Abstract
INTRODUCTION
We have recently demonstrated that in a rodent model of lipopolysaccharide (LPS)-induced shock, an increase in circulating citrullinated histone H3 (Cit H3) is associated with lethality of sepsis, and treatment with suberoylanilide hydroxamic acid (SAHA), a histone deacetylase (HDAC) inhibitor (HDACI), significantly improves survival. However, the role of Cit H3 in pathogenesis and therapeutics of sepsis are largely unknown. The present study was designed to test whether treatment with HDACI could inhibit cellular Cit H3 production, and inhibition of peptidyalarginine deiminase (PAD, an enzyme producing Cit H3) with Cl-amidine (PAD inhibitor) or neutralization of blood Cit H3 with anti-Cit H3 antibody could improve survival in a clinically relevant mouse model of cecal ligation and puncture (CLP) induced septic shock.
METHODS
Three experiments were carried out. In experiment I, HL-60 neutrophilic cells grown on a coverslip were treated with LPS (100 ng/ml) in the presence or absence of SAHA (5 µmol) for 3 h, and subjected to immuno-staining with anti-Cit H3 antibody to assess effect of SAHA on Cit H3 production under a fluorescence microscope. The ratio of Cit H3 positive cells was calculated as mean ± SD (n=3). In experiment II, male C57BL/6J mice were subjected to CLP, and 1 hour later randomly divided into three groups for intraperitoneal injection as follows: (1) dimethyl sulfoxide (DMSO), (2) SAHA (50 mg/kg) in DMSO, and (3) Cl-amidine (80 mg/kg) in DMSO (n=10/group). In experiment III, male C57BL/6J mice were divided into control and treatment groups, and subjected to CLP. Two hours later, immunoglobulin (IgG) and Cit H3 antibody (20 mg/kg iv; n=5/group) were injected into the control and treatment groups, respectively. Survival was monitored for up to 10 days.
RESULTS
In experiment I, LPS induced Cit H3 production in the HL-60 cells, while SAHA treatment inhibited H3 citrullination significantly (p<0.05). In experiment II, all vehicle injected mice died within 3 days with increased circulating Cit H3 levels, whereas treatment with HDACI or Cl-amidine notably improved long-term survival (p< 0.01). In experiment III, administration of IgG did not improve survival, but a single treatment with Cit H3 specific antibody significantly improved survival (p<0.014).
CONCLUSIONS
Inhibition of HDAC or PAD significantly suppresses Cit H3 production in vitro, and improves survival in vivo. Neutralization of Cit H3 significantly improves survival in septic mice. Collectively, our findings indicate for the first time that Cit H3 could not only serve as a potential biomarker but also a novel therapeutic target in sepsis.
Keywords: sepsis, citrullinated histone H3, suberoylanilide hydroxamic acid, peptidyl arginine deiminase inhibitor, Cl-amidine, anti-Cit H3 antibody
INTRODUCTION
Septic shock is a lethal complication of infection, characterized by dysregulated inflammatory and immune responses. Epigenetic mechanisms such as post-translational modification (PTM) of histones by acetylation are master regulators of gene expression and play a critical role in inflammatory and host defense responses.1, 2 Histone acetylation is controlled by histone acetyltransferases (HAT) and histone deacetylases (HDAC), which affect expression of genes and proteins involved in various key cellular functions.3,4 Numerous HDAC inhibitors (HDACI) are already in use for the treatment of cancers5,6, and are now undergoing testing for many other diseases. Recently, we have shown that an HDACI, suberoylanilide hydroxamic acid (SAHA), modulates the immune response,7–9 and improves survival in a mouse model of cecal ligation and puncture (CLP).10 Unfortunately, this non-selective HDAC inhibitor is not well tolerated, and may impair the innate immune response when given in therapeutically relevant doses.1
Citrullination of histones, another PTM catalyzed by peptidylarginine deiminase (PAD)-4 (PAD4), has recently been identified as an early step in a new type of cell death termed NETosis 11 or ETosis, 12 which is characterized by the release of neutrophil extracellular traps (NETs) or other immune cells’ extracellular traps (ETs).12,13 Furthermore, citrullination of histones, in particular histone H3, was revealed as a convergence point for diverse inflammatory signals that trigger the neutrophil response to infections.11 We have reported that citrullinated histone H3 (Cit H3) could be a potential serum biomarker for the early diagnosis of septic shock.14 It is not clear whether Cit H3 could also be a therapeutic target in sepsis.
In this study, we tested whether treatment with HDACI could inhibit Cit H3 production. In addition, we investigated whether inhibition of peptidyalarginine deiminase with Cl-amidine, a pan-PAD inhibitor, or neutralization of circulating Cit H3 with anti-Cit H3 antibody would improve survival in a mouse model of cecal ligation and puncture (CLP).
MATERIALS AND METHODS
Antibodies and supplies
LPS (from S. typhosa, Cat# L6386, Lot# 038k4005) and dimethyl sulfoxide (DMSO) were purchased from the Sigma Aldrich, Co (St. Louis, MO). Suberoylanilide hydroxamic acid was purchased from Enzo Life Sciences International, Inc (Plymouth Meeting, PA). Cl-amidine was purchased from Cayman Chemical (Ann Arbor, MI). Purified citrulinated histone H3 (citrulline 2 + 8 + 17) antibody and immunoglobulin G (Ig G, ab171870; control for the antibody) were purchased from abcam (Cambridge, MA). RPMI 1640 medium, fetal bovine serum (FBS), and phosphate buffered saline (PBS) were from Gibco-BRL (Grand Island, NY). L-glutamine, and fetal calf serum (FCS) were from Invitrogen (Carlsbad, CA). All-trans retinoic acid (ATRA) was purchased from Acros Organics (Geel, Belgium). All other chemicals in this study were of analytical grade and obtained from the Sigma-Aldrich unless mentioned otherwise.
Cell culture and treatment
HL-60 cells obtained from American Type Culture Collection (ATCC) were maintained in Iscove’s modified DMEM medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 20% fetal bovine serum (FBS). These cells were grown on a coverslip at 37 °C in a humidified incubator in 5% CO2 and 95% air, and were differentiated into granulocytes by culturing the cells in medium containing 1 µM ATRA for 3 days. The ATRA-differentiated HL-60 granulocytes were treated with 4 µM calcium ionophore in medium containing 1.5 mM calcium chloride, and then incubated with LPS (100 ng/ml) in the presence or absence of SAHA (10 µM) over 3 h. Following incubation, medium was collected, cells were fixed and subjected to immunostaining with anti-Cit H3 antibody following by the second antibody. The ratio of Cit H3 positive stained cells to all cells was counted and calculated as mean ± SD from three individual experiments.
Animals
All the research was conducted in compliance with the Animal Welfare Act, and was approved by the Animal Review Committee at the Massachusetts General Hospital. Male C57B1/6J mice (6–8 weeks) weighing 25–30 g were purchased from Jackson Labs (Bar Harbor, ME). All animals were housed in plastic cages and had access to chow and water throughout the experiment. They were kept at room temperature (24 ± 2°C) and exposed to alternative cycles of 12 h light and darkness. During the experiments the animals were monitored up to 10 days, and survival rate was compared between the experimental and control groups.
CLP-induced sepsis model
The CLP murine model, 15 modified by our laboratory, was used to induce fecal peritonitis.10 In brief, peritoneal cavity was opened under inhaled isoflurane anesthesia. Cecum was eviscerated, ligated below the ileocecal valve using a 5-0 suture, and punctured through and through (two holes) with a 20-gauge needle. The punctured cecum was squeezed to expel a small amount of fecal material and returned to the peritoneal cavity. The abdominal incision was closed in two layers with 4-0 silk suture. Animals were resuscitated by subcutaneous injection of 1 mL of saline. Sham-operated animals were handled in the same manner, except that the cecum was not ligated or punctured. The animals were then randomly divided into different groups for two separate in vivo experiments as follows.
Administration of inhibitors and experimental design
In one of the survival experiments, mice received intra-peritoneal SAHA (HDAC inhibitor, 50 mg/kg) 10 or Cl-amidine (PAD inhibitor, 80 mg/kg) 16 dissolved in dimethyl sulfoxide (DMSO), or vehicle DMSO 1 hour after CLP (n = 10/group). Mortality was recorded for up to 10 days post procedure.
Administration of antibody and experimental design
In the other survival experiment, mice received intravenous anti-Cit H3 antibody (20 mg/kg; abcam, Cambridge, MA) or immunoglobulin G (20 mg/kg; EMD Millipore, Billerica, MA) 2 hours after CLP (n=5/group). Mortality was recorded for up to 5 days.
Statistical analysis
Statistical differences were determined by Student t tests and ANOVA for two group and multiple group comparisons respectively (SPSS statistical software package, Chicago, Illinois). Kaplan-Meier survival curves were analyzed by using the MedCalc Statistical Software (Mariakerke, Belgium) for the in vivo studies. Differences were considered to be statistically significant when p values were <0.05.
RESULTS
1. SAHA suppresses LPS-induced ET formation
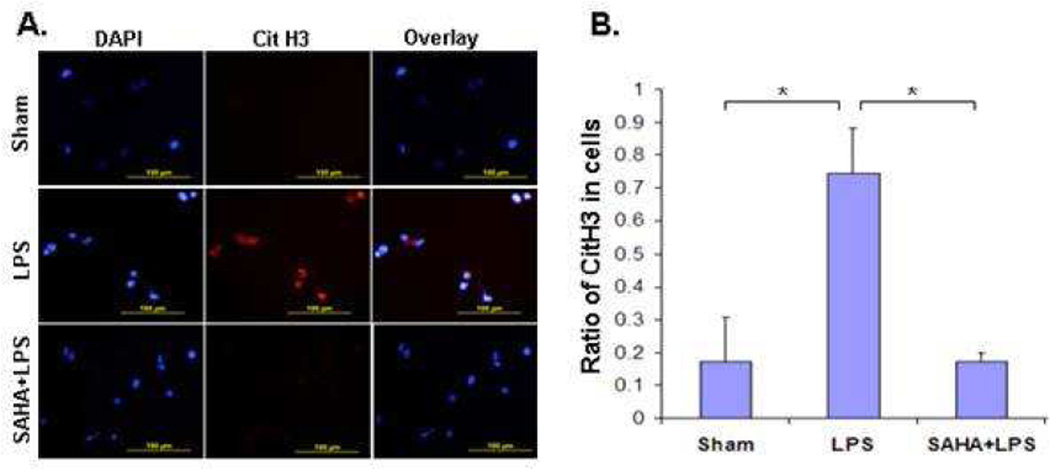
Given that LPS stimulates histone H3 citrullination and NETs formation, which in turn releases nuclear content (e.g., histones) into the extracellular milieu,17,18 we asked whether SAHA treatment could attenuate these alterations. As expected, LPS induced citrullination of H3, which spilled out of the cell during the formation of NETs (red color in Figure 1A). SAHA treatment significantly inhibited histone H3 citrullination and NETs formation in HL-60 neutrophilic cells after LPS insult (Figure 1 A and B).
Figure 1. SAHA suppresses LPS-induced Cit H3 production.
(A) A representative CitH3 staining. (B) Ratio of CitH3 positive cells to all cells. Cell culture and immunostaning are described in Materials and Methods. The red color denotes decondensed chromatin stained with the Cit H3 antibody. 4'-6-Diamidino-2-phenylindole (DAPI) was used for nuclei staining (blue color). Statistical analysis shows that SAHA significantly suppressed the LPS-induced Cit H3 production (n=3; p<0.05).
2. Inhibition of PAD with Cl-amidine improves survival in a mouse model of CLP-induced septic shock
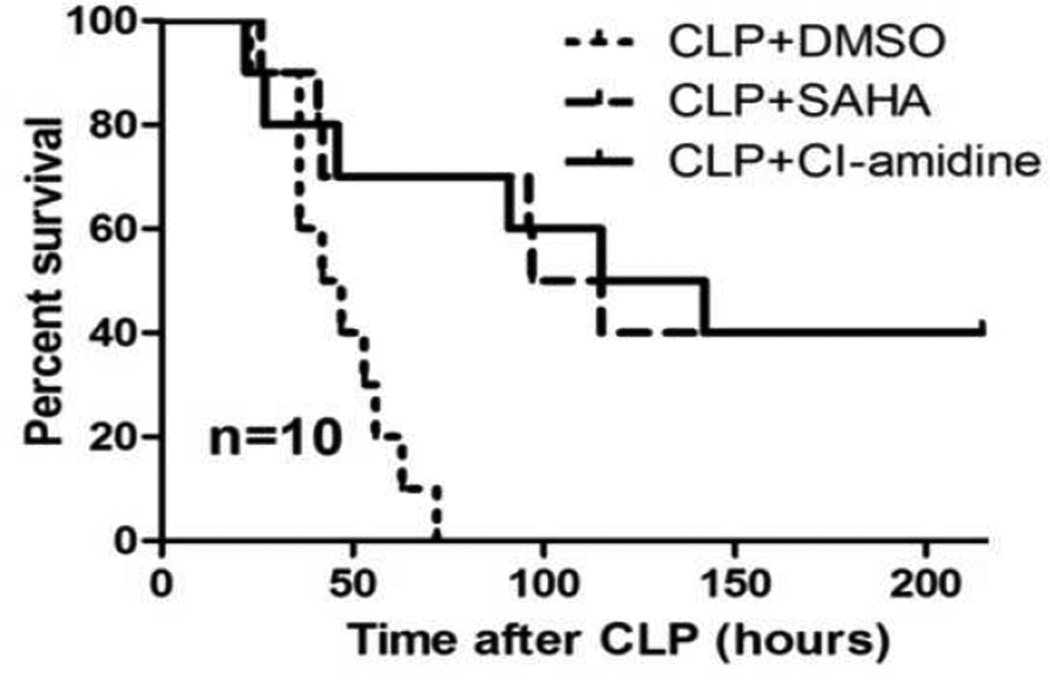
It is well known that inhibition of PAD by Cl-amidine can suppress Cit H3 expression. 19 To assess if decreased Cit H3 production could protect against lethality, we injected Cl-amidine (80 mg/kg, i.p.), a PAD inhibitor (PADI), into mice 1 hour after CLP. As a positive control, mice were given SAHA (50 mg/kg, i.p.). We found that all the mice from the vehicle control group died within 3 days. Treatment with Cl-amidine significantly improved survival (p < 0.01), similar to SAHA (Figure 2).
Figure 2. Cl-amidine decreases lethality in a septic model.
Mice were intraperitoneally administered 80mg/kg of Cl-amidine or vehicle DMSO 1h after CLP (n=10). SAHA treated animal (50 mg/kg) served as a positive control. Treatment with Cl-amidine significantly improved survival compared with DMSO vehicle group (42.5% versus 0% survival; p< 0.001).
3. Neutralization of circulating Cit H3 with anti-Cit H3 antibody improves survival in a mouse model of CLP-induced septic shock
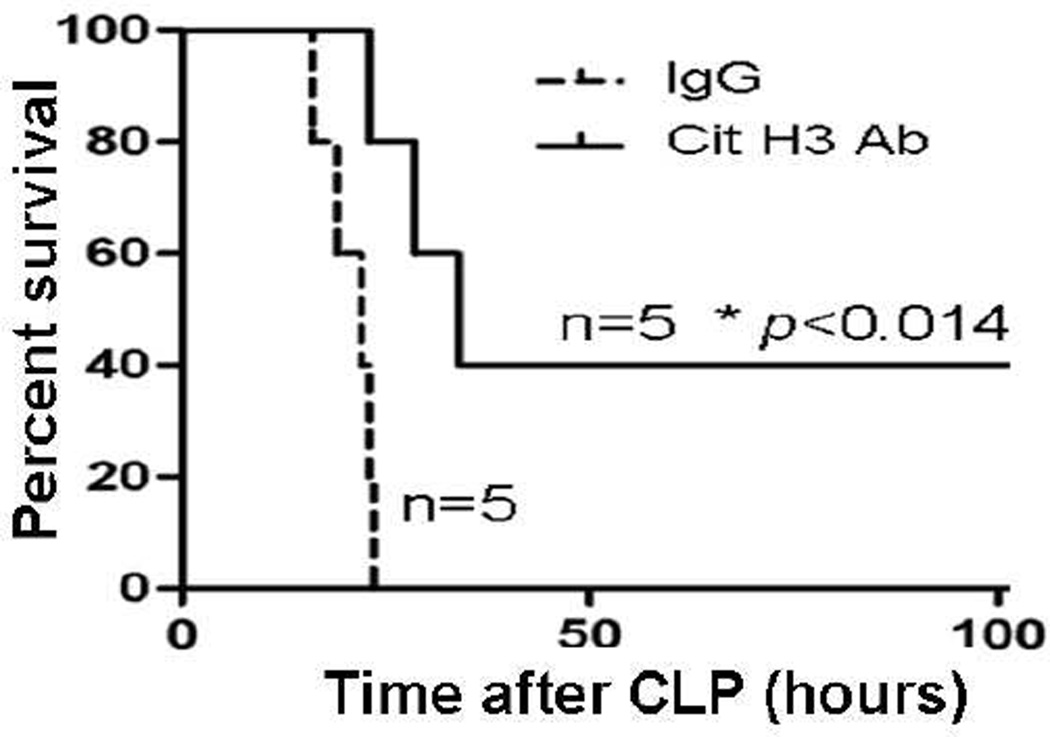
To determine whether blockade of Cit H3 activity could prolong survival, we intravenously injected anti-Cit H3 antibody 2 hours after CLP. Mouse immunoglobulin G was used as a control (n=5/group). As shown in Figure 3, all of the animals that received IgG died within 3 days. In contrast, antibody treated animals showed a significant improvement in survival (p< 0.014).
Figure 3. Neutralization of circulating Cit H3 with anti-Cit H3 antibody improves survival in a mouse model of CLP-induced sepsis.
Mice were intravenously administrated purified anti-Citrullinated H3 antibody (20 mg/kg) or immunoglobulin G (Ig G, 20 mg/kg) 2h after CLP (n=5/group). Treatment with the antibody against Cit H3 significantly improved survival compared to Ig G group (42% versus 9% survival, p< 0.014).
DISCUSSION
Recently, we have shown that treatment of septic mice with SAHA, a potent histone deacetylase inhibitor, improves survival. 7,10,20 In the current study, we discovered that LPS-induced histone H3 citrullination can be attenuated in vitro by SAHA treatment. In addition, a decrease in Cit H3 levels (through an inhibition of the PAD4 enzyme), or blockage of its actions (by specific antibody) improves survival in a lethal CLP model. This works builds upon our previous study where we used Western blots to demonstrate that SAHA decreases expression and secretion of Cit H3 protein in LPS-stimulated HL-60 granulocytes.14 In the present study, using immunofluorescence, we have discovered that SAHA suppresses LPS-induced NET formation, which in turn attenuates the production and secretion of of Cit H3 protein.
Research indicates that epigenetic mechanisms and, in particular, the PTM of histones may contribute to the unbalanced inflammatory and immune status that is often seen in the advanced stages of sepsis. Among the dozens of possible histone PTMs, this study suggests that citrullination of arginines on the N-terminal tail of histone H3 exhibits a particularly strong link with sepsis, and could serve as a potential therapeutic target. First, the HDAC inhibitor SAHA while increasing the acetylation of histone proteins, decreases their citrullination 7. Second, an inhibition of PAD activity with Cl-amidine can reduce the production of Cit H3 (data not shown) and improve survival in the CLP model. Third, neutralization of circulating Cit H3 with anti-Cit H3 antibody significantly decreases the lethality of this model. We have previously reported very high circulating levels of Cit H3 in an endotoxemia model14, but the current study suggests that in addition to being a biomarker, it may also play a key regulatory role in the pathogenesis of sepsis. .
Citrullination, also termed deimination, is a post-translational protein modification that leads to a charge loss that can alter its conformation and consequently its structure, function and interaction with other proteins. This PTM is catalyzed by the Ca2+-dependent peptidyl arginine deiminases (PADs). There are five PAD family members, but only PAD2 and PAD4 expression are closely linked with inflammatory diseases such as rheumatoid arthritis (RA).21,22 While PAD2 is broadly expressed across tissue types, including immune cells, expression of PAD4 is restricted to immune cells, in particular macrophages and granulocytes.21,23,24 Studies on neutrophils have demonstrated that PAD4 is essential to the histone citrullination that precedes formation of NETs — an important component of the inflammatory response to bacterial infections.18,25,26 Despite their obvious benefits, NET formation and histone H3 citrullination must be well regulated, to prevent immune-mediated tissue damage. We hypothesize that rapid and robust histone citrullination may lead to the death of cells such as neutrophils, which are the initial responders during sepsis. Following their release from the disintegrated neutrophils, citrullinated histones bind to and activate TLR2/4, creating a positive feedback loop that results in the release of additional Cit H3, establishing a vicious circle. Although this hypothesis is still under investigation, our recent finding that hyper-citrullinated histone H3 could be detected in the early stages of sepsis and is associated with lethality14 supports this possibility.
Cl-amidine, a recently described pan-PAD inhibitor, has been used as a potent PAD4 inhibitor. 26 Treatment with Cl-amidine can reduce the severity of murine collegen-induced arthritis. 27 Intriguingly, our study shows that this inhibitor can also improve survival in a model of lethal CLP-sepsis. Although the mechanisms are not entirely clear, this proof of concept study provides evidence for the first time that inhibition of PAD4 could serve as a target for future development of novel drugs for the treatment of sepsis.
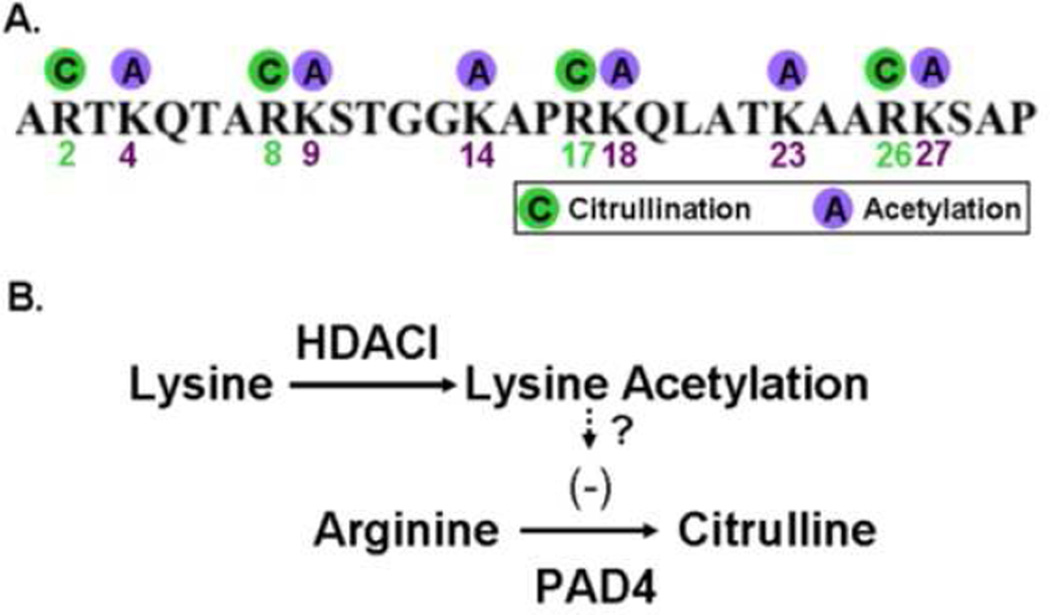
The relationship between histone acetylation and citrullination is unclear. Post-translational histone modifications are thought to regulate gene expression by facilitating the formation of open chromatin or serving as binding platform for additional effector proteins.28,29 Given that the arginine and lysine side chains on the N-terminal tail of histone H3, which have previously been identified as sites of citrullination and acetylation,29 are directly adjacent or in close vicinity, PTMs of the respective lysine side chains by acetylation or methylation can potentially alter the recognition motif of chromatin modifying enzymes that process adjacent amino acid residues (Figure 4).30,31 Arginine residues often play a central role in the structural integrity of a protein,due to their ability to participate in ionic interactions with negatively charged amino acid side chains, substrates, and cofactors, and to form multiple hydrogen bonds with the peptide backbone and other amino acid side chains.32 Arginine also has the most polar of all the common amino acid side chains and is therefore the amino acid that is most likely to be found on the surface of the proteins in an aqueous environment. 32 Citrullination would be expected to destroy the ionic interactions, interfere with hydrogen bonds, and create new interactions. Hence, the conversion of arginine into citrulline may result in an altered three-dimensional structure and function of the protein. 33 Similarly, lysine acetylation in histones is generally believed to allow chromatin to assume a more open state, permitting transcriptional activity. Our group and others have demonstrated that treatment of immune cells or animals with HDACI strongly inhibits proinflammatory cytokines.3,7 It is conceivable that binding of an effector protein to a specific site on the histone would prevent another PTM protein from binding the same histone.
Figure 4. Proposed hypothesis: histone acetylation could disrupt the PAD substrate recognition motif and thereby prevent the citrullination of adjacent arginine residues.
(A) Schematic drawing of the N-terminal tail of histone H3 showing that arginines 2 (R2), 8 (R8), 17 (R17), and 26 (R26) are the substrates of peptidyl arginine deiminase (PAD), and that lysines 4 (K4), 9 (K9), 14, (K14), 18 (K18), 23 (K23), and 27 (K27) are the substrates of histone acetyl transferase (HAT) / histone deacetylase (HDAC). (B) Due to amino acids of lysine and arginine are close to each other, increase in lysine acetylation by HDACI could suppress arginine citrullination by PAD.
In summary, these data taken together with our previous work, supports the hypothesis that microbial infection and/or endotoximia induce a rapid and robust increase in serum levels of CitH3 protein; and the elevated Cit H3 in circulation in turn aggravates sepsis. In this study, using a combination of in vitro and in vivo experiments, we have demonstrated that blockage of Cit H3 can be protective in the setting of lethal sepsis.
Acknowledgements
This work was funded by a grant from NIH RO1 GM084127 to HBA. Data presented at the 9th Annual Academic Surgical Congress in San Diego, CA, February 4–6, 2014.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFFERENCES
- 1.Roger T, Lugrin J, Le Roy D, Goy G, Mombelli M, Koessler T, Ding XC, Chanson AL, Reymond MK, Miconnet I, Schrenzel J, François P, Calandra T. Histone deacetylase inhibitors impair innate immune responses to Toll-like receptor agonists and to infection. Blood. 2011;117(4):1205–1217. doi: 10.1182/blood-2010-05-284711. [DOI] [PubMed] [Google Scholar]
- 2.Ciarlo E, Savva A, Roger T. Epigenetics in sepsis: targeting histone deacetylases. Int J Antimicrob Agents. 2013;42(Suppl):S8–S12. doi: 10.1016/j.ijantimicag.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Leoni F ZA, Bertolini G, Porro G, Pagani P, Pozzi P, Donà G, Fossati G, Sozzani S, Azam T, Bufler P, Fantuzzi G, Goncharov I, Kim SH, Pomerantz BJ, Reznikov LL, Siegmund B, Dinarello CA, Mascagni P. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci U.S.A. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Liu B, Gu X, Kochanek AR, Fukudome EY, Liu Z, Zhao T, Chong W, Zhao Y, Zhang D, Libermann TA, Alam HB. Creating a"pro-survival" phenotype through epigenetic modulation. Surgery. 2012;152(3):455–464. doi: 10.1016/j.surg.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler LM, Zhou X, Xu WS, Scher HI, Rifkind RA, Marks PA, Richon VM. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci U S A. 2002;99(18):11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks PA, Jiang X. Histone deacetylase inhibitors in programmed cell death and cancer therapy. Cell Cycle. 2005;4(4):549–551. doi: 10.4161/cc.4.4.1564. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Liu B, Zhao H, Sailhamer EA, Fukudome EY, Zhang X, Kheirbek T, Finkelstein RA, Velmahos GC, deMoya M, Hales CA, Alam HB. Protective effect of suberoylanilide hydroxamic acid against LPS-induced septic shock in rodents. Shock. 2009;32(5):517–523. doi: 10.1097/SHK.0b013e3181a44c79. [DOI] [PubMed] [Google Scholar]
- 8.Chong W, Li Y, Liu B, Zhao T, Fukudome EY, Liu Z, Smith WM, Velmahos GC, deMoya MA, Alam HB. Histone deacetylase inhibitor suberoylanilide hydroxamic acid attenuates Toll-like receptor 4 signaling in lipopolysaccharide-stimulated mouse macrophages. J Surg Res. 2012;178(2):851–859. doi: 10.1016/j.jss.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong W, Li Y, Liu B, Liu Z, Zhao T, Wonsey DR, Chen C, Velmahos GC, deMoya MA, King DR, Kung AL, Alam HB. Anti-inflammatory properties of histone deacetylase inhibitors: a mechanistic study. J Trauma Acute Care Surg. 2012;72(2):347–353. doi: 10.1097/TA.0b013e318243d8b2. discussion 353–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao T, Li Y, Liu B, Liu Z, Chong W, Duan X, Deperalta DK, Velmahos GC, Alam HB. Novel pharmacologic treatment attenuates septic shock and improves long-term survival. Surgery. 2013;154(2):206–213. doi: 10.1016/j.surg.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180:1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 12.Guimaraes-Costa AB, Nascimento MT, Wardini AB, Pinto-da-Silva LH, Saraiva EM. ETosis: A Microbicidal Mechanism beyond Cell Death. J Parasitol Res. 2012;2012:929743. doi: 10.1155/2012/929743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldmann O, Medina E. The expanding world of extracellular traps: not only neutrophils but much more. Front Immunol. 2012;3:420. doi: 10.3389/fimmu.2012.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Liu B, Fukudome EY, Lu J, Chong W, Jin G, Liu Z, Velmahos GC, Demoya M, King DR, Alam HB. Identification of citrullinated histone H3 as a potential serum protein biomarker in a lethal model of lipopolysaccharide-induced shock. Surgery. 2011;150:442–451. doi: 10.1016/j.surg.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange S, Gögel S, Leung KY, Vernay B, Nicholas AP, Causey CP, Thompson PR, Greene ND, Ferretti P. Protein deiminases: new players in the developmentally regulated loss of neural regenerative ability. Dev Biol. 2011;355(2):205–214. doi: 10.1016/j.ydbio.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun. 2009;1(3):194–201. doi: 10.1159/000206974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184(2):205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kan R, Jin M, Subramanian V, Causey CP, Thompson PR, Coonrod SA. Potential role for PADI-mediated histone citrullination in preimplantation development. BMC Dev Biol. 2012;12:19. doi: 10.1186/1471-213X-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Liu B, Fukudome EY, Kochanek AR, Finkelstein RA, Chong W, Jin G, Lu J, deMoya MA, Velmahos GC, Alam HB. Surviving lethal septic shock without fluid resuscitation in a rodent model. Surgery. 2010;148(2):246–254. doi: 10.1016/j.surg.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foulquier C, Sebbag M, Clavel C, Chapuy-Regaud S, Al Badine R, Mechin MC, Vincent C, Nachat R, Yamada M, Takahara H, Simon M, Guerrin M, Serre G. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007;56:3541–3553. doi: 10.1002/art.22983. [DOI] [PubMed] [Google Scholar]
- 22.Vossenaar ER, Radstake TR, van der Heijden A, van Mansum MA, Dieteren C, de Rooij DJ, Barrera P, Zendman AJ, van Venrooij WJ. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis. 2004;63:373–381. doi: 10.1136/ard.2003.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asaga H, Nakashima K, Senshu T, Ishigami A, Yamada M. Immunocytochemical localization of peptidylarginine deiminase in human eosinophils and neutrophils. J Leukoc Biol. 2001;70:46–51. [PubMed] [Google Scholar]
- 24.Rohrbach AS, Hemmers S, Arandjelovic S, Corr M, Mowen KA. PAD4 is not essential for disease in the K/BxN murine autoantibody-mediated model of arthritis. Arthritis Res Ther. 2012;14(3):R104. doi: 10.1186/ar3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss D, Weinrauch Y, Zychlinsky A. Neutrophil Extracellular Traps Kill Bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 26.Slack JL, Causey CP, Thompson PR. Protein arginine deiminase 4: a target for an epigenetic cancer therapy. Cell Mol Life Sci. 2011;68(4):709–720. doi: 10.1007/s00018-010-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, Luo Y, Levitt B, Glogowska M, Chandra P, Kulik L, Robinson WH, Arend WP, Thompson PR, Holers VM. N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186(7):4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 29.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Dieker J, Muller S. Epigenetic histone code and autoimmunity. Clin Rev Allergy Immunol. 2010;39:78–84. doi: 10.1007/s12016-009-8173-7. [DOI] [PubMed] [Google Scholar]
- 31.Nightingale KP BM, Eberharter A, Mamais A, Becker PB, Boyes J. Acetylation increases access of remodelling complexes to their nucleosome targets to enhance initiation of V(D)J recombination. Nucleic Acids Res. 2007;35:6311–6321. doi: 10.1093/nar/gkm650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borders CL, Jr, Broadwater JA, Bekeny PA, Salmon JE, Lee AS, Eldridge AM, Pett VB. A structural role for arginine in proteins: multiple hydrogen bonds to backbone carbonyl oxygens. Protein Sci. 1994;3(4):541–548. doi: 10.1002/pro.5560030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wegner N, Lundberg K, Kinloch A, Fisher B, Malmström V, Feldmann M, Venables PJ. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev. 2010;233(1):34–54. doi: 10.1111/j.0105-2896.2009.00850.x. [DOI] [PubMed] [Google Scholar]