Abstract
Objective: This study evaluated the effect of superpulse CO2 laser irradiation and deglazing of porcelain surfaces on the shear bond strength (SBS) of metal orthodontic brackets, and compared it with two conventional etching techniques. Methods: Forty-eight Feldspathic porcelain fused to metal specimens embedded in cylindrical acrylic resin tubes were fabricated, and all the specimens were divided into four groups. In Group 1, the specimens were roughened with a diamond bur and etched with hydrofluoric acid (HFA) gel for 4 min. In Group 2, the specimens were roughened with a bur and irradiated by a CO2 laser with a 2 W power setting for 20 sec. In Group 3, the specimens were only irradiated by a CO2 laser. In Group 4, the porcelain surface was sandblasted with 50 μm aluminum oxide. Before bonding, the bracket silane was applied on the porcelain surfaces. SBS was evaluated by a Universal testing machine (Zwickroll, Germany). The remaining adhesive after the bond failure was evaluated using an adhesive remnant index (ARI). Statistical analysis was conducted by analysis of variance (ANOVA), Tukey, and Kruskal–Wallis tests. Results: ANOVA revealed significant differences in SBS among the four groups (p<0.001). Group 1 demonstrated significantly higher bond strength (13.13±2.47) when compared with the other groups. Group 2 showed higher bond strength (9.60±1.91) when compared with group 4 (6.40±1.67) (p=0.016). Group 1 displayed the highest ARI scores among the groups. Conclusions: Deglazing combined with HFA etching produced the highest bond strength, but CO2 laser irradiation provided adequate bond strength and allowed for elimination of the HFA step. Deglazing is not recommended as a preliminary step before CO2 laser conditioning.
Introduction
One of orthodontists' problems is the need to bond orthodontic brackets to artificial surfaces such as porcelain, rather than the normal tooth surface.1–4 It has been proven that conventional acid etching is not effective in preparing the porcelain surface for the mechanical retention of orthodontic brackets.5,6
During the past few years, a number of methods have been introduced to overcome this problem.7–9 One of these techniques is deglazing the porcelain surface by roughening the surface with a diamond bur, or microetching the surface with aluminum oxide particles and after that bonding the bracket with or without a coupling agent.5,10 Whereas some laboratory studies have concluded that mechanical or chemical abrasion to deglaze the porcelain surface is necessary for enhanced bond strength,11–14 others report no advantage to deglazing, and suggest silane bonding.15,16 In a number of studies,17–19 silanes have been used to increase the bond strength to either glazed or roughened dental porcelain.6
Chemical preparation of the previously deglazed porcelain surface has been suggested.5 Application of orthophosphoric acid (OFA) as one of these chemical agents failed to present sufficient bond strength for clinical purposes.20,21 Hydrofluoric acid (HFA), on the other hand, seemed to be able to produce bond strength of acceptable values.4,20,21 It has even been reported that airborne-particle abrasion and acid etching with HFA increase the surface area of ceramic surfaces and create an irregular topography that enhances the potential for micromechanical retention of the adhesive cement.1
With the introduction of the laser, the idea of using it as a means of reinforcing bond strength has become popular. In previous studies, the efficacy of different types of lasers on porcelain surface preparation has been evaluated.5,22–26 Some studies claimed that Nd:YAG laser with appropriate parameters can be used as an alternative method for porcelain etching.22,23 The results on the efficacy of Er:YAG laser in porcelain conditioning is more controversial.22,24,25 Two studies evaluated the effect of CO2 laser irradiation of porcelain surfaces on the bond strength of metal orthodontic brackets.5,26 Akova et al. 5 concluded that superpulse CO2 laser irradiation provided adequate bonding strength between metal brackets and porcelain surfaces. Ahrari et al.26 evaluated the effect of fractional CO2 laser conditioning of porcelain surfaces on shear bond strength (SBS) of orthodontic brackets. This study recommended laser treatment as a suitable alternative to HFA for deglazed feldspathic porcelain.
Considering the challenge for orthodontists in preparation of adequate bond strength to the porcelain surfaces and the fact that there are few studies5,26 that evaluate the effect of new techniques such as CO2 laser eradiation in this regard, this study aimed to compare the effect of four different methods of porcelain surface treatment on the SBS of orthodontic metal brackets.
Materials and Methods
For this experimental study, 48 porcelain fused to metal specimens (Ceramco, Dentsply, York, PA; Heraus Kulzer, Hanau, Germany) were fabricated. Each specimen was formed in the form of a disc by the same operator. Feldspathic porcelain was placed on 3 mm nickel-chromium disc to produce 5-mm-thick specimens with a radius of 8 mm. A vacuum dental furnace (model: Centurion VPC, Ney Dental, Inc., Burlington, NJ) was used to fire the porcelain. The porcelain for the alloy had a heat up rate of 55°C/min to a peak firing temperature of 960°C and 0 min hold. All specimens were embedded in cylindrical acrylic resin tubes (Cold cure acrylic, Acropars, Marlic Inc., Iran) with a mounting jig. The mounting jig was used to align the direction of debonding force parallel to the surface of the porcelain during shear strength measurements. Metal standard edgewise maxillary central incisor brackets with 0.022 slot (American Orthodontics, Sheboygan, WI) and a surface area of 12.09 mm2 was placed on the porcelain surface. Before bonding the brackets, the surface area border on which the bracket was to be placed later was drawn by waterproof marker on the specimens so that the area that had to be treated could be controlled.
All the specimens were divided into four groups by systematic random sampling. The groups were divided as follows:
Deglazed+HFA group (Group1): The specimens were roughened with a diamond bur (863 Grit; Drendell and Zweilling, Berlin, Germany) to remove the glaze and then etched with 9.6% HFA gel (Porcelain Etch Gel, Pulpdent Corp., Watertown, MA) for 4 min, rinsed with distilled water for 30 sec, and then air dried for 15 sec.
Deglazed+CO2 group (Group2): The specimens were roughened with a diamond bur to remove the glaze, and then irradiated by superpulse CO2 laser (Ultradream pulse V, Daeshin enterprise, Hungary) with a 2 W power setting in the superpulse mode (15 msec, 2 Hz) for 20 sec as suggested by Akova et al.5 via contacting the laser probe on the porcelain surface.
CO2 group (Group 3): The specimens were only irradiated by CO2 laser as was done with the second group, without removing the glaze.
Sandblasted group (Group 4): The porcelain surface was sandblasted (Micro-etcher, Sandblaster, Masel, 2701 Bartram road, Bristol, USA) with 50 μm aluminum oxide at 80 psi for 5 sec. In order to remove the remnants of aluminum oxide, after the sandblasting procedure, all the specimens were cleaned with compressed air for 20 sec.
In the four groups, a silane coupling agent (Silane Bond Enhancer; Pulpdent Corp.) was applied after porcelain conditioning. Then, the brackets were bonded with light-curing orthodontic adhesive according to the manufacturer's instructions (Transbond XT, 3M Unitek, Monrovia, CA). The excess resin was removed by an explorer and the each surface was cured with light curing device (L.E.D. curing unit, Smartlife IQ2, Dentsply, Milford, USA) for a total of 40 sec from mesial, distal, occlusal, and gingival directions (10 sec each).
The specimens were then placed in an incubator (Vafaei, Iran) in water at 37°C for 24 h, and then SBS was evaluated by Universal testing machine (Zwickroll, Germany) with a crosshead speed of 1 mm/sec. During the debonding procedure, the bonded surface of the porcelain was parallel to the direction of force application, and the blade of the Instron was placed against the wing of the bracket. The shear force on the specimen was continued until debonding occurred. The maximum load applied to the surface (in Newtons) before debonding occurred was then divided by the cross-sectional area of the bracket (12.09 mm2) so that the results were obtained in megapascals (MPa). The process of applying the shear forces against the brackets and porcelain surface treatment was accomplished by operators who were blinded to the study.
After debonding the brackets, the porcelain specimens were examined under a stereomicroscope (SZ 40, Olympus, Tokyo, Japan) of×20 magnification to assess the mode of failure. Adhesive remnants indexes (ARI) were graded as suggested by Artun and Bergland27:
Grade 0: no adhesive remnant on former bracket site
Grade 1: adhesive remnants covering ≤50% of the former bracket site
Grade 2: adhesive remnants covering >50% of the former bracket site
Grade 3: all adhesive left behind on the former bracket site
Statistical analysis
To compare mean SBS among different groups of the study, statistical analysis was conducted by means of one way analysis of variance (ANOVA). At the next step (post-hoc), in order to compare the groups two by two, Tukey test with a correction in p value was used. Kruskal–Wallis and Mann–Whitney tests were used to compare the ARI scores. The statistical significance level was established at 0.05. All the statistical analysis was performed by SPSS software (Statistical Package for the Social Sciences, Version 11.5, SPSS, Chicago, IL).
Results
The descriptive statistics of the shear bond strength in all four groups are summarized in Table 1. ANOVA revealed significant differences in SBS among the four groups (p<0.001). Two by two comparison of the mean SBS in the study groups is outline in Table 2. Multiple comparisons by Tukey test demonstrated that the highest bond strength was in the deglazed+HFA group (Group 1) and that the lowest was in the sandblasted group (Group 4).
Table 1.
Descriptive Statistics of Shear Bond Strength (MPa) in Four Groups
| Study group | Mean (MPa) | SD | Range |
|---|---|---|---|
| 1 (Deglazed+HFA) | 13.13 | 2.47 | 9.59–17.70 |
| 2 (Deglazed+CO2) | 9.60 | 1.91 | 6.10–12.03 |
| 3 (CO2) | 8.38 | 3.74 | 4.17–18.53 |
| 4 (Sandblast) | 6.40 | 1.67 | 4.05–9.93 |
HFA, hydrofluoric acid.
Table 2.
Two by Two Comparison of the Mean Shear Bond Strength in the Study Groups
| Study groups | Mean difference | Significance |
|---|---|---|
| 1 (Deglazed+HFA), 2 (Deglazed+CO2) | 3.53598 | 0.009* |
| 1 (Deglazed+HFA), 3 (CO2) | 4.75738 | 0.000** |
| 1 (Deglazed+HFA), 4 (sandblast) | 6.72801 | 0.000** |
| 2 (Deglazed+CO2), 3 (CO2) | 1.22140 | 0.655 |
| 2 (Deglazed+CO2), 4 (sandblast) | 3.19203 | 0.021* |
| 3 (CO2), 4 (sandblast) | 1.97064 | 0.256 |
p<0.05, **p<0.001.
HFA, hydrofluoric acid.
The distribution of ARI scores is shown in Table 3. Kruskal–Wallis showed that there were significant differences among the four groups (p<0.001) regarding the ARI scores. In the deglazed+HFA group (Group 1), an increased frequency of ARI score 2 was reported. In the deglazed+CO2 group (Group 2), a higher frequency of ARI score 1 was present, and none of the failures occurred in ARI scores of 2 or 3. In the sandblasted and CO2 laser groups (Groups 3 and 4), most of the bond failures happened at ARI scores of 0. No ARI scores of 3 were reported in any of the specimens (Fig. 1).
Table 3.
Descriptive Statistics of ARI Scores in All Groups
| Adhesive remnant index (ARI) scores | ||||
|---|---|---|---|---|
| Study Group | 0 | 1 | 2 | 3 |
| 1 (Deglazed+HFA) | 0 (0%) | 2 (16.7%) | 10 (83.3%) | 0 (0%) |
| 2 (Deglazed+CO2) | 2 (16.7%) | 10 (83.3%) | 0 (0%) | 0 (0%) |
| 3 (CO2) | 7 (58.3%) | 4 (33.3%) | 1 (8.3%) | 0 (0%) |
| 4 (Sandblast) | 8 (66.7%) | 4 (33.3%) | 0 (0%) | 0 (0%) |
HFA, hydrofluoric acid.
FIG. 1.
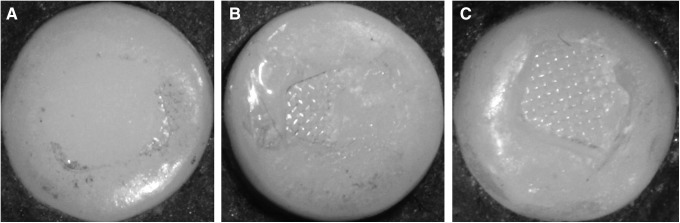
After debonding the brackets, we found three modes of bond failure in the porcelain specimens under the stereomicroscope. (A) Adhesive remnant index (ARI) 0: no adhesive remnant on the former bracket site. (B) ARI 1: adhesive remnants covering ≤50% of the former bracket site. (C) ARI 2: adhesive remnants covering >50% of former bracket site.
Discussion
Because of the chemical properties of bonding agents as well as the glazed surfaces of porcelain, bonding orthodontic brackets to porcelain surfaces has always been an issue for orthodontists.5 Conventional methods such as acid etching provide an inadequate bond strength for orthodontic brackets and, therefore, are not as effective.7 A number of alternative methods have been suggested to overcome this problem, such as deglazing and roughening the porcelain surface with a diamond bur, microetching the surface with aluminum oxide particles, conditioning the porcelain surface with the application of silane, and, most recently, lasers.5,10,13,14,18,21–26
Some of the previous studies22–25 have evaluated the effect of Nd:YAG and Er:YAG lasers on porcelain surface treatment for efficient bracket bonding, whereas some other studies5,26 have assessed the effectiveness of CO2 laser for this purpose.
During the process of heat induction of porcelain surfaces with a focused CO2 laser, conchoidal tears, a typical effect of surface warming, appear. It is said that these tears provide mechanical retention between the composite resin and porcelain.5,28 Considering several advantages CO2 lasers provide, such as time saving and biocompatibility,5 their effectiveness on the SBS of orthodontic brackets was compared with that of sandblasting and HFA etching in our study. The deglazed+HFA group (Group 1) demonstrated significantly higher bond strength (13.13±2.47) when compared with other groups; whereas the deglazed+CO2 group (Group 2) showed higher bond strength (9.60±1.91) when compared with the sandblasted group (Group 4) (6.40±1.67) (p=0.016).
According to Reynolds,29 a minimum bond strength of 6–8 MPa is required for efficient clinical orthodontic bonding, whereas 13 MPa has been considered the maximum authorized strength between porcelain and adhesive to prevent cohesive porcelain fracture.30 In our study, the mean SBS in the deglazed+HFA group (group 1) was 13.13, which is not only higher than the values reported for other groups, but also higher than the maximum authorized strength between porcelain and adhesive.30 The values reported in the rest of the groups in our study were well within acceptable limits.
The effectiveness of different types of lasers on porcelain surface treatment has been evaluated in previous studies.5,22–26
It has been proven that CO2 wavelength is absorbed almost totally by porcelain, suggesting that it as a good candidate for porcelain surface treatment.5,28 Some investigators5,26 used the CO2 laser as the present study, and reported satisfying results in SBS as a result of conditioning the porcelain surface by CO2 laser. Akova et al.5 studied the effect of conventional CO2 laser with the output energy of 2 W on the bond strength of orthodontic brackets to porcelain surfaces, and reported that the average debonding force was 6.26±0.58 MPa. All the samples in their study were deglazed by grinding. It has been stated that this method has the capability of introducing microcracks along the porcelain and, therefore, is not recommended by some investigators.5,7 Ahrari et al.26 used a fractional CO2 laser with different output energies on glazed and deglazed porcelain surfaces. This study showed that deglazing followed by CO2 laser treatment at 10 W produced the highest mean bond strength (11.4 MPa), whereas the glazed surfaces treated by CO2 laser at 20 W yielded the lowest SBS value (4.4 MPa).
In our study, laser irradiation with an output energy of 2 W was used for treating the porcelain surface. The power of the laser used in the study of Akova et al.5 was also 2 W, which was obtained from a pilot study comparing the effect of 2, 3, 5, 10, and 15 W powers on surface bond strength. That pilot study found that output energies other than 2 W were not suitable for porcelain surface treatment and produced surface cracks, which could reduce the fracture resistance of porcelain. However, in the study of Ahrari et al.,26 porcelain surfaces were irradiated with the different powers of 10, 15, and 20 W, using a fractional CO2 laser device. Their results indicated that the bond strengths of the aforementioned groups were not significantly different from each other.
In the present study, the deglazed and laser-irradiated group (Group 2) showed higher SBS than the group only irradiated by laser (Group 3), but the difference was not statistically significant. This finding is in agreement with that of Akova et al.5 who did not find any significant differences between the SBS of glazed and deglazed surfaces. Ahrari et al.,26 however, reported that the SBS of the deglazed and laser-irradiated group was higher than that of the glazed group, and concluded that CO2 laser can produce higher bond strength on deglazed porcelain when compared with HFA. The difference between the results of the present study and the study of Ahrari et al. can be attributed to different parameters and duration of laser application. The specimens in the latter study were treated with a fractional CO2 laser in dynamic mode for 10 sec, with an output energy of 10 mJ, a frequency of 200 Hz, and powers of 10 and 15 W, which were different from the parameters used in our study [2W power setting in the superpulse mode (15 msec, 2 Hz) for 20 sec]. Moreover, the bond strength of the specimens treated by HFA were lower in the study of Ahrari et al.26 than in our study, which may be related to different etching time (2 vs. 4 min). In the study conducted by Ahrari et al.,26 the type of bracket and adhesive used was the same as our study; moreover, none of the specimens were thermocycled, whereas in the study conducted by Akova et al.,5 a different type of bracket and adhesive was used and the specimens underwent thermocycling prior to SBS testing. However, the results of our study were mostly in agreement with the results of Akova et al., rather than with those of Ahrari et al. Therefore, it can be deduced that surface conditioning techniques as well as laser parameters play a more crucial role in the bond strength of brackets to ceramic surfaces than do the aforementioned factors.
Deglazing may increase the risk of crack propagation and probability of porcelain fracture during debonding,31 and the aesthetic characteristics may be irreversibly damaged by the roughening process.32 Considering the comparable bond strength of the glazed and deglazed porcelain surfaces irradiated by laser in the present study, grinding the porcelain surface before bonding in cases in which CO2 laser is to be applied cannot be justified.
The findings of the present study indicate that porcelain treated with either CO2 laser irradiation or sandblasting can yield bond strength values within the acceptable clinical range. Considering the need for rinsing and drying the porcelain surface following the application of HFA, it is obvious that a more time-consuming process is required when working with HFA (∼4 min) than when working with CO2 laser (20 sec). Moreover, HFA requires extra care to avoid soft tissue damage.
The evaluation of the remaining composite after the shear bond test showed that none of the specimens had an ARI score of 3, which means that in none of the experimental groups did the entire adhesive remain on the porcelain surface. The highest frequency of an ARI score of 2 was found in the deglazed+HFA group (Group1), which had the highest reported SBS as well. It can be concluded that HFA produces the best wettability of porcelain surface. This finding could also indicate that more time is needed to remove the remaining adhesive from the porcelain surface treated by HFA than with CO2 laser after bracket debonding.
It has been proven that adhesive failure during debonding is preferred to cohesive failure, because the latter may lead to porcelain fracture.33 If the bond strength between the porcelain and composite resin is >13 MPa, the risk of cohesive fracture increases.30 This is in agreement with the results of our study, because in the deglazed+HFA group (Group 1), the reported bond strength was >13 MPa, and all of the bond failures were cohesive, whereas in the CO2 and sandblasted groups (Groups 3 and 4) most of the bond failures were adhesive. A higher frequency of adhesive failure in the latter two groups compared with HFA can be attributed to the lower bond strength.
It is highly recommended that further in vivo studies be conducted to obtain further information regarding different surface treatment procedures.
Conclusions
The results of this study indicated that HFA provides a higher-than-recommended SBS, whereas CO2 laser irradiation with or without porcelain deglazing provides adequate bond strength between metal brackets and porcelain surfaces. Therefore, considering the more time-consuming process required for porcelain etching with HFA, possible soft tissue injuries, and the excessive mean bond strength, CO2 laser can be recommended as an appropriate substitute for HFA in porcelain surface treatment, with a diminished risk of porcelain fracture. Considering the comparable bond strength in the laser-treated groups with or without porcelain deglazing, and the risks engendered by deglazing on the integrity of porcelain, deglazing is not recommended prior to CO2 laser irradiation. Moreover, based on the results of the ARI scores, albeit HFA provides the best porcelain surface wetting, complete adhesive removal following a bond failure will be more time-consuming.
Acknowledgments
The authors thank the vice-chancellor of Shiraz University of Medical Sciences for supporting the research. as well as Dr. Mehrdad Vossoughi from the Dental Research Development Center for the statistical analysis. The authors also gratefully acknowledge the Shiraz orthodontic and Shiraz biomaterial and research assistance of Shiraz Dental School, for support of this research (grant number 90-01-37-3751).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Yadav S., Upadhyay M., Borges G.A., and Roberts W.E. (2010). Influence of ceramic (feldspathic) surface treatments on the micro-shear bond strength of composite resin. Angle Orthod. 80, 765–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heydecke G., Thomason J.M., Lund J.P., and Feine J.S. (2005). The impact of conventional and implant supported prostheses on social and sexual activities in edentulous adults. Results from a randomized trial 2 months after treatment. J. Dent. 33, 649–657 [DOI] [PubMed] [Google Scholar]
- 3.Merrill S.W., Oesterle L.J., and Hermesch C.B. (1994). Ceramic bracket bonding: a comparison of shear, tensile and torsional bond strengths of ceramic brackets. Am. J. Orthod. Dentofacial Orthop. 106, 159–164 [DOI] [PubMed] [Google Scholar]
- 4.Barbosa V.L.T., Almedia M.A., Chevitarese O., and Keith O. (1995). Direct bonding to porcelain. Am. J. Orthod. Dentofacial Orthop. 107, 159–164 [DOI] [PubMed] [Google Scholar]
- 5.Akova T., Yoldas O., Toroglu S., and Uysal H. (2005). Porcelain surface treatment by laser for bracket-porcelain bonding. Am. J. Orthod. Dentofacial Orthop. 128, 630–637 [DOI] [PubMed] [Google Scholar]
- 6.Kocadereil I., Canay S., and Akac K. (2001). Tensile bond strength of ceramic orthodontic brackets bonded to porcelain surfaces. Am. J. Orthod. Dentofacial Orthop. 119, 617–620 [DOI] [PubMed] [Google Scholar]
- 7.Eustaquio R., Garner L.D., and Moorre B.K. (1988). Comparative tensile strengths of brackets bonded to porcelain with orthodontics adhesive and porcelain repair systems. Am. J. Orthod. Dentofacial Orthop. 94, 421–425 [DOI] [PubMed] [Google Scholar]
- 8.Kao E.C., Boltz K.C., and Johnson W.M. (1988). Direct bonding of orthodontic brackets to porcelain venner laminates. Am. J. Orthod. Dentofacial Orthop. 94, 458–468 [DOI] [PubMed] [Google Scholar]
- 9.Zelos L., Bevis R.P., and Keenan K.M. (1994). Evaluation of the ceramic/ceramic interface. Am. J. Orthod. Dentofacial Orthop. 106, 10–21 [DOI] [PubMed] [Google Scholar]
- 10.Zachrisson B.U., and Buyukyilmaz T. (1993). Recent advances in bonding to gold, amlgam and porcelain. J. Clin Orthod. 27, 661–675 [Google Scholar]
- 11.Withlock B.O., Eick D.J., Ackerman R., Galros A.G., and Chappele R.P. (1994). Shear strength of ceramic brackets bonded to porcelain. Am. J. Orthod. Dentofacial Orthop. 106, 358–364 [DOI] [PubMed] [Google Scholar]
- 12.Kao E.C., and Johnson W.M. (1991). Fracture incidence on debonding of orthodontic brackets from porcelain venner laminates. J. Prosthet. Dent. 66, 631–637 [DOI] [PubMed] [Google Scholar]
- 13.Zacharisson Y.O., Zaharisson W.M., and Buyukuilmaz t. (1996). Surface preparation for orthodontic bonding to porcelain. Am. J. Orthod. Dentofacial Orthop. 109, 420–430 [DOI] [PubMed] [Google Scholar]
- 14.Ajlouni R., Bishara S.E., Oonsombat C., Soliman M., and Laffoon J. (2005). The effect of porcelain surface conditioning on bonding orthodontic brackets. Angle Orthod. 75, 858–864 [DOI] [PubMed] [Google Scholar]
- 15.Major P.W., Koehler J.R., and Manning K.E. (1995). 24-Hour shear bond strength of metal orthodontic brackets bonded to porcelain using various adhesive promoters. Am. J. Orthod. Dentofacial Orthop. 108, 322–329 [DOI] [PubMed] [Google Scholar]
- 16.Bourke B.M., and Rock W.P. (1999). Factors affecting the shear bond strength of orthodontic brackets to porcelain. Br. J. Orthod. 26, 285–290 [DOI] [PubMed] [Google Scholar]
- 17.Bowen R.L., and Rodriguez M.S. (1962). Tensile strength and modulus of elasticity of tooth structure and several restorative materials. J. Am. Dent Assoc. 64, 378–387 [DOI] [PubMed] [Google Scholar]
- 18.Lacy A.M., LaLuz J., Watanabe L.G., and Dellinges M. (1988). Effect of porcelain surface treatment on the bond to composite. J. Prosthet. Dent. 60, 288–291 [DOI] [PubMed] [Google Scholar]
- 19.Wolf D.M., Powers J.M., and O'Keef K.L. (1992). Bond strength of composite to porcelain treated with new porcelain repair agents. Dent. Mater. 8, 158–161 [DOI] [PubMed] [Google Scholar]
- 20.Gillis I., and Redlich M. (1998). The effect of different porcelain conditioning techniques on shear bond Strength of stainless steel brackets. Am. J. Orthod. Dentofacial Orthop. 114, 387–392 [DOI] [PubMed] [Google Scholar]
- 21.Shahverdi S., Canay S., Sahin E., and Bilge A. (1998). Effect of different surface treatment methods on the bond Strength of composite resin to porcelain. J. Oral. Rehabil. 25, 699–705 [DOI] [PubMed] [Google Scholar]
- 22.Poosti M., Jahanbin A., Mahdavi P., and Mehrnoush S. (2012). Porcelain conditioning with Nd:YAG and Er:YAG laser for bracket bonding in orthodontics. Lasers. Med Sci. 27, 321–324 [DOI] [PubMed] [Google Scholar]
- 23.Hosseini M.H., Sobouti F., Etemadi A., Chiniforush N., and Shariati M. (2013). Shear bond strength of metal brackets to feldspathic porcelain treated by Nd:YAG laser and hydrofluoric acid. Lasers. Med. Sci. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Yassaei S., Moradi F., Aghili H., and Kamran M.H. (2013). Shear bond strength of orthodontic brackets bonded to porcelain following etching with Er:YAG laser versus hydrofluoric acid. Orthodontics (Chic.). 14, e82–e87 [DOI] [PubMed] [Google Scholar]
- 25.Topcuoglu T., Oksayan R., Topcuoglu S., Coskun M.E., and Isman N.E. (2013). Effect of Er:YAG laser pulse duration on shear bond strength of metal brackets bonded to a porcelain surface. Photomed. Laser Surg. 31, 240–246 [DOI] [PubMed] [Google Scholar]
- 26.Ahrari F., Heravi F., and Hosseini M. (2012). CO2 laser conditioning of porcelain surface for bonding metal orthodontic brackets. Lasers Med. Sci. 28, 1091–1097 [DOI] [PubMed] [Google Scholar]
- 27.Artun J., and Bergland S. (1984). Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am. J. Orthod. 85, 333–340 [DOI] [PubMed] [Google Scholar]
- 28.Dobberstein H., Dobberstein H., Schwarz A., Zuhurt R., and Tani Y. (1989). Laser processing of dental materials. Lasers in dentistry. Elsevier Science, University of Illinois, Chicago, IL [Google Scholar]
- 29.Reynolds I.R. (1975). A review of direct orthodontic bonding. Br. J. Orthod. 2, 171–178 [Google Scholar]
- 30.Thurmond J.W., Barkmeier W.W., and Wilwerding T.M. (1994). Effect of porcelain surface treatments on bond strengths of composite resin bonded to porcelain. J. Prosthet. Dent. 72, 355–359 [DOI] [PubMed] [Google Scholar]
- 31.Jarvis J., Zinelis S., Eliades T., and Bradley T.G. (2006). Porcelain surface roughness, color and gloss changes after orthodontic bonding. Angle Orthod. 76, 274–277 [DOI] [PubMed] [Google Scholar]
- 32.Herion D.T., Ferracane J.L., and Covell D.A., Jr (2010). Porcelain surface alterations and refinishing after use of two orthodontic bonding methods. Angle Orthod. 80, 167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith G.A., McInnes–Ledoux P., Ledoux W.R., and Weinberg R. (1988). Orthodontic bonding to porcelain: bond strength and refinishing. Am. J. Orthod. Dentofacial Orthop. 94, 245–252 [DOI] [PubMed] [Google Scholar]


