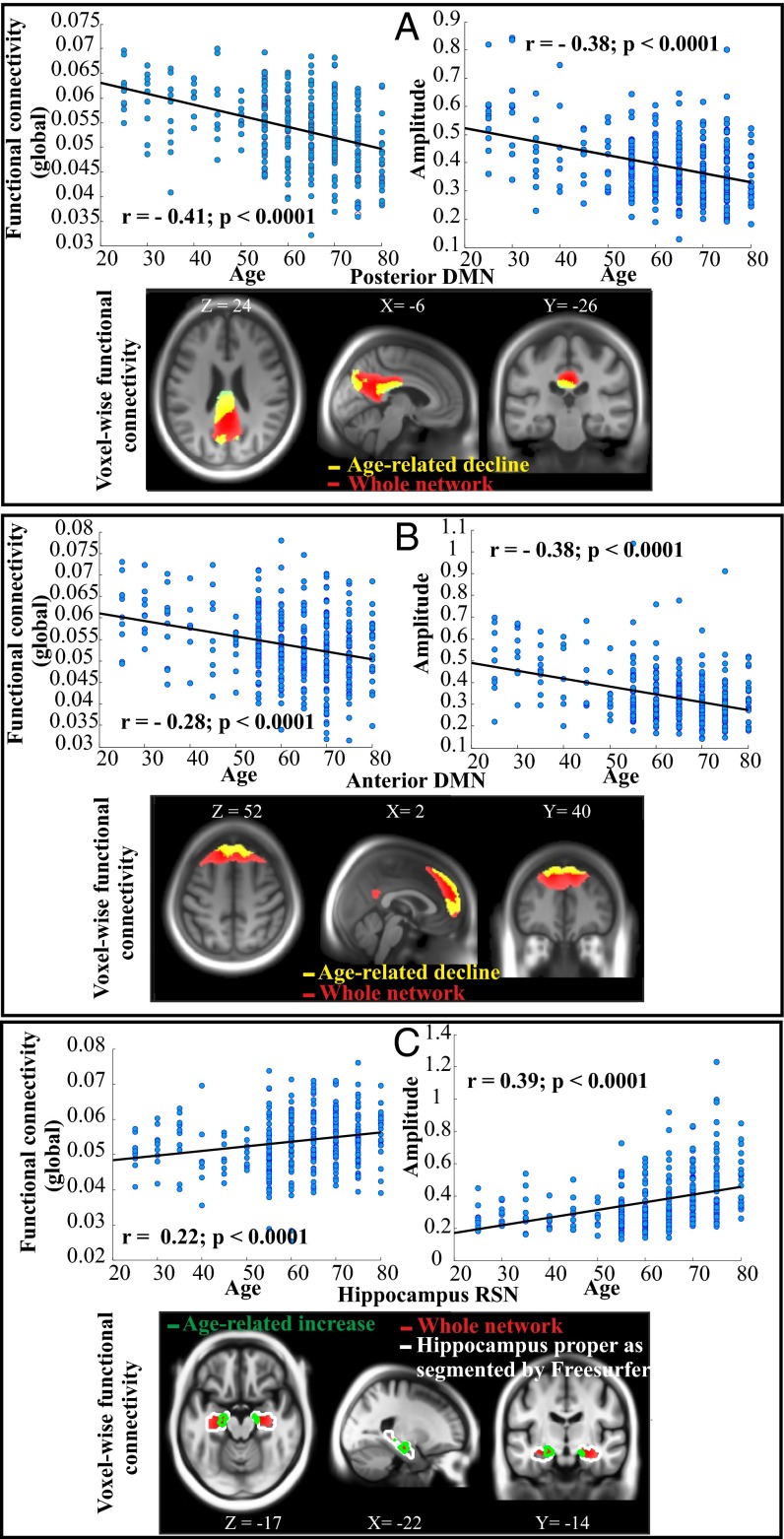
Consistent with our prediction, age had a significant negative association with the anterior and posterior cortical DMN (Fig. 1 A and B; see also Fig. S4). For both the posterior and the anterior DMN components, the results of the voxelwise analyses (i.e., regions in yellow on the slice figure) converged with amplitude and global connectivity indices (across the whole network shown in red) in showing age-related decline. For the posterior DMN, however, the voxelwise effect of age was more expressed in the posterior cingulate cortex but less evinced in the angular gyrus. The observed negative relation between age and cortical DMN components is consistent with several previous reports (4, 5, 16, 17).
Fig. 1.
Age-related alterations of the posterior (A) and anterior (B) DMN and the HC RSN (C) for three different ICA-driven measures (voxelwise connectivity, global connectivity, and amplitude) after post-ICA motion correction. For each network, scatter plots display global functional connectivity (based on the whole network) and amplitude as a function of chronological age (n = 339). The slice panels indicate brain regions (in yellow) exhibiting age-related decline (for DMNs) and age-related increase (green contour, for HC component; k = 375 voxels within the entire MTL; hippocampus proper: LHC: k = 47; RHC: k = 25) in voxelwise functional connectivity overlaid on the sample-specific template created using DARTEL. Hippocampus proper as segmented by Freesurfer (surfer.nmr.mgh.harvard.edu/fswiki/FreeSurferWiki) is shown in white contour. Note that regions in yellow and green are subregions of the whole network shown in red, and global functional connectivity was measured across the whole RSN network (in red). The unit for the global functional connectivity is percent signal changes (intensity normalization followed by no scaling; SI Text).
Age-Related Increase in the Hippocampus Subsystem.
For the HC subsystem, a significant positive relation between age and RSN measures was observed (Fig. 1C). As illustrated in Fig. 1C, with increasing age the coupling between left and right HC was strengthened, as shown by both the voxelwise (in green contour) and the global connectivity (scatter plot based on regions in red) measures. Similarly, there was an age-related increase in amplitude for the HC component.
DMN and Head Motion.
Head motion may have systematic effects on functional connectivity (37, 39). To explore the influence of head motion on observed age effects on functional connectivity, a set of control analyses (including scrubbing and the Friston 24-parameter model) was conducted before ICA. All control analyses confirmed that the main findings on the influence of age on cortical DMN and the HC component were maintained regardless of whether motion was regressed out pre- or post-ICA (Fig. S5; see SI Text for details).
HC–Cortical DMN Internetwork Connectivity.
Next, the connectivity among DMN components was explored. These analyses revealed significant internetwork connectivity between posterior/anterior DMN and the HC [mean correlation: r (posterior DMN; HC) = 0.30, p < 0.0001; r (anterior DMN; HC) = 0.23, p < 0.001]. The degree of internetwork connectivity was positively associated with performance on the EM task (corrected for age) for both the posterior (r = 0.17, p < 0.001) and anterior (r = 0.11, p < 0.05) DMN. Together, these findings support the notion that the HC component interacts with cortical components of the DMN, and that such interactions promote EM performance. Critically, the degree of internetwork connectivity significantly decreased as a function of increasing age for posterior (r = −0.33, p < 0.0001) and anterior (r = −0.32, p < 0.001) DMN. Thus, with increasing age, the anterior and posterior cortical components and the HC tended to operate more independent of each other.
Increased Hippocampal Connectivity in Relation to Brain Structure.
Increased HC connectivity may reflect age-related degenerative effects on the brain’s gray and white matter (18). We therefore examined whether the observed age-related changes in HC coupling were related to structural brain changes across the examined age span. First, we computed the influence of age on gray matter (GM). Hippocampal RSN connectivity and GM volume were integrated in a voxelwise multiple-regression approach. Of chief interest was to investigate whether elevated hippocampal coupling in aging could be accounted for by age-related local changes in GM volume. Results showed that age-related increases in voxelwise functional connectivity of the HC RSN persisted [left HC (LHC): xyz = −18 −14 −18, t = 9.29; right HC (RHC): xyz = 16 −10 −14, t = 5.60] after controlling for voxelwise GM volume (Fig. S6), suggesting that increases in functional connectivity were not driven by HC GM loss.
For white matter, we found a significant negative association between HC functional connectivity and the integrity of white matter (measured by fractional anisotropy; FA) in the fornix (Table S1). The negative association between hippocampus and fornix integrity remained after statistically correcting for age, sex, and head motion, suggesting that elevated HC coupling, at least in part, could be accounted for by alterations in white matter integrity of specific tracts. Fornix is a main efferent neural pathway of the hippocampus, but the cingulum bundles are also important for connecting the HC with posterior cortex. No significant correlation was found between HC RSN functional connectivity and FA of descending cingulum (p > 0.5). We did observe a positive correlation between the level of internetwork functional connectivity (between HC and posterior DMN) and FA of descending cingulum (r = 0.14; p < 0.005), although this correlation did not remain significant after controlling for age.
DMN–Performance Relations.
We considered cognitive tasks associated with posterior cortical areas (block design; BD) and HC (an episodic memory composite score). The cortical DMN components had positive associations with EM and BD (Table S1). By contrast, for the HC, which was positively related to chronological age, a negative relation was observed with EM and BD (Table S1). Only the EM association remained significant after controlling for age (r = −0.17, p < 0.005). Moreover, in addition to the global connectivity measures presented in Table S1, a voxelwise analysis of the relation between HC connectivity and EM performance revealed a strong negative association in bilateral HC [LHC: xyz = −15 −6 −14, t = 5.48, p < 0.01 (corrected for false-discovery rate; FDR); RHC: xyz = 16 −16 −18, t = 3.73, p < 0.01 (corrected for FDR)], which remained significant in the left HC after controlling for age [LHC: xyz = −26 −18 −10, t = 3.76, p < 0.01 (corrected for FDR)]. Collectively, these findings support our prediction that age-related increases in connectivity within the HC subsystem are detrimental to EM performance.
Hippocampal RSN in Relation to Longitudinal Memory Change.
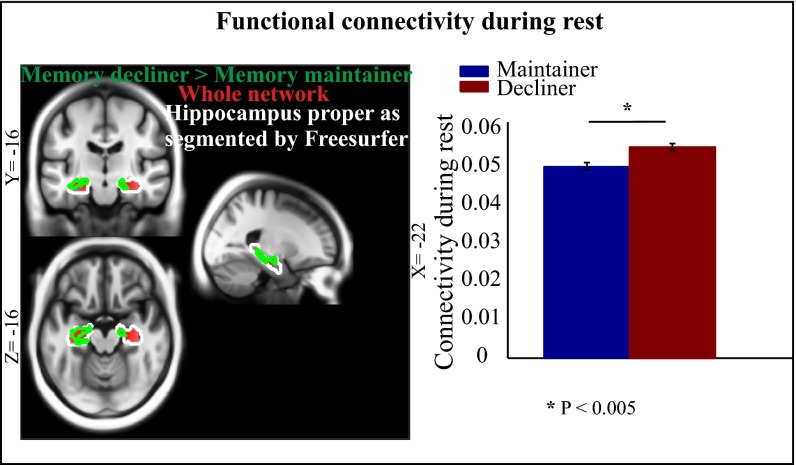
We hypothesized that elevated connectivity of the HC RSN would negatively correlate with longitudinal decline in EM. To test this hypothesis, we compared individuals who maintained EM relatively intact over 20 y with individuals showing typical age-related decline (36). There was no significant difference (t < 0.6, p > 0.5) in head motion or age between the two groups. We predicted that individuals who maintained memory would show less elevation of HC resting-state coupling than those with declining memory. In support of this prediction, we noticed a significant group difference in voxelwise (left HC: xyz = −24 −20 −10, t48 = 3.74; right HC: xyz = 16 −26 −10, t48 = 3.55; p < 0.05; FDR-corrected) as well as global (t = 3.16, p < 0.005) functional connectivity (Fig. 2). This finding provides further evidence that an age-related increase in connectivity of the HC subsystem is associated with declining EM performance.
Fig. 2.
HC RSN coupling in relation to longitudinal memory changes (36). Individuals (n = 51 subjects) who showed age-related decline over 20 y exhibited greater HC RSN coupling than their age-matched individuals (n = 51 subjects) who maintained episodic memory (age, 68.8 ± 7.1 y). The slice panels indicate brain regions (in green contour; P < 0.05 for demonstration purposes) exhibiting greater bilateral HC RSN coupling in the decliner than in the maintainer group. The bar graph indicates greater global connectivity of the HC network (based on the whole HC RSN shown in red) in decliners than maintainers. Error bars reflect standard error.
Hippocampal RSN in Relation to Task-Induced Recruitment.
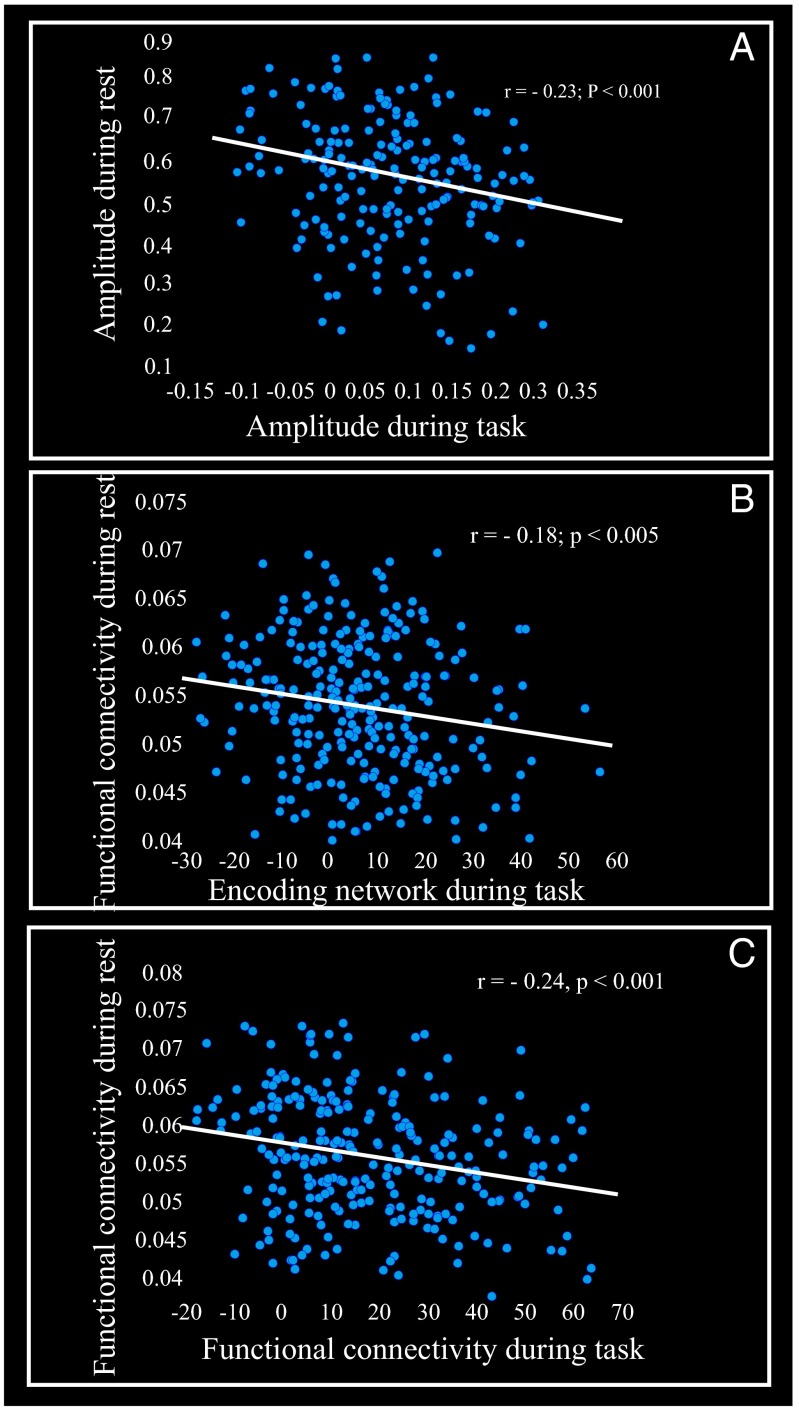
Our results showed that elevated connectivity of HC RSN is negatively linked to EM performance (composite score of episodic memory) as well as to longitudinal change in EM. We tested the prediction that such negative correlations could be driven by an inability to engage the HC and functionally associated areas during EM tasks by relating within-person HC RSN to task-induced hippocampal (and cortical) recruitment during an EM-encoding fMRI task. The magnitude of recruitment of the HC and associated brain networks during encoding has been found to promote EM performance (accuracy during the face–name fMRI task) in this sample (35). We first focused on left and right HC regions (these regions were previously identified to be part of a network that facilitated EM performance during the fMRI task for this sample; table 2 in ref. 35) that spatially overlapped with the HC RSN. A significant negative correlation was observed between the amplitude of the HC RSN and the degree of task-induced left HC recruitment (Fig. 3A). A similar relation was seen for right HC (r = −0.20, p < 0.001). Next, connectivity of the HC RSN was related to a large-scale functional network including bilateral HC (35). A similar negative relation between HC connectivity during rest and task-induced HC–cortical functional engagement was seen (Fig. 3B). Third, we related connectivity of the HC RSN to HC functional connectivity during EM encoding (Fig. S7) and again observed that high HC coupling during rest was negatively associated with the strength of HC–cortical functional connectivity during the EM task (Fig. 3C). These three analyses converge to show that strong HC coupling during rest predicts weaker engagement of HC, and associated brain regions, during active memory processing.
Fig. 3.
HC RSN coupling in relation to hippocampus and memory-related networks during an encoding task. (A) The scatter plot displays amplitude (percent signal changes) of the left hippocampus (xyz = −28 −14 −16), which promoted episodic encoding as a function of HC RS amplitude. Amplitude was computed as a joint metric of the SD of the HC time course (TC) and the maximum value of the HC spatial map (SM; SI Text). Both TC and SM are in units of percent signal changes. (B) The scatter plot displays the strength of a large-scale network (measured by brain score) that promoted episodic encoding as a function of HC connectivity during rest. Brain score is a subject-specific measure at network level that indicates how strongly an individual recruits an encoding network (SI Text). Here, a brain score represents a level of activation across the entire network that facilitated EM performance during the fMRI task (figure 1 e and f and table 2 in ref. 35). Functional connectivity during rest was computed from subject-specific SMs of the HC network according to a suggestion by Glahn and colleagues (46) (SI Text). (C) The scatter plot displays the relation between the strength of a network functionally connected to LHC (xyz = −28 −14 −16), which promoted the episodic encoding task, and HC connectivity during rest. Here a brain score represents a connectivity index across the entire network that facilitated EM performance during the fMRI task (Fig. S7).
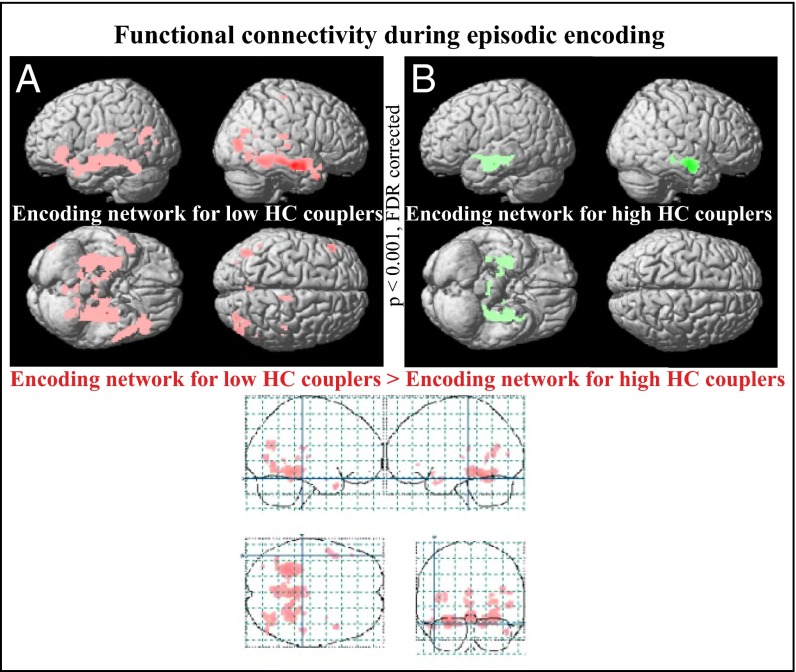
To further test the notion that strong HC connectivity at rest restricts the degree to which the HC interacts with other brain regions during active mnemonic processing, we subdivided the older participants (≥55 y), for whom elevated connectivity of the HC RSN was apparent (Fig. 1C), into high vs. low couplers (SI Text). There was no significant difference (t < 0.2, p > 0.9) in head motion between the two groups. We conducted a functional connectivity analysis of left HC with the rest of the brain during EM encoding (Fig. S7). Results showed that functional connectivity of the left HC was essentially restricted to the right HC for high couplers, whereas low couplers displayed a large-scale cortical network that interacted with the left HC during EM encoding (Fig. 4). Thus, individuals who displayed high HC synchronicity during resting state had a less extensive task-induced functional connectivity pattern with the HC. A similar pattern was seen when functional connectivity patterns during EM encoding were compared for individuals with a stable versus declining longitudinal memory performance (Fig. S8).
Fig. 4.
HC RSN in relation to memory-related network recruitment during encoding. Older participants (55 y and older; n = 212 subjects) were subdivided into two age-matched groups of high- and low-HC couplers according to a median-split analysis based on the degree of HC coupling during rest. (A) Regions (in red) functionally connected to left HC (xyz = −28 −14 −16) during episodic encoding in the low-coupler group (n = 106; 66.5 ± 7.41 y of age). (B) Regions (in green) functionally connected to left HC (xyz = −28 −14 −16) during episodic encoding in the high-coupler group (n = 106; 66.5 ± 7.41 y of age). Regions with p < 0.0001 (FDR-corrected) are shown in red and green. The glass brain demonstrates brain regions that exhibited greater functional connectivity to the left HC during the encoding task for the low couplers than for the high couplers (left fusiform: xyz = −26 −36 −18; right fusiform: xyz = 28 −44 −16; left hippocampus: xyz = −28 −30 −16; right hippocampus: xyz = 24 −36 4; left inferior frontal cortex: xyz = −46 32 −4).
Discussion
We investigated the influence of age on the cortical DMN and the HC subsystem in 339 healthy individuals between 25 and 80 y of age using group ICA and dual regression. Consistent with past studies (4, 5, 16, 17), we observed age-related decrements in functional connectivity for the anterior and posterior DMN components. In contrast, functional connectivity within the HC subsystem exhibited a positive association with advancing age. We found no evidence that elevated connectivity of the HC was driven by age-related HC volume reductions. The observation that age has opposite effects on the major cortical nodes of the DMN and the HC subsystem provides evidence that the influence of advancing age is not homogeneous across components of the DMN (18).
The analyses of relations between the connectivity of various DMN components and cognitive performance revealed a selective pattern. The posterior DMN, which was negatively linked to advancing age, was positively associated with block design and to a weaker degree with EM. By contrast, for the hippocampal RSN, which was positively related to chronological age, a negative relation was observed with the EM composite score and to a lesser degree with block design. Importantly, the negative relation between functional connectivity and EM, but not BD, remained after controlling for age in both the global and voxelwise analyses. Moreover, in the voxelwise analyses, which provide a more locally sensitive measure of brain–performance relations, the negative correlation between HC connectivity and performance on the EM composite task was found to be sizable and significant. Also, individuals with declining longitudinal EM performance had stronger age-related elevation of HC connectivity, which concurs with a previous result that elevated HC activation predicted impending hippocampal failure and subsequent memory decline (40). This notion is also consistent with cross-sectional findings that hyperactivity and/or increased functional connectivity are present at early stages of mild cognitive impairment and then followed by reduced activation as cognitive impairment worsens, as seen in individuals with AD (41). With this view, it is reasonable to predict that elderly individuals with higher HC connectivity at rest will exhibit a more rapid cognitive decline or faster conversion to AD. Although firm conclusions must await longitudinal follow-up studies, the present observations strongly indicate that age-related elevation in HC RSN amplitude and connectivity is detrimental for the integrity of the EM system.
The observed negative correlation between elevated HC RSN and cross-sectional and longitudinal episodic memory performance might reflect an inability to flexibly recruit the HC together with other task-relevant areas during EM. In successful EM encoding, the HC interacts with lateral prefrontal regions (35). The present results showed that elevated hippocampal coupling during rest was associated with reduced functional integration of HC with other regions, such as the prefrontal cortex, during EM encoding (Fig. 3). Moreover, older adults with no or weaker elevation of HC RSN coupling displayed a more extensive functional connectivity pattern with the left HC during active memory encoding (Fig. 4). Critically, this network was associated with superior EM performance (accuracy during fMRI tasks; Fig. S7), suggesting that more extensive interactions are beneficial to performance. Although the causal direction cannot be determined, these findings imply that elevated HC RSN coupling in aging contributes to age-related decline in EM performance by preventing efficient hippocampal–cortical connectivity during task performance.
In line with the present findings, a combined EEG/fMRI study also found opposing age–activity relations for cortical DMN and the HC (28), with an age decrease in cortical DMN beta power along with an age increase in HC beta power. The basis for differential influences of advancing age on key components of the DMN remains to be determined. A negative relation of age with the cortical DMN has been linked to amyloid deposition (30, 32). The positive influence of age on the HC RSN could in turn relate to age-related cortical changes, such that amyloid-induced destabilization of cortical neural network activity (42) distally drives the up-regulation of HC connectivity. Specifically, increased HC functional connectivity might be due to progressively less inhibitory cortical input. Reduced inhibitory input has been shown to lead to increased RSN functional connectivity in studies of amyotrophic lateral sclerosis (ALS) (43). Critically, in the context of aging, it has also been proposed that HC subregions are vulnerable to age-related loss of inhibitory input, which cascades into HC hyperactivation (34). In a study of ALS patients (43), increased functional connectivity was linked to decreased structural connectivity, which is in keeping with our observation that age-related changes in white matter integrity of the fornix were negatively related to the magnitude of HC RSN elevation. Finally, it has been argued that hubs that are relatively more isolated may display increased local connectivity (44). Thus, in response to age-related reduction in cortical DMN–HC structural and functional connectivity, leading to reduced cortical inhibitory input to the HC, the HC component may begin to operate more in isolation and exhibit elevated interhippocampal synchrony.
Resting-state fMRI has emerged as a powerful tool for mapping intrinsic functional connectivity in large-scale networks. Still, this method is susceptible to some technical artifacts as well as to being influenced by the mental states of participants that may confound interpretation (39). In addition, aging may affect cardiovascular dynamics, and although ICA can help to disentangle such confounding variables from RSNs, it cannot be ruled out that the observed differences in RSNs at least in part reflected nonneural factors. Critically, however, the related observation of an age-related EEG increase in HC RSN activity (28) suggests that our finding of an age-related increase in HC–fMRI connectivity has a neural origin. We used ICA components to define RS networks (defined as spatially independent but temporally correlated sets of regions), but note that there are various ways to characterize and estimate networks. An interesting task for future studies is to consider alternative network science approaches, such as graph theory, to describe neural systems in terms of graphs of compromising nodes (brain regions) and edges [synaptic connections (6)]. The correlations for the global measures of DMN components were rather weak, but the voxelwise measure of each component, which is the most typical measure used, exhibited strong and reliable correlations with offline measures even at a stringent statistical threshold. This finding suggests that not all voxels within a component exhibited correlations with offline performance measures. In other words, the global measure (which is a measure across the majority of voxels within a network) may not be as strongly correlated with performance as outcomes from voxelwise analyses. A longer duration of RS scans would likely boost correlations with the global measures.
In conclusion, with increasing age, alterations in functional connectivity emerge within cortical DMNs and the associated HC network. Our findings suggest a model where age-related cortico–hippocampal functional connectivity disruption leads to a more functionally isolated HC at rest, which translates into aberrant hippocampal decoupling and deficits in active mnemonic processing.
Methods
Subjects and Experimental Procedures.
All participants in the present study (n = 339, age = 61.5 ± 13.3 y, 176 females) were part of the Betula prospective cohort study on memory, health, and aging. From the initial sample of 357 participants, 18 were excluded due to technical error (five participants) and/or severe motion (2 SD above the mean) that corrupted fMRI data (13 participants). All remaining participants were native Swedish speakers, had normal or corrected-to-normal vision, and had no history of severe neurological illness or events that might cause dementia. fMRI time series data from each of the 339 participants were acquired at rest over a 6-min period. Participants were instructed to keep their eyes open during the scan and look at a presented fixation cross. In addition, 292 of the participants performed an episodic face–name paired-associate (FN-PA) encoding and retrieval task as well as an active baseline task. Full details of the episodic memory paradigm are given elsewhere (35).
Imaging Methods.
Structural and functional imaging was performed on a 3-T General Electric scanner equipped with a 32-channel head coil. Functional data (resting-state and FN-PA task) were acquired with a gradient echo-planar imaging sequence [37 transaxial slices; thickness, 3.4 mm; gap, 0.5 mm; repetition time (TR), 2,000 ms; echo time (TE), 30 ms; flip angle, 80°; field of view, 25 × 25 cm]. Ten dummy scans were collected and discarded before experimental image acquisition. High-resolution T1-weighted structural images were collected with a 3D fast spoiled gradient echo sequence (180 slices; thickness, 1 mm; TR, 8.2 ms; TE, 3.2 ms; flip angle, 12°; field of view, 25 × 25 cm). Finally, white matter integrity was assessed with a diffusion tensor imaging (DTI) T2-weighted spin-echo planar sequence (64 slices; TR, 8,000 ms; flip angle, 90°; field of view, 25 × 25 cm; b = 1,000 s/mm2; 32 directions; six B0 images).
Functional MRI Data Analysis.
All preprocessing steps were carried out using Statistical Parametric Mapping software (SPM8; Wellcome Department of Imaging Science, Functional Imaging Laboratory). fMRI data were first corrected for acquisition time differences between slices within each volume and then were motion-corrected. Using diffeomorphic anatomical registration using exponentiated lie algebra (DARTEL) (45) (SI Text), the realigned fMRI images were nonlinearly normalized to the sample-specific group template, affine-aligned into stereotactic space of the Montreal Neurological Institute, and smoothed using an 8.0-mm full width at half maximum Gaussian filter. Preprocessed resting-state data were analyzed using temporal concatenation independent component analysis as implemented in the GIFT toolbox (4). Both the cortical and the HC DMN were identified and their ICA-driven measures (amplitude, voxelwise, and global connectivity) were extracted. A detailed description of the ICA, RSN selection, and ICA-driven measures of RSNs is provided in SI Text.
Supplementary Material
Acknowledgments
We thank the staff of the Betula project and the staff at the Umeå Center for Functional Brain Imaging. We thank Randy Buckner, Peter Fransson, Vince Calhoun, Edward Bulmore, Gwenaelle Douaud, Lars Bäckman, and Michael Rugg for valuable comments. This study was supported by the Göran Gustafsson Award in Medicine, the Swedish Science Council, the UmU-KI Strategic Neuroscience Network, a Wallenberg Scholar Grant, and Torsten and Ragnar Söderberg's Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410233111/-/DCSupplemental.
References
- 1.Ingvar DH. “Hyperfrontal” distribution of the cerebral grey matter flow in resting wakefulness; on the functional anatomy of the conscious state. Acta Neurol Scand. 1979;60(1):12–25. doi: 10.1111/j.1600-0404.1979.tb02947.x. [DOI] [PubMed] [Google Scholar]
- 2.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 3.Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen EA, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswal BB, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17(12):683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Zuo XN, et al. Reliable intrinsic connectivity networks: Test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49(3):2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 10.Fransson P, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci USA. 2007;104(39):15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 14.Ward AM, et al. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. 2014;35(3):1061–1073. doi: 10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 16.Damoiseaux JS, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 17.Andrews-Hanna JR, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. 2013;37(3):384–400. doi: 10.1016/j.neubiorev.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleisher AS, et al. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. Neuroimage. 2009;47(4):1678–1690. doi: 10.1016/j.neuroimage.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, et al. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus. 2010;20(3):345–351. doi: 10.1002/hipo.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller SL, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 2008;105(6):2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westlye ET, Lundervold A, Rootwelt H, Lundervold AJ, Westlye LT. Increased hippocampal default mode synchronization during rest in middle-aged and elderly APOE ε4 carriers: Relationships with memory performance. J Neurosci. 2011;31(21):7775–7783. doi: 10.1523/JNEUROSCI.1230-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filippini N, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hafkemeijer A, et al. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect. 2013;3(4):353–362. doi: 10.1089/brain.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell KL, Grigg O, Saverino C, Churchill N, Grady CL. Age differences in the intrinsic functional connectivity of default network subsystems. Front Aging Neurosci. 2013;5:73. doi: 10.3389/fnagi.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ystad M, Eichele T, Lundervold AJ, Lundervold A. Subcortical functional connectivity and verbal episodic memory in healthy elderly—A resting state fMRI study. Neuroimage. 2010;52(1):379–388. doi: 10.1016/j.neuroimage.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 28.Balsters JH, et al. Changes in resting connectivity with age: A simultaneous electroencephalogram and functional magnetic resonance imaging investigation. Neurobiol Aging. 2013;34(9):2194–2207. doi: 10.1016/j.neurobiolaging.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Rentz DM, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckner RL, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mormino EC, et al. Alzheimer’s Disease Neuroimaging Initiative Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperling RA, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedden T, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29(40):12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leal SL, Yassa MA. Perturbations of neural circuitry in aging, mild cognitive impairment, and Alzheimer’s disease. Ageing Res Rev. 2013;12(3):823–831. doi: 10.1016/j.arr.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salami A, Eriksson J, Nyberg L. Opposing effects of aging on large-scale brain systems for memory encoding and cognitive control. J Neurosci. 2012;32(31):10749–10757. doi: 10.1523/JNEUROSCI.0278-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pudas S, et al. Brain characteristics of individuals resisting age-related cognitive decline over two decades. J Neurosci. 2013;33(20):8668–8677. doi: 10.1523/JNEUROSCI.2900-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan CG, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16(7):832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien JL, et al. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74(24):1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sperling R. Potential of functional MRI as a biomarker in early Alzheimer’s disease. Neurobiol Aging. 2011;32(Suppl 1):S37–S43. doi: 10.1016/j.neurobiolaging.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat Neurosci. 2010;13(7):812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Douaud G, Filippini N, Knight S, Talbot K, Turner MR. Integration of structural and functional magnetic resonance imaging in amyotrophic lateral sclerosis. Brain. 2011;134(Pt 12):3470–3479. doi: 10.1093/brain/awr279. [DOI] [PubMed] [Google Scholar]
- 44.Meunier D, Achard S, Morcom A, Bullmore E. Age-related changes in modular organization of human brain functional networks. Neuroimage. 2009;44(3):715–723. doi: 10.1016/j.neuroimage.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 45.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Glahn DC, et al. Genetic control over the resting brain. Proc Natl Acad Sci USA. 2010;107(3):1223–1228. doi: 10.1073/pnas.0909969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.