Abstract
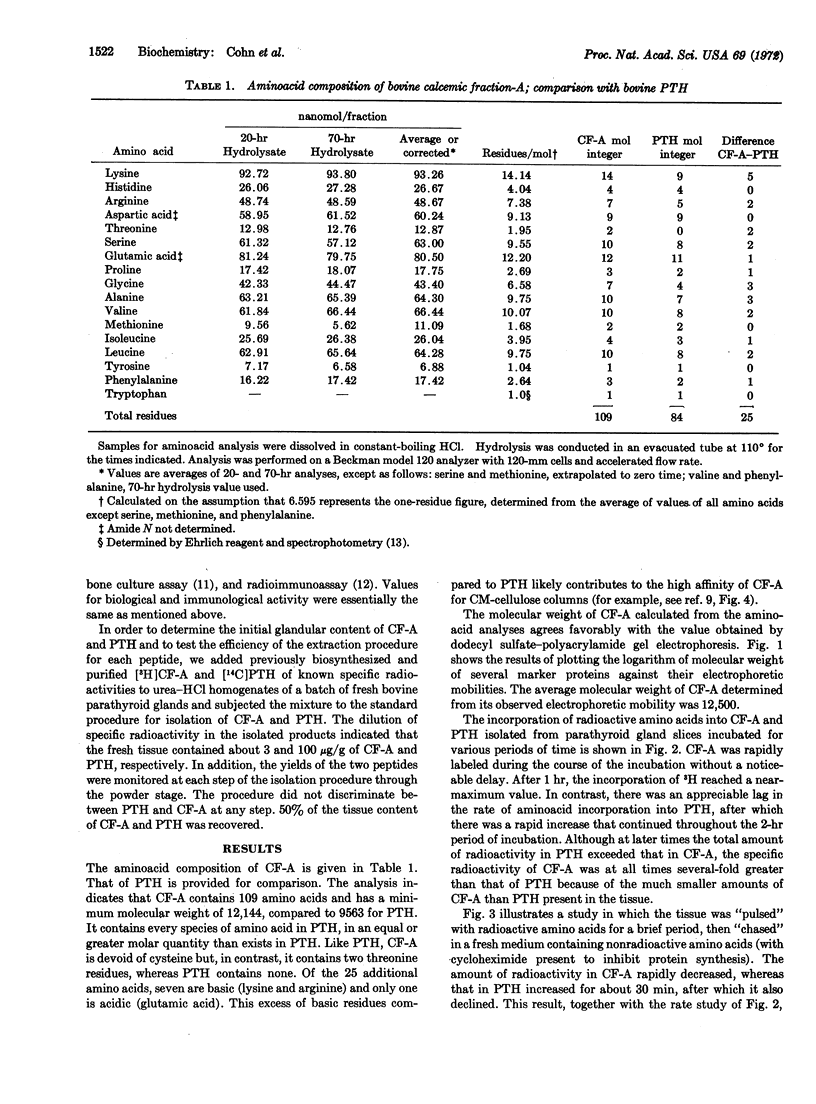
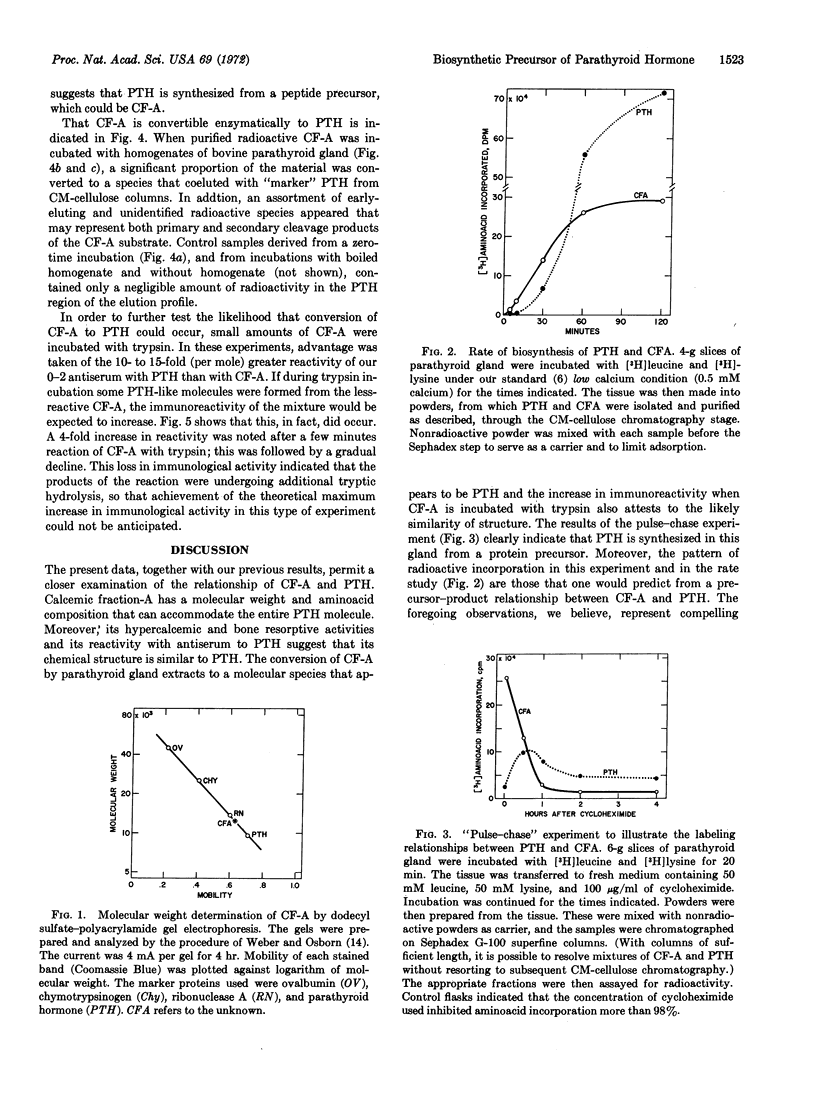
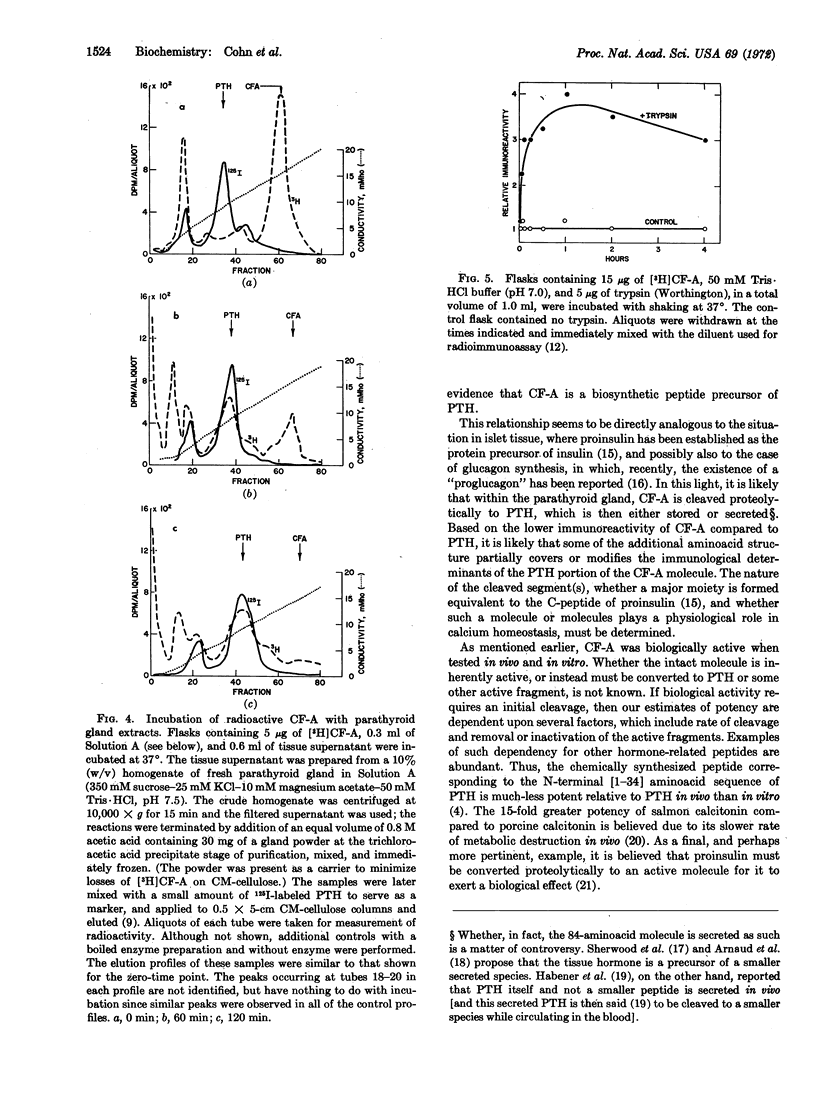
Calcemic fraction-A (CF-A) is a biologically active, hypercalcemic and bone resorptive peptide, which was detected in, and isolated from, bovine parathyroid glands [Hamilton et al. (1971) Endocrinology 89, 1440-1447]. It has been further purified, and its relationship to parathyroid hormone clarified. The peptide is present in fresh glands at a concentration of about 3 μg/g (parathyroid hormone, 100 μg/g). It contains 109 amino acids (hormone, 84), each of which is present in equal or greater molar ratio than in the hormone. Its molecular weight, calculated from amino-acid composition, is 12,144; determined by dodecyl sulfate-polyacrylamide gel electrophoresis, it is 12,500 (hormone, 9563). Per mole, it reacts with antiserum to parathyroid hormone to an extent of 7-10% that of the hormone, and is about 50% as active in its hypercalcemic and bone resorptive properties in the appropriate assays. Time course and pulse-chase experiments with parathyroid gland slices, in which the incorporation of amino acid into isolated peptide and hormone were measured, indicate that the hormone is made from a protein precursor; the patterns of incorporation of radioactivity are those that would be predicted from a precursor-product relationship. When the large peptide was incubated with parathyroid gland extracts it was partially converted to a molecule that appeared to be the hormone, as based upon its coelution with marker hormone from ion-exchange columns. Finally, tryptic digestion of the peptide increased the immunoreactivity of the sample in accord with the known greater immunoreactivity of the hormone than the peptide. On the basis of these results, it is proposed that the peptide is a biosynthetic precursor of the hormone in bovine parathyroid gland.
Keywords: bovine, 109-aminoacid precursor, molecular weight, gel electrophoresis, calcium
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud C. D., Sizemore G. W., Oldham S. B., Fischer J. A., Tsao H. S., Littledike E. T. Human parathyroid hormone: glandular and secreted molecular species. Am J Med. 1971 May;50(5):630–638. doi: 10.1016/0002-9343(71)90118-5. [DOI] [PubMed] [Google Scholar]
- Brewer H. B., Jr, Ronan R. Bovine parathyroid hormone: amino acid sequence. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1862–1869. doi: 10.1073/pnas.67.4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L. L., Macgregor R. R., Hamilton J. W., Cohn D. V. A bioassay for parathyroid hormone based on hormonal inhibition of CO2 production from citrate in mouse calvarium. Endocrinology. 1971 Dec;89(6):1425–1431. doi: 10.1210/endo-89-6-1425. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Powell D., Murray T. M., Mayer G. P., Potts J. T., Jr Parathyroid hormone: secretion and metabolism in vivo. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2986–2991. doi: 10.1073/pnas.68.12.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F., Singer F. R., Deftos L. J., Neer R. M., Potts J. T., Jr Explanation for unusual potency of salmon calcitonin. Nat New Biol. 1971 Jul 21;232(29):91–92. doi: 10.1038/newbio232091a0. [DOI] [PubMed] [Google Scholar]
- Hamilton J. W., Cohn D. V. Studies on the biosynthesis in vitro of parathyroid hormone. I. Synthesis of parathyroid hormone by bovine-parathyroid gland slices and its control by calcium. J Biol Chem. 1969 Oct 25;244(20):5421–5429. [PubMed] [Google Scholar]
- Hamilton J. W., Macgregor R. R., Chu L. L., Cohn D. V. The isolation and partial purification of a non-parathyroid hormone calcemic fraction from bovine parathyroid glands. Endocrinology. 1971 Dec;89(6):1440–1447. doi: 10.1210/endo-89-6-1440. [DOI] [PubMed] [Google Scholar]
- Hamilton J. W., Spierto F. W., MacGregor R. R., Cohn D. V. Studies on the biosynthesis in vitro of parathyroid hormone. II. The effect of calcium and magnesium on synthesis of parathyroid hormone isolated from bovine parathyroid tissue and incubation medium. J Biol Chem. 1971 May 25;246(10):3224–3233. [PubMed] [Google Scholar]
- Keutmann H. T., Aurbach G. D., Dawson B. F., Niall H. D., Deftos L. J., Potts J. T., Jr Isolation and characterization of the bovine parathyroid isohormones. Biochemistry. 1971 Jul 6;10(14):2779–2787. doi: 10.1021/bi00790a020. [DOI] [PubMed] [Google Scholar]
- Melani F., Rubenstein A. H., Steiner D. F. Human serum proinsulin. J Clin Invest. 1970 Mar;49(3):497–507. doi: 10.1172/JCI106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niall H. D., Keutmann H., Sauer R., Hogan M., Dawson B., Aurbach G., Potts J., Jr The amino acid sequence of bovine parathyroid hormone I. Hoppe Seylers Z Physiol Chem. 1970 Dec;351(12):1586–1588. [PubMed] [Google Scholar]
- Noe B. D., Bauer G. E. Evidence for glucagon biosynthesis involving a protein intermediate in islets of the anglerfish (Lophius americanus). Endocrinology. 1971 Sep;89(3):642–651. doi: 10.1210/endo-89-3-642. [DOI] [PubMed] [Google Scholar]
- Potts J. T., Jr, Tregear G. W., Keutmann H. T., Niall H. D., Sauer R., Deftos L. J., Dawson B. F., Hogan M. L., Aurbach G. D. Synthesis of a biologically active N-terminal tetratriacontapeptide of parathyroid hormone. Proc Natl Acad Sci U S A. 1971 Jan;68(1):63–67. doi: 10.1073/pnas.68.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopman W., Hackeng W. H., Lequin R. M. A radioimmunoassay for parathyroid hormone in man. I. Development of a radioimmunoassay for bovine PTH. Acta Endocrinol (Copenh) 1970 Apr;63(4):643–654. doi: 10.1530/acta.0.0630643. [DOI] [PubMed] [Google Scholar]
- Shaw W. N., Chance R. E. Effect of porcine proinsulin in vitro on adipose tissue and diaphragm of the normal rat. Diabetes. 1968 Dec;17(12):737–745. doi: 10.2337/diab.17.12.737. [DOI] [PubMed] [Google Scholar]
- Sherwood L. M., Mayer G. P., Ramberg C. F., Jr, Kronfeld D. S., Aurbach G. D., Potts J. T., Jr Regulation of parathyroid hormone secretion: proportional control by calcium, lack of effect of phosphate. Endocrinology. 1968 Nov;83(5):1043–1051. doi: 10.1210/endo-83-5-1043. [DOI] [PubMed] [Google Scholar]
- Sherwood L. M., Rodman J. S., Lundberg W. B. Evidence for a precursor to circulating parathyroid hormone. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1631–1638. doi: 10.1073/pnas.67.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L., Nolan C., Rubenstein A. H., Margoliash E., Aten B., Oyer P. E. Proinsulin and the biosynthesis of insulin. Recent Prog Horm Res. 1969;25:207–282. doi: 10.1016/b978-0-12-571125-8.50008-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


