Abstract
Background
Higher levels of small low-density lipoprotein (LDL) and lower levels of high-density lipoprotein (HDL) subclasses have been associated with increased risk of cardiovascular disease. The extent to which HIV infection and HIV/HCV coinfection are associated with abnormalities of lipoprotein subclasses is unknown.
Methods
Lipoprotein subclasses were measured by nuclear magnetic resonance (NMR) spectroscopy in plasma samples from 569 HIV-infected and 5948 control participants in the FRAM, CARDIA and MESA studies. Multivariable regression was used to estimate the association of HIV and HIV/HCV coinfection with lipoprotein measures with adjustment for demographics, lifestyle factors, and waist-to-hip ratio.
Results
Relative to controls, small LDL levels were higher in HIV-monoinfected persons (+381 nmol/L, p<.0001), with no increase seen in HIV/HCV coinfection (−16.6 nmol/L). Levels of large LDL levels were lower (−196 nmol/L, p<.0001) and small HDL were higher (+8.2 μmol/L, p<.0001) in HIV-monoinfection with intermediate values seen in HIV/HCV-coinfection. Large HDL levels were higher in HIV/HCV-coinfected persons relative to controls (+1.70 μmol/L, p<.0001), whereas little difference was seen in HIV-monoinfected persons (+0.33, p=0.075). Within HIV-infected participants, HCV was associated independently with lower levels of small LDL (−329 nmol/L, p<.0001) and small HDL (−4.6 μmol/L, p<.0001), even after adjusting for demographic and traditional cardiovascular risk factors.
Conclusion
HIV-monoinfected participants had worse levels of atherogenic LDL lipoprotein subclasses compared with controls. HIV/HCV coinfection attenuates these changes, perhaps by altering hepatic factors affecting lipoprotein production and/or metabolism. The effect of HIV/HCV coinfection on atherosclerosis and the clinical consequences of low small subclasses remain to be determined.
Keywords: HIV infection, HCV infection, lipoproteins, cardiovascular disease
INTRODUCTION
Both HIV and HCV monoinfection are associated with an increased risk of cardiovascular disease (CVD) [1-4]. Some studies also report that HIV/HCV coinfection may confer an increased risk of CVD when compared to those with HIV monoinfection, [5, 6] while another study reported little association of HIV/HCV coinfection with subclinical atherosclerosis relative to those with HIV monoinfection [7].
Dyslipidemia is an important risk factor for CVD, and both HIV and HCV monoinfection have been independently associated with alterations in lipid metabolism [3, 8, 9]. HIV monoinfection is known to be accompanied by an atherogenic lipoprotein phenotype (ALP) including increased triglycerides (TG), and decreased high-density lipoprotein cholesterol (HDL-C), but also decreased low-density lipoprotein cholesterol (LDL-C) [8, 10, 11],. HCV monoinfection has been associated with decreased total cholesterol, TG, and LDL-C when compared to those with neither HIV nor HCV infection [3]. However, the relationship of HIV/HCV coinfection with lipoprotein levels is unclear.
Some studies have suggested that changes in lipoprotein subclass particle concentration may be a stronger predictor of CVD than the standard lipid panel [12, 13]. The key lipoprotein classes, LDL-C and HDL-C, are comprised of many subclasses based on size and density [14]. Small LDL is believed to have atherogenic properties. In the general population, increased levels of small LDL often accompany other metabolic derangements including high TG, low HDL-C, and insulin resistance,[8, 10, 15] that are similar to changes found in HIV-monoinfected individuals. Increased relative number of LDL particles (LDL-P), total and small LDL-P concentration, and smaller LDL size have been directly associated with CVD in studies in the general population and can be independent of LDL-C [16-21].
HDL particle (HDL-P) concentrations have also been associated with subclinical atherosclerosis and incident CVD in the general population [22]. Lower baseline total, large and small HDL-P concentrations were associated with a higher risk of CVD in HIV-monoinfected participants [2]. These findings are of concern because HIV infection is associated with lower HDL-P numbers with reductions in large and small HDL-P concentrations [23, 24]. To date, no large, nationally representative multiethnic study has taken the effects of HCV infection into account when examining concentrations of lipoprotein subclasses in HIV-infected participants. Therefore, we evaluated lipoprotein levels in HIV/HCV-coinfected persons, HIV-monoinfected persons, and those with neither infection from the Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) and the Multi-Ethnic Study of Atherosclerosis (MESA).
MATERIALS AND METHODS
Study Population
FRAM HIV-infected participants were initially recruited from 16 HIV or infectious disease clinics or cohorts, and were demographically nationally representative. The first FRAM exam enrolled 1183 HIV-infected and 297 HIV-uninfected controls from 2000-2002 [25]. The second exam conducted approximately five years later, included lipoprotein measures and 581 HIV-infected and 246 HIV-uninfected controls that had been seen at the first exam. By the second exam, the FRAM HIV-infected participants were highly treated with 97% having received some form of antiretroviral therapy(ART).
Control participants were recruited from two centers of the Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA participants were originally recruited in 1985-1986 as a population-based sample of healthy 18-30 year old Caucasian and African-American women and men to longitudinally study cardiovascular risk factors. At the second FRAM exam, the CARDIA controls from the original FRAM study were 37-50 years old.
Controls from the MESA study who had lipoproteins measured and met inclusion criteria were also used for controls in this analysis as done previously [26]. This additional pool of control participants was included to supplement the CARDIA control participant pool with individuals in the upper age range (45-78 years) of the HIV-infected FRAM participants. MESA was initiated in July 2000 to investigate the prevalence, correlates, and progression of CVD in a population-based sample of 6,814 men and women aged 45-84 free of clinical CVD at enrollment, recruited from six U.S. field centers. The baseline MESA visit was conducted from 2000-2002.
All three cohorts were nationally representative and the control groups were population-based. FRAM and MESA study protocols were approved by institutional review boards at all respective sites.
Analyses of HIV-infected participants alone included all 569 participants with non-missing lipoprotein measures. To ensure comparability of the HIV-infected and control groups, participants included in analyses comparing HIV-infected and controls were restricted to Caucasian, African-American, and Hispanic men and women aged 37-78 years. All HIV-infected FRAM (n=483), MESA control (n=5712), and CARDIA control (n=236) participants that met these criteria and had lipoprotein measurements available were included in the analysis. The linearity assumption was tested for continuous measures by adding quadratic terms to the models and by examining generalized additive models [27].
NMR lipoprotein measurements
Lipoprotein particle concentrations and size were measured on frozen plasma specimens (−70°C) by proton NMR spectroscopy (LipoScience Inc., North Carolina). Lipoprotein subclass particle concentrations of different sizes were directly obtained from the measured amplitudes of their spectroscopically distinct lipid methyl group NMR signals [21]. Weighted-average lipoprotein particle sizes are derived from the sum of the diameter of each subclass multiplied by its relative mass percentage based on the amplitude of its methyl NMR signal. As outlined in previous MESA papers [19], the concentrations in nanomoles of particles/liter(nmol/L) of the following subclasses were measured: small LDL (diameter of 18.0–21.2nm), large LDL (21.2–23.0nm), intermediate-density lipoprotein (IDL) (23.0–27.0nm), large HDL (8.8–13.0nm), medium HDL (8.2–8.8nm), small HDL (7.3–8.2nm), large very low-density lipoprotein (VLDL) (>60nm), medium VLDL (35.0–60.0nm), and small VLDL (27.0–35.0nm). [19, 21]. Inter-assay reproducibility, determined from replicate analyses of plasma pools, is indicated by the following coefficients of variation: <2% for VLDL size and <0.5% for LDL and HDL size, <10% for VLDL-P subclasses, <4% for total LDL-P, <8% for large and small LDL-P, and <5% for large and small HDL-P, with higher variation (<30%) for medium HDL-P and IDL-P [28].
Other Measurements
Height and weight were measured by standardized protocols. Standardized questionnaires determined demographic characteristics; medical history; HIV risk factors; and use of alcohol and tobacco. Research associates interviewed participants and reviewed medical charts regarding ART use. A diagnosis of AIDS was made by history of opportunistic infection or CD4 count <200c/μL. We classified participants as having diabetes if they had a fasting blood glucose level of ≥126mg/dL (7.0mmol/L) or reported use of insulin or oral hypoglycemic medication.
HCV RNA testing was performed on frozen sera using the Bayer Versant 3.0 branched DNA assay (Leverkusen, Germany) in the entire FRAM cohort [25]. CD4 lymphocyte count and percent, HIV RNA level (lower limit of detection: 400copies/ml) in HIV-infected participants, and other blood specimens from FRAM participants were analyzed in a single centralized laboratory (Covance, Indianapolis, IN).
Covariates
Candidate covariates included demographic characteristics, lifestyle factors, and measures of body composition. Demographic characteristics included age, sex, and race. Lifestyle factors included smoking, alcohol use, and diabetes. Measures of body composition included waist-to-hip ratio (WHR), waist circumference, hip circumference, and body mass index (BMI). HIV-related factors included HIV duration, HCV status, HIV RNA level, current and nadir CD4 count, history of AIDS, and current ART use.
Statistical Analysis
We compared demographic and clinical characteristics of HIV-infected and uninfected participants using the Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Distributions of lipoprotein levels were examined using smoothed curves from kernel density estimates and were compared using Levene’s test for homogeneity of variance. Tests of the residuals found violations of normality and homoscedasticity assumptions; therefore, we used quantile regression to compare median lipoprotein levels by HIV and HCV status [29].
To determine whether HIV and HCV infection were independently associated with lipoprotein levels, multivariable models were sequentially adjusted for (1) demographics alone, and (2) demographics, lifestyle factors, and body composition.
Separate models were constructed in HIV-infected individuals alone, adjusting for HIV-related factors as well as demographics, lifestyle factors, and body composition. Factors forced in the full model included age, gender, and race/ethnicity. We used stepwise backward selection with a significance level of α=0.05 to remove candidate covariates that did not appear to be strongly associated with the outcome. All analyses were conducted using the SAS system, version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
The characteristics of the participants studied are presented in Table 1. For comparison of HIV-infected participants with controls, the age range was restricted to 38-79 years, where there was overlap between the HIV-infected and control participants. The median age was 49 years for HIV-infected participants and 61 years for controls. Among HIV-infected participants, 28% were female, 51% Caucasian, 42% African-American and 6.4% Hispanic. Due to the design of the control cohorts, the controls were evenly divided by gender, and had a higher proportion of Hispanics than the HIV-infected cohort. Differences in smoking, diabetes, and other traditional CVD risk factors have been described previously [25]. Of the FRAM participants, 11% of HIV/HCV-coinfected, 33% of HIV monoinfected, and 16% of the controls were on lipid lowering therapy.
Table 1.
Demographic and Clinical Characteristics of HIV-infected and Control Participants
| Parameter | Age- and race-restricted* |
All FRAM HIV+ (n = 569) |
|
|---|---|---|---|
| Controls** (n = 5948) |
FRAM HIV+ (n = 483) |
||
| Age (y)† | 61 (52-69) | 49 (44-55) | 47 (42-53) |
| Female | 52.8% | 28.2% | 29.9% |
| Race | |||
| African-American | 32.1% | 42.4% | 40.9% |
| Hispanic | 23.8% | 6.4% | 7.4% |
| Caucasian | 44.1% | 51.1% | 46.7% |
| Other | 0% | 0% | 4.9% |
| Smoking Status | |||
| Current | 14.2% | 37.3% | 36.2% |
| Past | 38.1% | 26.3% | 24.6% |
| Never | 47.7% | 36.4% | 39.2% |
| Diabetes | 12.3% | 9.9% | 9.3% |
| Alcohol user | 55.7% | 42.4% | 42.4% |
| BMI (kg/m2) | 28.2 (25.2-31.9) | 25.1 (22.2-27.8) | 25.1 (22.2-28.0) |
| Waist Circumference (cm) | 98.5 (90.0-108.0) | 91.2 (83.5-99.4) | 90.7 (83.0-99.1) |
| Hip Circumference (cm) | 105.4 (99.7-113.0) | 95.7 (90.2-101.7) | 95.8 (90.2-102.0) |
| WHR | 0.94 (0.87-0.98) | 0.95 (0.89-1.01) | 0.94 (0.89-1.01) |
| Current Lipid Lowering Use | 16.3% | 27.6% | 25.2% |
| Current CD4 (cells/uL) | 422 (268-626) | 424 (264-623) | |
| HIV RNA (1000/mL) | 0.4 (0.4-1.1) | 0.4 (0.4-1.6) | |
| Hepatitis C (HCV) | 23.3% | 22.6% | |
| Current HAART use | 82.6% | 81.5% | |
| Current NRTI use | 87.0% | 85.8% | |
| Current NNRTI use | 38.7% | 39.4% | |
| Current PI use | 56.7% | 54.7% | |
Restricted on age (38-79 years) and race/ethnicity (African-American, Caucasian, Hispanic)
Pooled controls from MESA and CARDIA
Data for continuous variables are presented as median (IQR).
Abbreviations: BMI = body mass index; WHR = waist to hip ratio
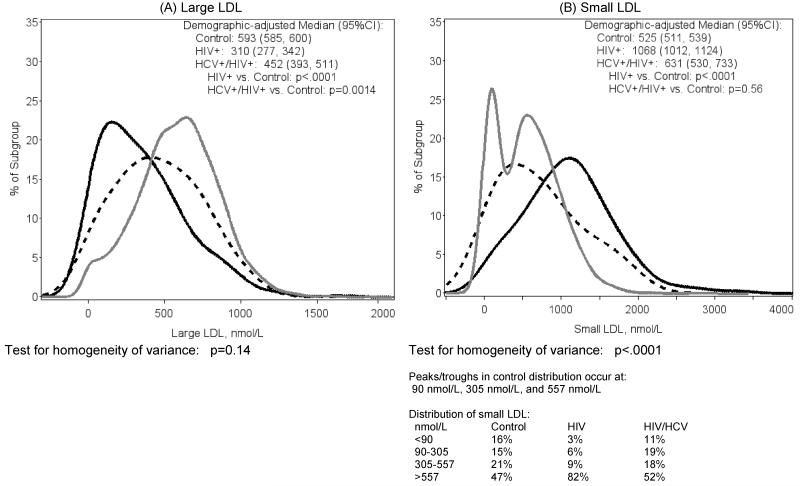
Distributions of lipoprotein measures are shown in Figure 1. HIV-monoinfected participants had lower levels of large LDL-P (Figure 1A) compared to controls (median 310 vs. 593 nmol/L, p<.0001), while the values for HIV/HCV-coinfected were intermediate (demographic-adjusted median 452, p=0.0014 vs. controls). Analysis of the distribution found no strong evidence for a difference between the groups (p=0.14, test for homogeneity of variance).
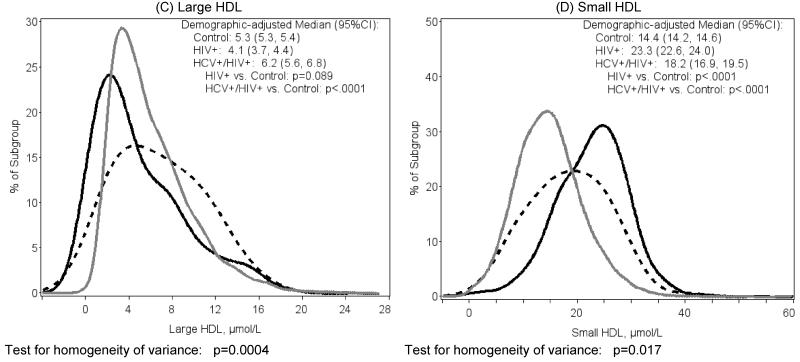
Figure 1.
Distribution of NMR lipoprotein measures by HIV Status
(solid gray=Control, solid black=HIV-monoinfected, dashed black=HCV/HIV-coinfected
By contrast, HIV-monoinfected participants had higher levels of small LDL-P (Figure 1B) compared to controls (1068 vs. 525nmol/L, p<.0001), while the values for HIV/HCV-coinfected were closer to controls (median 631, p=0.56 vs. controls). The distribution of small LDL-P in the control participants was bimodal, showing separate populations of low and high levels of small LDL-P. The distributions of small LDL-P in the HIV-monoinfected and HIV/HCV-coinfected reflected a skewed distribution relative to the controls (p<.0001, test for homogeneity of variance). The distribution in HIV-monoinfected participants was unimodal and was right-shifted relative to the high mode in the control group, whereas the distribution in HIV/HCV-coinfected participants was also unimodal but largely overlapped with the middle of the control group.
Large HDL-P levels (Figure 1C) trended lower in HIV-monoinfected than control (4.1 vs. 5.3μmol/L, p=0.089), but were higher in HIV/HCV-coinfected (6.2, p<0.0001 vs. control). Variability in distribution of large HDL was greater in the two HIV-infected groups relative to controls (p=0.0004).
Small HDL-P levels (Figure 1D) were higher in HIV-monoinfected participants compared with control (23.3 vs. 14.4μmol/L, p<.0001), while levels in HIV/HCV-coinfected were intermediate (18.2, p<.0001 vs. control). The test for homogeneity of variance showed a greater spread in the HIV/HCV-coinfected group (p=0.017).
The association of lifestyle factors and waist-to-hip ratio (WHR) with lipoprotein measures in HIV infection and HIV/HCV coinfection
The demographic-adjusted median levels of NMR lipoprotein measurements from controls, HIV-monoinfected and HIV/HCV-coinfected participants are summarized in Supplemental Table 1. After multivariable adjustment for demographics, lifestyle factors, and body composition, HIV monoinfection remained associated with lower large LDL-P and with higher small LDL-P and small HDL-P relative to controls, although the difference was somewhat attenuated (Table 2). Additionally, HIV monoinfection remained associated with lower large LDL-P (−102, 95% CI: −132, −71.6, p<0.0001) and higher small LDL-P (281, 95% CI: 236, 325, p<0.0001) after further adjustment for triglycerides and HDL-C.
Table 2.
Association of HIV monoinfection and HCV/HIV coinfection with key NMR lipoprotein measures¶
| Outcome | Model | HIV+ vs. Control Estimate (95%CI) |
HCV+/HIV+ vs. Control Estimate (95%CI) |
|---|---|---|---|
| Large LDL, nmol/L | Demographic-adjusted a | −230.0 (−264.3, −195.6) *** | −96.6 (−155.9, −37.3) * |
| Final model b | −195.9 (−230.8, −161.1) *** | −86.4 (−146.1, −26.7) * | |
|
| |||
| Small LDL , nmol/L | Demographic-adjusted a | 443.7 (380.4, 507.0) *** | 32.6 (−76.6, 141.7) |
| Final model b | 381.2 (331.1, 431.3) *** | −16.6 (−102.4, 69.1) | |
|
| |||
| Large HDL, μmol/L | Demographic-adjusted a | −0.33 (−0.71, 0.050) | 1.44 (0.78, 2.1) *** |
| Final model b | 0.33 (−0.034, 0.70) | 1.70 (1.07, 2.3) *** | |
|
| |||
| Small HDL, μmol/L | Demographic-adjusted a | 8.7 (8.0, 9.5) *** | 3.5 (2.1, 4.8) *** |
| Final model b | 8.2 (7.4, 9.0) *** | 3.3 (1.99, 4.7) *** | |
|
| |||
| LDL Cholesterol, mg/dL ‡ | Demographic-adjusted a | −4.9 (−9.0, −0.85) + | −27.2 (−34.3, −20.1) *** |
| Final model b | −5.5 (−9.6, −1.30) + | −26.9 (−34.0, −19.7) *** | |
Demographic adjusted model includes HIV/HCV status and demographics(age, sex, and race).
Final model includes demographics, lifestyle factors (smoking, alcohol, and diabetes), and body composition (WHR).
denotes p<0.05 for association of HIV status with lipoprotein measures
denotes p<0.01 for association of HIV status with lipoprotein measures
denotes p<0.001 for association of HIV status with lipoprotein measures
denotes p<0.0001 for association of HIV status with lipoprotein measures
Standard lab -- not measured by Liposciences
Estimates from median regression analysis
When adjusted for demographics in HIV/HCV-coinfected participants, there was a weak trend to an increase in small LDL-P when compared to controls; however, this trend disappeared after further adjustment for lifestyle factors and WHR. The differences between HIV/HCV coinfection and control remained significant for large LDL-P and large and small HDL-P even after adjustment in the final model.
Association of HIV-related factors with lipoprotein measures
Because HIV infection was associated with abnormal levels of lipoprotein measures even after multivariable adjustment, we constructed models to investigate the associated factors in HIV-infected participants alone. The association of HIV infection with all NMR lipoprotein measures is summarized in Supplemental Table 2. Female gender, increasing age, and African-American race were independently associated with higher median levels of large LDL-P (Table 3). By contrast, diabetes and WHR were associated with lower large LDL-P. Within all HIV-infected participants, HCV was also associated with higher large LDL-P in unadjusted analyses, but decreased in significance after full multivariable adjustment (77.1, 95%CI: −26.4, 180.5, p=0.14).
Table 3.
Multivariable regression analysis of factors associated with LDL lipoprotein particles in all HIV-infected participants
| Large LDL | Small LDL | |
|---|---|---|
|
| ||
| Parameter | Adjusted Estimate (95%CI)a |
Adjusted Estimate (95%CI)a |
| Female | 148.8 (75.9, 221.6), p<.0001 | −245.1 (−385.2, −105.0), p=0.0016 |
| Age (per decade) | 57.7 (20.4, 94.9), p=0.0028 | −30.9 (−89.9, 28.2), p=0.25 |
| African American | 62.4 (4.6, 120.2), p=0.036 | 5.3 (−116.0, 126.5), p=0.93 |
| Hispanic | 6.6 (−100.3, 113.5), p=0.91 | 40.7 (−287.5, 368.9), p=0.79 |
| Diabetes | −181.6 (−273.3, −89.9), p=0.0002 | 210.2 (−6.7, 427.1), p=0.039 |
| WHR (per 0.1 unit increase) | −71.4 (−107.8, −35.0), p<.0001 | 155.4 (93.8, 217.0), p<.0001 |
| HCV | −329.0 (−439.6, −218.5), p<.0001 | |
| Current NRTI Use | 183.0 (35.4, 330.6), p=0.0078 | |
Estimates from median regression analysis
Factors independently associated with higher levels of small LDL-P included diabetes, WHR, and current NRTI use (Table 3). In contrast, female gender and HCV infection were independently associated with lower levels of small LDL-P. When we examined the association of specific NRTIs with small LDL-P, we found that nearly all were positively associated, including tenofovir, although not as strong as for the class itself. Current protease inhibitor(PI) and lopinavir/ritonavir use were initially associated with higher levels of small LDL-P in unadjusted analyses (127.4, 95% CI: 13.0, 241.8, p=0.029; 184.0, 95% CI: 12.6, 355.5, p=0.036), but decreased in significance after adjustment (24.6, 95% CI: −86.1, 135.3, p=0.66; 9.3, 95% CI: −140.5, 159.2, p=0.90).
Factors associated with higher levels of large HDL (Table 4) included female gender, age, African-American race, alcohol use, HCV infection and the use of the NNRTIs nevirapine and efavirenz. Factors independently associated with lower levels of small HDL (Table 4) included greater HIVRNA, HCV, and lopinavir/ritonavir use. Abacavir use was associated with higher levels of small HDL.
Table 4.
Multivariable regression analysis of factors associated with HDL lipoprotein particles in all HIV-infected participants
| Large HDL | Small HDL | |
|---|---|---|
|
| ||
| Parameter | Adjusted Estimate (95%CI)a |
Adjusted Estimate (95%CI)a |
| Female | 2.8 (1.87, 3.8), p<.0001 | −1.21 (−2.6, 0.16), p=0.084 |
| Age (per decade) | 0.77 (0.33, 1.21), p=0.0006 | 0.63 (−0.039, 1.29), p=0.065 |
| African American | 1.46 (0.70, 2.2), p=0.0002 | 0.70 (−0.37, 1.77), p=0.20 |
| Hispanic | −0.26 (−1.34, 0.83), p=0.64 | 1.75 (−1.20, 4.7), p=0.24 |
| Diabetes | −1.99 (−3.0, −0.99), p=0.0001 | |
| Alcohol (drinks per week) | 0.074 (0.027, 0.12), p=0.0020 | |
| WHR (per 0.1 unit increase) | −1.07 (−1.55, −0.58), p<.0001 | |
| HIVRNA (per 10-fold increase) | −2.3 (−3.0, −1.65), p<.0001 | |
| Duration of HIV infection (y) | −0.077 (−0.14, −0.0093), p=0.026 | |
| HCV | 1.58 (0.78, 2.4), p=0.0001 | −4.6 (−5.9, −3.2), p<.0001 |
| Current Nevirapine Use | 2.2 (0.97, 3.5), p=0.0005 | |
| Current Efavirenz Use | 0.77 (0.072, 1.47), p=0.031 | |
| Current Lopinavir/Ritonavir Use | −2.4 (−4.0, −0.85), p=0.0027 | |
| Current Abacavir Use | 3.1 (1.85, 4.4), p<.0001 | |
Estimates from median regression analysis
DISCUSSION
Our study is the first, to our knowledge, to directly examine lipoprotein subclass concentrations and distributions in HIV/HCV-coinfected participants. Persons with HIV/HCV coinfection are thought to be at higher risk of CVD compared to those with HIV monoinfection or those with neither infection [5, 6]. However, our findings suggest that HIV/HCV coinfection attenuates the atherogenic changes in lipoprotein subclass concentrations observed in HIV monoinfection. We found that HIV monoinfection was associated with higher levels of small LDL-P compared with controls, but HIV/HCV coinfection blunted this increase in small LDL-P such that the levels were similar to those found in controls after multivariable adjustment. HIV-monoinfected individuals had the highest levels of small HDL-P while levels in HIV/HCV-coinfected individuals were intermediate to HIV-monoinfected individuals and controls. Compared to controls, HIV-monoinfected had lower concentrations of large LDL-P while HIV/HCV-coinfected individuals had levels that were also lower than controls but intermediate when compared to HIV monoinfection. In contrast, there was little change in large HDL-P in HIV monoinfection, while HIV/HCV coinfection was associated with substantially higher levels of large HDL-P relative to HIV monoinfection and controls, even after adjusting for demographic and other risk factors. These results add to the growing evidence that HIV/HCV coinfection has a direct effect on lipid profiles and lipoprotein concentrations [30-33] and suggest that HCV status should be considered when evaluating CVD risk factors such as lipoprotein subclasses in HIV-infected persons.
The observed association of HIV monoinfection with higher levels of small LDL-P and TG, with decreased large LDL-P compared to controls are consistent with an ALP [34] described in HIV-monoinfected men on ART. Additionally, HIV monoinfection remained associated with higher levels of small LDL-P and decreased large LDL-P even after adjustment for TG and HDL-C. Thus, alteration in small LDL-P and large LDL-P levels were independent of hypertriglyceridemia and low HDL-C seen in HIV-monoinfection. Results from the Swiss HIV Cohort Study suggest that in HIV-infected patients treated with antiretroviral therapy, small LDL particles were associated with coronary events [1]. The consequences of the attenuation by HIV/HCV coinfection are unknown.
Our findings showed that small HDL-P levels were higher in HIV-monoinfected participants compared with control with intermediate levels seen in HIV/HCV coinfection. A previous study found that HIV infection was associated with lower small HDL-P in women even after adjustment for standard lipids and ART use [24] and lower small HDL-P concentrations were associated with a higher risk of CVD in HIV-monoinfected participants [2]. Small HDL-P may play a role in mitigating the risk of CVD given their preference over larger HDL-P as initial acceptors of cholesterol from peripheral cells in reverse cholesterol transport and their anti-inflammatory properties [34, 35]. However, in studies of the general population, the clinical consequences of low levels of small HDL-P on CVD risk have been inconsistent [17, 36, 37].
Several traditional risk factors for CVD can be impacted by HIV infection and its treatment. However, there is limited data regarding the extent to which ART induced abnormalities in the lipoprotein subclass concentrations. We examined the association of ART class with lipoprotein concentration in all HIV-infected participants. We found that NRTI use was associated with higher small LDL-P. The association of NRTI use with higher levels of small LDL-P appeared to be specific to this class as there was little association when other classes or ART use in general were examined in the model.
While PI use was initially associated with higher small LDL-P, after adjustment, the association was attenuated and no longer reached statistical significance. These findings are similar to results from Tien el al. that showed that ART use, mainly PI-based, was associated with greater small LDL-P but after adjustment for standard lipids, ART use was no longer associated [24].
Among the individual antiretroviral drugs, we found that the NNRTIs nevirapine and efavirenz were associated with higher large HDL-P perhaps reflecting the increase in HDL-C that has been previously described [38]. Lopinavir/ritonavir was associated with lower small HDL-P similar to the trend of decreased HDL-C observed in some studies of ritonavir-boosted PIs [38]. Interestingly, abacavir use was associated with higher small HDL-P, which might suggest lower risk. The effect of specific ART and alterations in lipoprotein particles on CVD needs further study.
These findings have potential clinical implications for the management of both HIV/HCV-coinfected and HIV-monoinfected participants. First, HIV/HCV-coinfected participants appear to have attenuated abnormalities in lipoprotein subclasses levels that have a reported association with CVD in HIV-monoinfected and healthy patients—in particular, HIV/HCV-coinfected have lower levels of small dense LDL-P versus HIV-monoinfected. This seems to imply that HIV/HCV-coinfected participants have less atherogenic lipid profiles and would have less CVD risk. However, previous studies have found that HIV/HCV coinfection is associated with an increased risk of CVD events [5, 6]. Thus, these changes in lipoprotein particles do not appear to protect against CVD and suggest that they should not be used clinically. These results imply that factors other than lipid and lipoprotein perturbations likely account for the increased reports of CVD in the HIV/HCV-coinfected population.
Also, because lipid profiles and lipoprotein subclass particle concentrations appear to be less atherogenic in HIV/HCV-coinfected compared with HIV-monoinfected persons, it may be that clinicians underutilize lipid-lowering medications in such patients. Indeed, in our study, HIV/HCV-coinfected participants were only one-third as likely as HIV-monoinfected participants to be treated with statins. Prospective studies on CVD outcomes are needed to guide treatment targets in the HIV/HCV-coinfected population.
One possible explanation for the observed difference in lipid profiles between the HIV-monoinfected and HIV/HCV-coinfected groups is the direct effect of HCV on modulation of lipoprotein production in hepatocytes [39]. HCV infection increases intra-hepatocyte lipid deposition and utilizes lipids to promote HCV viral replication and secretion of lipoviroparticles. HCV-apoE containing lipoprotein particles span the range of VLDL to HDL. Clearance of VLDL and LDL containing HCV and apoE has been shown to be jointly mediated by both the LDL receptor and another receptor, such as the scavenger receptor SR-B1 [39-42], with these receptors playing a role in HCV infectivity by serving as a potential entry factor [40] as well as a modulator of replication of the HCV genome [43]. The role of HCV containing lipoprotein particles in atherogenesis needs further study.
The strengths of our study are direct assessment of HCV infection and the inclusion of a large control group with extensive data on CVD risk factors that enabled us to adjust for relevant traditional CVD risk factors, including age, which was our major aim. Limitations of our study include its cross-sectional design, which complicates the evaluation of the potential causality of the reported associations. We did not use matched controls, but used a large population-based cohort, adjusting for multiple determinants of lipoprotein levels; however, we cannot exclude the possibility of residual confounding. Injection drug use status was not available in MESA, so we are unable to determine whether this variable may influence the association of HCV status with lipoprotein levels. We do not have data on adequate numbers of HCV-monoinfected subjects, which should be the focus of future studies. Finally, our study lacks cardiovascular events to correlate with alterations in lipoproteins.
In summary, after adjusting for traditional CVD risk factors, HIV infection is associated with abnormal lipoprotein levels. This risk appears to be attenuated partially by the presence of HIV/HCV coinfection. Although HIV/HCV coinfection is associated with a less atherogenic lipid profile than HIV monoinfection, the long-term effects of HIV/HCV coinfection on vascular disease are unclear with limited studies suggesting an increased risk of CVD. Additional studies are needed to determine whether HIV/HCV coinfection is associated with increased CVD events beyond that of HIV infection. In the interim, lack of elevated small LDL in a patient with HIV/HCV-coinfection should be interpreted cautiously with regard to lowering risk. Also, given that lipoprotein alterations secondary to coinfection may not be protective, other novel markers of CVD risk in HIV/HCV-coinfected persons should be investigated. Our findings emphasize the importance of determining HCV status when assessing CVD risk factors in HIV-infected patients.
Supplementary Material
Acknowledgments
Supported by NIH grants RO1-DK57508, HL74814, and HL 53359, and NIH GCRC grants M01-RR00036, RR00051, RR00052, RR00054, RR00083, RR0636, RR00865, UL1 RR024131, P30 DK 026687-269012, and NHLBI contracts N01-HC-95095, N01-HC-48047, and N01-HC-48050, the Albert L. and Janet A. Schultz Supporting Foundation; and with resources and the use of facilities of the Veterans Affairs Medical Centers of, Atlanta, District of Columbia, New York and San Francisco. The funding agency had no role in the collection or analysis of the data. Also supported by R01-HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Clinicaltrials.gov ID: NCT00331448
Role of the Funder:
The funder played no role in the conduct of the study, collection of the data, management of the study, analysis of data, interpretation of the data or preparation of the manuscript. A representative of the funding agent participated in planning the protocol.
Appendix
Sites and Investigators
University Hospitals of Cleveland (Barbara Gripshover, MD); Tufts University (Abby Shevitz, MD (deceased) and Christine Wanke, MD); Stanford University (Andrew Zolopa, MD); University of Alabama at Birmingham (Michael Saag, MD); Johns Hopkins University (Joseph Cofrancesco Jr., MD and Adrian Dobs, MD); University of Colorado Health Sciences Center (Lisa Kosmiski, MD and Constance Benson, MD); University of North Carolina at Chapel Hill (David Wohl, MD and Charles van der Horst, MD*); University of California at San Diego (Daniel Lee, MD and W. Christopher Mathews, MD*); Washington University (E. Turner Overton, MD and William Powderly, MD); VA Medical Center, Atlanta (David Rimland, MD); University of California at Los Angeles (Judith Currier, MD); VA Medical Center, New York (Michael Simberkoff, MD); VA Medical Center, Washington DC (Cynthia Gibert, MD); St Luke’s-Roosevelt Hospital Center (Donald Kotler, MD and Ellen Engelson, PhD); Kaiser Permanente, Oakland (Stephen Sidney, MD); University of Alabama at Birmingham (Cora E. Lewis, MD).
FRAM 2 Data Coordinating Center
University of Washington, Seattle (Richard A. Kronmal, PhD, Mary Louise Biggs, PhD, J. A. Christopher Delaney, Ph.D. and John Pearce).
Image Reading Centers
St Luke’s-Roosevelt Hospital Center: (Steven Heymsfield, MD, Jack Wang, MS and Mark Punyanitya). Tufts Medical Center, Boston: (Daniel H. O’Leary, MD, Joseph F. Polak MD MPH, Anita P. Harrington).
Office of the Principal Investigator
University of California, San Francisco, Veterans Affairs Medical Center and the Northern California Institute for Research and Development: (Carl Grunfeld, MD, PhD, Phyllis Tien, MD, Peter Bacchetti, PhD, Michael Shlipak, MD, Rebecca Scherzer, PhD, Mae Pang, RN, MSN, Heather Southwell, MS, RD)
Footnotes
Conflicts of Interest:
None. All authors received funding from some of the supporting grants.
References
- 1.Bucher HC, Richter W, Glass TR, Magenta L, Wang Q, Cavassini M, et al. Small dense lipoproteins, apolipoprotein B, and risk of coronary events in HIV-infected patients on antiretroviral therapy: the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2012;60:135–142. doi: 10.1097/QAI.0b013e31824476e1. [DOI] [PubMed] [Google Scholar]
- 2.Duprez DA, Kuller LH, Tracy R, Otvos J, Cooper DA, Hoy J, et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 2009;207:524–529. doi: 10.1016/j.atherosclerosis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225–232. doi: 10.1086/599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roed T, Lebech AM, Kjaer A, Weis N. Hepatitis C virus infection and risk of coronary artery disease: a systematic review of the literature. Clin Physiol Funct Imaging. 2012;32:421–430. doi: 10.1111/j.1475-097X.2012.01152.x. [DOI] [PubMed] [Google Scholar]
- 5.Freiberg MS, Chang CC, Skanderson M, McGinnis K, Kuller LH, Kraemer KL, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes. 2011;4:425–432. doi: 10.1161/CIRCOUTCOMES.110.957415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med. 2010;11:462–468. doi: 10.1111/j.1468-1293.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 7.Tien PC, Schneider MF, Cole SR, Cohen MH, Glesby MJ, Lazar J, et al. Association of hepatitis C virus and HIV infection with subclinical atherosclerosis in the women’s interagency HIV study. AIDS. 2009;23:1781–1784. doi: 10.1097/QAD.0b013e32832d7aa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feingold KR, Krauss RM, Pang M, Doerrler W, Jensen P, Grunfeld C. The hypertriglyceridemia of acquired immunodeficiency syndrome is associated with an increased prevalence of low density lipoprotein subclass pattern B. J Clin Endocrinol Metab. 1993;76:1423–1427. doi: 10.1210/jcem.76.6.8501146. [DOI] [PubMed] [Google Scholar]
- 9.Sosner P, Wangermez M, Chagneau-Derrode C, Le Moal G, Silvain C. Atherosclerosis risk in HIV-infected patients: the influence of hepatitis C virus co-infection. Atherosclerosis. 2012;222:274–277. doi: 10.1016/j.atherosclerosis.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Feingold KR, Grunfeld C, Pang M, Doerrler W, Krauss RM. LDL subclass phenotypes and triglyceride metabolism in non-insulin-dependent diabetes. Arterioscler Thromb. 1992;12:1496–1502. doi: 10.1161/01.atv.12.12.1496. [DOI] [PubMed] [Google Scholar]
- 11.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 12.Cromwell WC, Otvos JD. Low-density lipoprotein particle number and risk for cardiovascular disease. Curr Atheroscler Rep. 2004;6:381–387. doi: 10.1007/s11883-004-0050-5. [DOI] [PubMed] [Google Scholar]
- 13.Hsia J, Otvos JD, Rossouw JE, Wu L, Wassertheil-Smoller S, Hendrix SL, et al. Lipoprotein particle concentrations may explain the absence of coronary protection in the women’s health initiative hormone trials. Arterioscler Thromb Vasc Biol. 2008;28:1666–1671. doi: 10.1161/ATVBAHA.108.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982;23:97–104. [PubMed] [Google Scholar]
- 15.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 16.Blake GJ. Low-Density Lipoprotein Particle Concentration and Size as Determined by Nuclear Magnetic Resonance Spectroscopy as Predictors of Cardiovascular Disease in Women. Circulation. 2002;106:1930–1937. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 17.Kuller LH, Grandits G, Cohen JD, Neaton JD, Prineas R. Multiple Risk Factor Intervention Trial Research G. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis. 2007;195:122–128. doi: 10.1016/j.atherosclerosis.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, et al. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547–553. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 19.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, Jr., et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;192:211–217. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol. 2002;90:89–94. doi: 10.1016/s0002-9149(02)02427-x. [DOI] [PubMed] [Google Scholar]
- 21.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab. 2002;48:171–180. [PubMed] [Google Scholar]
- 22.Mackey RH, Greenland P, Goff DC, Jr., Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2012;60:508–516. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker J, Ayenew W, Quick H, Hullsiek KH, Tracy R, Henry K, et al. High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis. 2010;201:285–292. doi: 10.1086/649560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tien PC, Schneider MF, Cox C, Cohen M, Karim R, Lazar J, et al. HIV, HAART, and lipoprotein particle concentrations in the Women’s Interagency HIV Study. AIDS. 2010;24:2809–2817. doi: 10.1097/QAD.0b013e32833fcb3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tien PC, Benson C, Zolopa AR, Sidney S, Osmond D, Grunfeld C. The study of fat redistribution and metabolic change in HIV infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860–869. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23:1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hastie T, Tibshirani R. Generalized Additive Models (Chapman & Hall/CRC Monographs on Statistics & Applied Probability) Chapman and Hall; New York: 1990. [Google Scholar]
- 28.Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, et al. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111:3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 29.Koenker R, Hallock K. Quantile regression. Journal of Economic Perspectives. 2001:143–156. [Google Scholar]
- 30.Floris-Moore M, Howard AA, Lo Y, Schoenbaum EE, Arnsten JH, Klein RS. Hepatitis C infection is associated with lower lipids and high-sensitivity C-reactive protein in HIV-infected men. AIDS Patient Care STDS. 2007;21:479–491. doi: 10.1089/apc.2006.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patroni A, Torti C, Tomasoni L, Roldan EQ, Bertelli D, Puoti M, et al. Effect of highly active antiretroviral therapy (HAART) and hepatitis C Co-infection on hyperlipidemia in HIV-infected patients: a retrospective longitudinal study. HIV Clin Trials. 2002;3:451–461. doi: 10.1310/w024-qc4t-nxu0-tkyt. [DOI] [PubMed] [Google Scholar]
- 32.Duong M, Petit JM, Piroth L, Grappin M, Buisson M, Chavanet P, et al. Association between insulin resistance and hepatitis C virus chronic infection in HIV-hepatitis C virus-coinfected patients undergoing antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:245–250. doi: 10.1097/00126334-200107010-00005. [DOI] [PubMed] [Google Scholar]
- 33.Reingold J, Wanke C, Kotler D, Lewis C, Tracy R, Heymsfield S, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48:142–148. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barter P, Kastelein J, Nunn A, Hobbs R. Future Forum Editorial B. High density lipoproteins (HDLs) and atherosclerosis; the unanswered questions. Atherosclerosis. 2003;168:195–211. doi: 10.1016/s0021-9150(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 35.Kontush A, Chapman MJ. Antiatherogenic small, dense HDL--guardian angel of the arterial wall? Nat Clin Pract Cardiovasc Med. 2006;3:144–153. doi: 10.1038/ncpcardio0500. [DOI] [PubMed] [Google Scholar]
- 36.Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of Lipoprotein Subclasses as Measured by Proton Nuclear Magnetic Resonance Spectroscopy to Coronary Artery Disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18:1046–1053. doi: 10.1161/01.atv.18.7.1046. [DOI] [PubMed] [Google Scholar]
- 37.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 38.Grunfeld C. Dyslipidemia and its Treatment in HIV Infection. Top HIV Med. 2010;18:112–118. [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu Y, Hishiki T, Ujino S, Sugiyama K, Funami K, Shimotohno K. Lipoprotein component associated with hepatitis C virus is essential for virus infectivity. Curr Opin Virol. 2011;1:19–26. doi: 10.1016/j.coviro.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Molina S, Castet V, Fournier-Wirth C, Pichard-Garcia L, Avner R, Harats D, et al. The low-density lipoprotein receptor plays a role in the infection of primary human hepatocytes by hepatitis C virus. J Hepatol. 2007;46:411–419. doi: 10.1016/j.jhep.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 41.Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1999;96:12766–12771. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartosch B, Vitelli A, Granier C, Goujon C, Dubuisson J, Pascale S, et al. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem. 2003;278:41624–41630. doi: 10.1074/jbc.M305289200. [DOI] [PubMed] [Google Scholar]
- 43.Albecka A, Belouzard S, Op de Beeck A, Descamps V, Goueslain L, Bertrand-Michel J, et al. Role of low-density lipoprotein receptor in the hepatitis C virus life cycle. Hepatology. 2012;55:998–1007. doi: 10.1002/hep.25501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.