Abstract
The nuclear synthesis of adenovirus-specific RNA late in the infectious cycle in the presence of toyocamycin (an adenosine analogue) has been investigated. There is reduced synthesis of viral RNA with an accumulation of virus-specific RNA in the molecular weight range of at least 4 to 8 × 106. No new viral RNA associates with cytoplasmic polyribosomes. In addition, hybridization competition experiments indicate a 70% competition between these large nuclear transcripts and polyribosome-associated viral RNA that was synthesized in the absence of inhibitor. These data are consistent with the following interpretations: complete nuclear processing of viral RNA is necessary for polyribosome association, and precursor viral message(s) contain sequences that are lost normally during post-transcriptional processing.
Keywords: toyocamycin, HeLa cells, viral transcripts
Full text
PDF
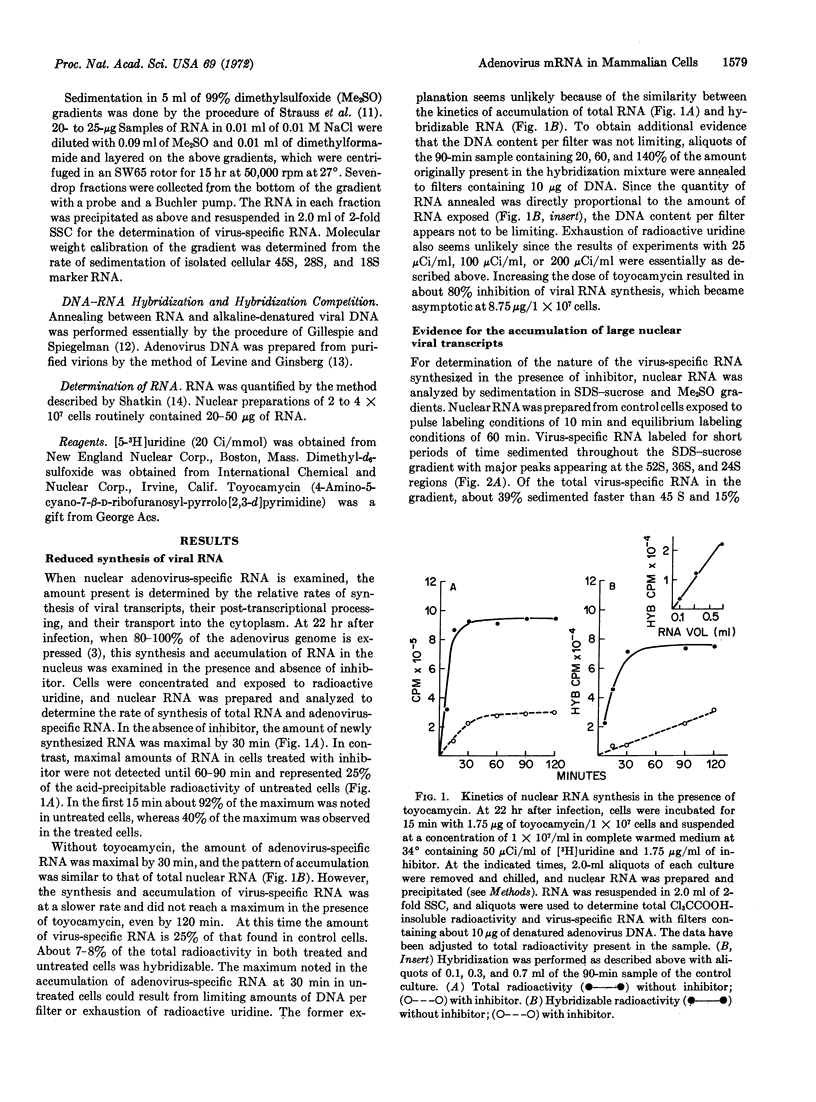
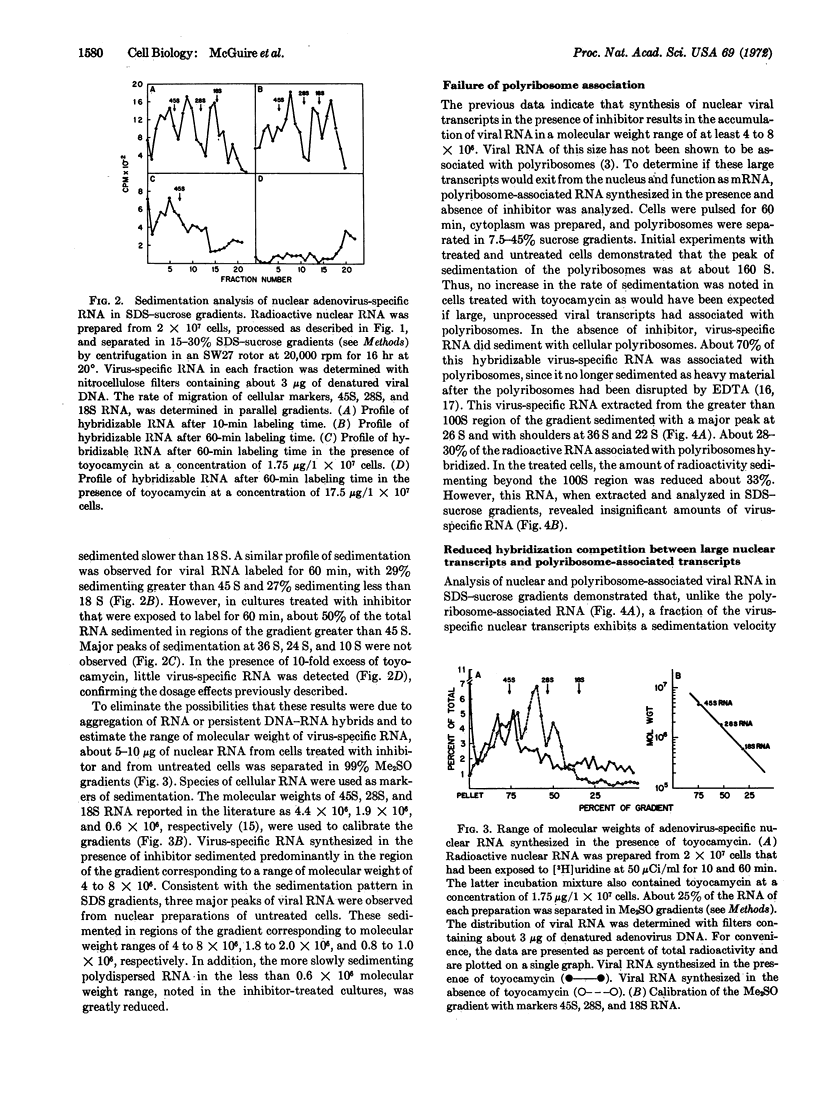
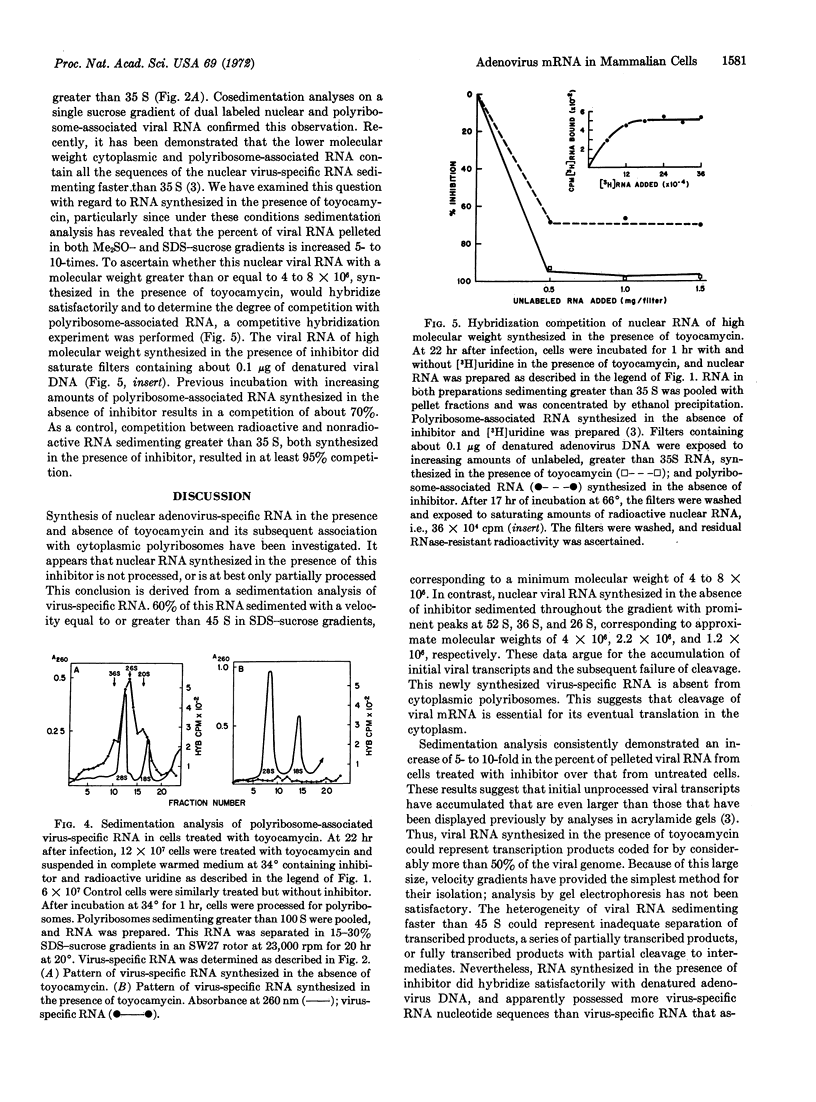

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hodge L. D., Scharff M. D. Effect of adenovirus on host cell DNA synthesis in synchronized cells. Virology. 1969 Apr;37(4):554–564. doi: 10.1016/0042-6822(69)90273-6. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Hodge L. D. Gene expression in synchronized lymphocytes: studies on the control of synthesis of immunoglobulin polypeptides. J Cell Physiol. 1971 Apr;77(2):265–276. doi: 10.1002/jcp.1040770215. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Ginsberg H. S. Role of adenovirus structural proteins in the cessation of host-cell biosynthetic functions. J Virol. 1968 May;2(5):430–439. doi: 10.1128/jvi.2.5.430-439.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- McConkey E. H., Hopkins J. W. Molecular weights of some HeLa ribosomal RNA's. J Mol Biol. 1969 Feb 14;39(3):545–550. doi: 10.1016/0022-2836(69)90144-2. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Gardner J., Green M. Biochemical studies on adenovirus multiplication, XIX. Resolution of late viral RNA species in the nucleus and cytoplasm. Proc Natl Acad Sci U S A. 1971 Mar;68(3):557–560. doi: 10.1073/pnas.68.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro R., Darnell J. E. A comparison between heterogeneous nuclear RNA and polysomal messenger RNA in HeLa cells by RNA-DNA hybridization. J Cell Biol. 1970 Mar;44(3):467–475. doi: 10.1083/jcb.44.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Kelly R. B., Sinsheimer R. L. Denaturation of RNA with dimethyl sulfoxide. Biopolymers. 1968 Jun;6(6):793–807. doi: 10.1002/bip.1968.360060604. [DOI] [PubMed] [Google Scholar]
- Tavitian A., Uretsky S. C., Acs G. Selective inhibition of ribosomal RNA synthesis in mammalian cells. Biochim Biophys Acta. 1968 Mar 18;157(1):33–43. doi: 10.1016/0005-2787(68)90261-x. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B. RNA synthesis in cells infected with herpes simplex virus. II. Evidence that a class of viral mRNA is derived from a high molecular weight precursor synthesized in the nucleus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):626–633. doi: 10.1073/pnas.64.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]


