Abstract
Objectives
To study the body mass index (BMI) trajectory in patients with incident end-stage kidney disease and its association with all-cause mortality.
Methods
This longitudinal cohort study included 17022 adult patients commencing hemodialysis [HD] (n = 10860) or peritoneal dialysis [PD] (n = 6162) between 2001 and 2008 and had ≥6-month follow-up and ≥2 weight measurements, using the Australia and New Zealand Dialysis and Transplant Registry data. The association of time-varying BMI with all-cause mortality was explored using multivariate Cox regression models.
Results
The median follow-up was 2.3 years. There was a non-linear change in the mean BMI (kg/m2) over time, with an initial decrease from 27.6 (95% confidence interval [CI]: 27.5, 27.7) to 26.7 (95% CI: 26.6, 26.9) at 3-month, followed by increments to 27.1 (95% CI: 27, 27.2) at 1-year and 27.2 (95% CI: 26.8, 27.1) at 3-year, and a gradual decrease subsequently. The BMI trajectory was significantly lower in HD patients who died than those who survived, although this pattern was not observed in PD patients. Compared to the reference time-varying BMI category of 25.1–28 kg/m2, the mortality risks of both HD and PD patients were greater in all categories of time-varying BMI <25 kg/m2. The mortality risks were significantly lower in all categories of time-varying BMI >28.1 kg/m2 among HD patients, but only in the category 28.1–31 kg/m2 among PD patients.
Conclusions
BMI changed over time in a non-linear fashion in incident dialysis patients. Time-varying measures of BMI were significantly associated with mortality risk in both HD and PD patients.
Introduction
Nearly two-thirds of the adult population in developed countries is either overweight or obese, defined as body-mass index (BMI) ≥25 kg/m2 [1], [2]. High BMI is a risk factor for end-stage kidney disease (ESKD) [3]. Patients with ESKD receiving dialysis are no exception to the obesity epidemic [4]. In the USA, the mean BMI of incident ESKD patients has increased from 25.7 kg/m2 in 1995 to 27.5 kg/m2 in 2002 [4], and to 28.9 kg/m2 in 2007–2009 [5]. In the general population, obesity is associated with increased risks of all-cause and cardiovascular mortality [6], [7]. Therefore, weight loss therapy is recommended for people with BMI ≥30 kg/m2 or BMI between 25 and 29.9 kg/m2 with ≥2 absolute cardiovascular risk factors [8], [9]. However, there are currently no such treatment guidelines for ESKD patients receiving chronic dialysis for two main reasons. First, malnutrition is extremely common in dialysis patients and is a major risk factor of mortality [10]. Second, high BMI is associated with a survival advantage in dialysis patients [11]. The paradox of better survival with higher BMI has been well described in ESKD patients receiving hemodialysis (HD) [11]–[15]. The association between obesity and survival is less clear in ESKD patients receiving peritoneal dialysis (PD), with studies variably showing better [16], [17], comparable [11], [18], [19], or worse [14], [20] survival. Due to this conflicting evidence, except for severely obese patients who are waiting for kidney transplant surgery, it is largely unclear whether to recommend weight loss therapy to dialysis patients who are either overweight or obese. The studies evaluating BMI and mortality are based on a BMI value at baseline or at the commencement of dialysis therapy. Studies using follow-up measures of BMI (time-varying) are sparse in both HD and PD patients [12], [21]–[27]. Therefore, we examined the BMI trajectory and its association with all-cause mortality in incident ESKD patients on both HD and PD using data from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry.
Methods
This longitudinal cohort study was undertaken in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) Statement [28]. The study included 20524 patients from the ANZDATA Registry who commenced chronic dialysis therapy between January 1, 2001 and December 31, 2008, comprising all incident patients with ESKD in Australia and New Zealand during that time. Patients were excluded if they were younger than 18 years at the time of renal replacement therapy (RRT) commencement or did not complete at least 6 months on dialysis since commencement of RRT (due to death or recovery of kidney function or transplantation or follow-up<6 months) or underwent pre-emptive kidney transplantation. Patients with <2 available follow-up measurements of weight or typographical error (for example, 9 kg instead of 90 kg) were also excluded. Final analysis included 17022 patients (Figure S1 in S1 Information).
The ANZDATA Registry collects information on all patients receiving RRT from all renal units throughout Australia and New Zealand, and has been extensively used for clinical epidemiological studies [29], [30]. The data collection is conducted in accordance with the Australian Commonwealth Privacy Act and associated state legislation governing health data collection; and individual patient consent is not required for the registry data. The anonymity of patient information is maintained by the coding of data during compilation; only anonymized data is released by the registry to the researchers. The ANZDATA Registry has approved this study and the submission of this manuscript. The data were collected every 6 months until March 31, 2004 (survey dates 31st March and 30th September) and annually since December 31, 2004 (survey date 31st December). The structure of the ANZDATA Registry, the methods of data collection and validation are described in detail on its website (http://www.anzdata.org.au). In summary, the collection is complete from the first RRT procedure in Australia and New Zealand in 1963 and includes all patients from all renal units in both countries. The data collected included demographic details, underlying cause of ESKD, a limited range of comorbidities (the presence of coronary artery disease, peripheral vascular disease, cerebrovascular disease, chronic lung disease, diabetes mellitus, hypertension, and cigarette smoking), late nephrologist referral (<3 months before dialysis start), serum creatinine at dialysis start, the type and dates of each dialysis episode, details about kidney transplantation, and measurements of height and dry weight.
RRT modality was classified into HD (including hospital-, satellite-, and home-based HD), PD (including continuous ambulatory PD and automated PD), and renal transplantation. Dialysis modality was assigned as modality used at 90 days after commencement of RRT. BMI was calculated from the quotient of the weight and the square of the height at the commencement of RRT. The values of BMI were divided into 9 categories (≤19, 19.1–22, 22.1–25, 25.1–28, 28.1–31, 31.1–34, 34.1–37, 37.1–40 and >40.1 kg/m2).
Statistical analysis
The basic statistics on study variables were expressed as number (%), mean (SD) or median (IQR), as appropriate. The distributions of categorical and continuous study variables by two dialysis modalities were compared using χ2 test and non-parametric Mann-Whitney U test, respectively.
The BMI trajectory was constructed by plotting the mean (95% CI) of baseline and follow-up measures of BMI against time points. For the BMI trajectory analysis, data at the following time points during follow-up were utilized: baseline; 3-, 6-, 9-months; 1-, 1.5-, 2-, 2.5-, 3-, 4-, 5-, 6-, 7- and 8-years. These time periods were rounded to the nearest survey date of the ANZDATA Registry data collection. Complete case analysis with follow-up measures of BMI was conducted, and no missing data imputation was employed. Generalized Estimating Equation based multivariate regression models were used to compare the BMI trajectories. Multivariate Cox regression models were used to evaluate risk factors of all-cause mortality. To evaluate the association between follow-up BMI measures and all-cause mortality, BMI was used as a time-varying covariate. Other covariates used in the multivariate regression models were: gender, age, dialysis modality, ethnicity, cause of ESKD, smoking status, diabetes mellitus, chronic lung disease, coronary artery disease, cerebrovascular disease, peripheral vascular disease, and late referral. Due to estimation convergence problems, the time-varying dialysis modality could not be used, and a fixed dialysis modality variable (modality used at 90 days after commencement of RRT) was used as a covariate instead. Robust standard errors of the hazard ratios (HR) were estimated after adjusting for the effects of study centres as random effects. The “proportional hazard” assumption was tested using standard likelihood ratio test. Survival time was calculated from the date of commencement of RRT to the date of event or censoring. Survival analyses were censored for kidney transplantation, loss of follow-up, recovery of renal function or December 31, 2008.
Results
Patient characteristics
Baseline patient characteristics are described in Table 1. Compared to those on PD, HD patients were more likely to be males, Caucasians, Aboriginals or Torres Strait Islanders, referred late to nephrologist before commencement of dialysis and to have comorbid conditions, including diabetes mellitus, chronic lung disease and coronary artery disease (Table 1). PD patients had lower average weight and BMI values, and were less likely to have BMI >34 kg/m2 compared to HD patients. Distributions of BMI groups according to the ethnicity and dialysis modality are described in Table S1 in S1 Information.
Table 1. Baseline patient characteristics.
| Characteristic | All | Hemodialysis | Peritoneal dialysis |
| n* | 17,022 | 10,860 (63.8) | 6,162 (36.2) |
| Men* | 10,249 (60.2) | 6,793 (62.6) | 3,456 (56.1) |
| Age (years)# | 60.4 (15) | 60.6 (15.1) | 60.3 (14.7) |
| Age categories* | |||
| 18 to 45 years | 2,820 (16.6) | 1,782 (16.4) | 1,038 (16.9) |
| 45 to 65 years | 6,791 (39.9) | 4,330 (39.9) | 2,461 (39.9) |
| >65 years | 7,411 (43.5) | 4,748 (43.7) | 2,663 (43.2) |
| Ethnicity* | |||
| Caucasians | 12,324 (72.4) | 8,030 (73.9) | 4,294 (69.7) |
| Aboriginal or Torres Strait Islander | 1,397 (8.2) | 1,046 (9.6) | 351 (5.7) |
| Maori or Pacific Islander | 1,840 (10.8) | 1,063 (9.8) | 777 (12.6) |
| Asian | 785 (4.6) | 370 (3.4) | 415 (6.7) |
| Other | 676 (4) | 351 (3.2) | 325 (5.3) |
| Primary cause of end-stage kidney disease* | |||
| Chronic glomerulopathy | 4,155 (24.4) | 2,591 (23.9) | 1,564 (25.4) |
| Diabetic nephropathy | 5,729 (33.7) | 3,627 (33.4) | 2,102 (34.1) |
| Renovascular disease | 2,966 (17.4) | 1,862 (17.2) | 1,104 (17.9) |
| Polycystic disease | 1,033 (6.1) | 688 (6.3) | 345 (5.6) |
| Reflux or obstructive nephropathy | 827 (4.9) | 542 (5) | 285 (4.6) |
| Other | 1,256 (7.4) | 871 (8) | 385 (6.3) |
| Unknown | 1,056 (6.2) | 679 (6.3) | 377 (6.1) |
| Smoking status* † | |||
| Non-smoker | 7,688 (45.2) | 4,807 (44.3) | 2,881 (46.8) |
| Former smoker | 6,965 (40.9) | 4,533 (41.8) | 2,432 (39.5) |
| Current smoker | 2,365 (13.9) | 1,517 (14) | 8,48 (13.8) |
| Weight (kg)# | 77.4 (19.9) | 79.5 (21.4) | 73.6 (16.5) |
| Body mass index (kg/m2)# | 27.6 (6.4) | 28.2 (6.9) | 26.6 (5.3) |
| Body mass index category* | |||
| ≤19 kg/m2 | 750 (4.4) | 480 (4.4) | 270 (4.4) |
| 19–22 kg/m2 | 2,182 (12.8) | 1,295 (11.9) | 887 (14.4) |
| 22–25 kg/m2 | 3,563 (20.9) | 2,161 (19.9) | 1,402 (22.8) |
| 25–28 kg/m2 | 3,615 (21.2) | 2,180 (20.1) | 1,435 (23.3) |
| 28–31 kg/m2 | 2,711 (15.9) | 1,707 (15.7) | 1,004 (16.3) |
| 31–34 kg/m2 | 1,795 (10.6) | 1,179 (10.9) | 616 (10) |
| 34–37 kg/m2 | 1,008 (5.9) | 712 (6.6) | 296 (4.8) |
| 37–40 kg/m2 | 615 (3.6) | 458 4.2) | 157 (2.6) |
| >40 kg/m2 | 783 (4.6) | 688 (6.3) | 95 (1.5) |
| Comorbid conditions* | |||
| Diabetes mellitus | 7,382 (43.4) | 4,773 (44) | 2,609 (42.3) |
| Chronic lung disease | 2,679 (15.7) | 1,775 (16.3) | 904 (14.7) |
| Coronary artery disease | 6,807 (40) | 4,470 (41.2) | 2,337 (37.9) |
| Cerebrovascular disease | 2,492 (14.6) | 1,583 (14.6) | 909 (14.8) |
| Peripheral vascular disease | 4,278 (25.1) | 2,762 (25.4) | 1,516 (24.6) |
| Late referral‡ † | 4,057 (23.8) | 2,757 (25.4) | 1,300 (21.1) |
*n (%).
mean (standard deviation).
Some missing data.
Late referral defined as referral to nephrologist >3 months before dialysis start.
BMI trajectory in incident dialysis patients
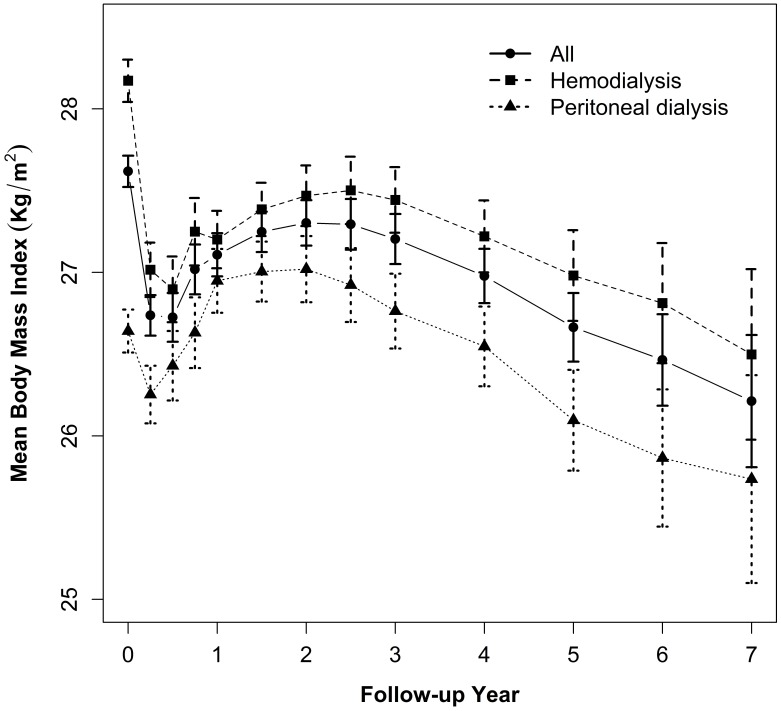
The median follow-up time was 2.3 years (range: 6 months to 8 years) and the median number of weight measurements per patient was 4 with a range from 2 to 13 (See Table S2 in S1 Information). BMI changed over time in a non-linear fashion (Fig. 1). Mean BMI initially decreased significantly from 27.6 (95% confidence interval [CI]: 27.5, 27.7) kg/m2 at baseline to 26.7 (95% CI: 26.6, 26.9) kg/m2 at 3 months, then increased to 27.1 (95% CI: 27, 27.2) kg/m2 at 1 year. It then remained stable over the next 2 years with a mean BMI of 27.2 (95% CI: 26.8, 27.1) kg/m2 at 3 years. Thereafter, mean BMI decreased gradually. The average baseline BMI in the HD group was significantly higher by 1.6 kg/m2 (95% CI: 1.4, 1.8, P<0.001) than the PD group, and the BMI trajectory in the HD group remained at a significantly higher level than the PD group throughout the follow-up period (Fig. 1). Despite a significant difference in the baseline BMI, the patterns of non-linear change in BMI were similar in both groups. In PD patients, the average BMI at 1 year of follow-up was 0.4 kg/m2 (95% CI: 0.2, 0.6) higher than the baseline level. However, in HD patients, follow-up BMI values were consistently below the baseline level.
Figure 1. Body Mass Index Trajectories in All Incident Dialysis Patients and According to Dialysis Modality.
Baseline BMI and all-cause mortality
A total of 5,971 (35%) patients died during follow-up (3,671[34%] in the HD group vs. 2,300[37%] in the PD group) with a death rate of 12.7 (95% CI: 12.4, 13.1) per 100 person-years. The death rates per 100 person-years in the HD and PD groups were 12.2 (95% CI: 11.8, 12.6) and 13.7 (95% CI: 13.1, 14.3), respectively (See Table S3 in S1 Information for causes of death).
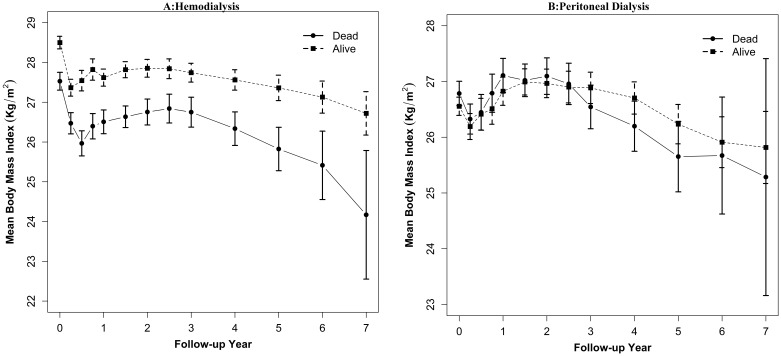
The baseline BMI was higher by 0.6 kg/m2 (95% CI: 0.4, 0.8, P<0.001) in patients who remained alive than those who died. At baseline, the surviving cohort had higher BMI by 1 kg/m2 (P<0.01) in HD patients (Fig. 2, panel A). However, the baseline BMI was not different by mortality status in the PD group (Fig. 2, panel B). Compared to the reference BMI category of 25–28 kg/m2 at baseline, all categories of BMI <25 kg/m2 were associated with increased mortality risk for all dialysis patients (Table 2). However, the risk estimates were not consistent between the HD and PD groups. Higher baseline BMI was associated with significantly lower mortality risk for HD patients with BMI between 28 and 37 kg/m2. The mortality risk was significantly higher in the PD group with BMI 34–37 kg/m2.
Figure 2. Body Mass Index Trajectory and Mortality in Incident Dialysis Patients: A, Hemodialysis; B, Peritoneal dialysis.
Table 2. The association between baseline BMI and all-cause mortality.
| All patients | HD patients | PD patients | |||||||
| BMI (kg/m2) | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P |
| ≤19 | 1.55 | 1.37, 1.77 | <0.001 | 1.62 | 1.37, 1.92 | <0.001 | 1.43 | 1.15, 1.78 | <0.01 |
| 19 to 22 | 1.14 | 1.05, 1.23 | <0.01 | 1.16 | 1.03, 1.30 | 0.02 | 1.08 | 0.96, 1.22 | 0.2 |
| 22 to 25 | 1.12 | 1.04, 1.20 | 0.003 | 1.07 | 0.98, 1.17 | 0.1 | 1.17 | 1.03, 1.32 | 0.02 |
| 25 to 28 | Reference | Reference | Reference | ||||||
| 28 to 31 | 0.96 | 0.89, 1.03 | 0.3 | 0.90 | 0.81, 0.99 | 0.03 | 1.05 | 0.94, 1.18 | 0.4 |
| 31 to 34 | 0.92 | 0.84, 1.01 | 0.08 | 0.82 | 0.74, 0.90 | <0.001 | 1.11 | 0.96, 1.28 | 0.2 |
| 34 to 37 | 0.95 | 0.86, 10.4 | 0.3 | 0.78 | 0.68, 0.89 | <0.001 | 1.33 | 1.12, 1.57 | <0.01 |
| 37 to 40 | 0.97 | 0.82, 1.14 | 0.7 | 0.90 | 0.73, 1.11 | 0.3 | 1.05 | 0.76, 1.46 | 0.8 |
| >40 | 0.96 | 0.83, 1.11 | 0.6 | 0.89 | 0.77, 1.03 | 0.1 | 1.11 | 0.77, 1.61 | 0.6 |
Time-varying BMI and all-cause mortality
The BMI trajectory was significantly higher on average by 1.22 kg/m2 (95% CI: 1.2, 1.3) in those who remained alive compared to those who died. The BMI trajectories in HD and PD patients who survived were higher on average by 1.5 kg/m2 (95% CI: 1.4, 1.6) and 0.5 kg/m2 (95% CI: 0, 0.6), respectively, than those who died. The adjusted risks of mortality associated with different categories of time-varying BMI for all patients and separately for dialysis modality are presented in Table 3. Compared to the reference time-varying BMI category of 25–28 kg/m2, there was significantly increased mortality risk in all categories with time-varying BMI<25 kg/m2. There was also a decreased risk of mortality for all categories of time-varying BMI >28 kg/m2, except for 37–40 kg/m2. When analyzed separately for dialysis modalities, increased mortality risks with all categories of time-varying BMI <25 kg/m2 were evident in both HD and PD groups. Decreased mortality risk was evident with all categories of time-varying BMI >28 kg/m2 in the HD group. However, decreased mortality risk in the PD group was observed only up to the time-varying BMI category of 28–31 kg/m2. In the PD group, there were no significant differences in the risk estimates for higher time-varying BMI categories (up to 37 kg/m2); although a trend of increasing mortality risk was observed for the time-varying BMI category 37–40 kg/m2. The risk estimates for other risk factors from the multivariate Cox regression models are presented in Table S4 in S1 Information.
Table 3. The association between time-varying BMI and all-cause mortality.
| All patients | HD patients | PD patients | |||||||
| BMI (kg/m2) | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P |
| ≤19 | 3.30 | 2.91, 3.74 | <0.001 | 3.72 | 3.27, 4.24 | <0.001 | 2.56 | 2.10, 3.12 | <0.001 |
| 19 to 22 | 1.70 | 1.53, 1.89 | <0.001 | 1.74 | 1.54, 1.98 | <0.001 | 1.63 | 1.41, 1.89 | <0.001 |
| 22 to 25 | 1.23 | 1.13, 1.34 | <0.001 | 1.25 | 1.11, 1.40 | <0.001 | 1.22 | 1.06, 1.40 | <0.01 |
| 25 to 28 | Reference | Reference | Reference | ||||||
| 28 to 31 | 0.80 | 0.73, 0.88 | <0.001 | 0.80 | 0.71, 0.91 | 0.001 | 0.80 | 0.69, 0.94 | <0.01 |
| 31 to 34 | 0.82 | 0.74, 0.90 | <0.001 | 0.72 | 0.65, 0.81 | <0.001 | 0.96 | 0.80, 1.14 | 0.6 |
| 34 to 37 | 0.82 | 0.71, 0.94 | <0.01 | 0.69 | 0.59, 0.81 | <0.001 | 1.06 | 0.83, 1.34 | 0.7 |
| 37 to 40 | 0.94 | 0.81, 1.09 | 0.4 | 0.76 | 0.61, 0.95 | 0.02 | 1.30 | 1.05, 1.62 | 0.02 |
| >40 | 0.76 | 0.64, 0.90 | <0.01 | 0.74 | 0.63, 0.87 | <0.001 | 0.72 | 0.51, 1.01 | 0.06 |
Discussion
This large registry study showed that BMI changed over time in a non-linear fashion in incident dialysis patients. Although the patterns of non-linear change in BMI were similar in HD and PD groups, the average BMI in PD patients at 1 year of follow-up was slightly higher than the baseline level. Time-varying measures of BMI were significantly associated with mortality risk in both HD and PD patients.
High BMI is a risk factor for ESKD and the prevalence of obesity is increasing among ESKD patients needing dialysis [3]. Most of the studies evaluating the association between BMI and mortality have used a single BMI value at baseline. Few studies have assessed follow-up measures of BMI or weight [12], [21]–[27]. Using data from a large dialysis provider, Kalantar-Zadeh and colleagues reported a significantly increased mortality risk in HD patients with time-varying BMI values below the reference category of 23 to 25 kg/m2 and a decreased mortality risk with high BMI categories, including very high BMI values of >45 kg/m2 [12], [21], [22], [25]. Kotanko and colleagues observed a marked decrease in body weight in 3 months preceding death in HD patients [26]. Using serum creatinine as a surrogate for muscle mass, decline in muscle mass was a stronger predictor of mortality than weight loss [25]. The patient characteristics from these studies were different to those from our study. First, the previous studies included prevalent dialysis patients that may have led to the introduction of selective survival bias [31]. Second, the ethnic compositions of the cohort were different as our study predominantly included Caucasian patients. Third, except one [27], all studies included patients receiving HD only. Previously reported analyses of the effect of intra-individual change in (gain or loss of) BMI or weight over time on mortality could have been affected by regression to the mean [32] and an incorrect assumption of linear and unidirectional change of weight.
A key strength of our study was the inclusion of all incident adult ESKD patients receiving both HD and PD. The initial decrease in BMI in the first year of starting dialysis could be due to the excess burden of illness that these patients experience at the time of reaching ESKD necessitating dialysis. Indeed, mortality is highest in the first year of starting dialysis with reported rates ranging from 17.5% to 25% [5], [33]. Due to the absence of data on the assessment of volume status, we could not differentiate between weight loss due to fluid removal and true weight loss. Subsequent weight gain could be due to improved appetite and nutrition once patients are stabilised on dialysis therapy. As with HD patients, there was an increased risk of mortality in PD patients with lower time-varying BMI. In contrast, the survival benefit associated with high BMI in PD patients was limited to the time-varying BMI category of 28 to 31 kg/m2. There were no significant differences in the risk estimates for time-varying BMI up to 37 kg/m2, although a trend of increasing mortality risk was observed that achieved statistical significance for the time-varying BMI category of 37 to 40 kg/m2. This discrepancy in mortality risks between the two dialysis modalities at higher BMI values may be due to differential development of abdominal obesity, which is in turn associated with increased mortality in ESKD patients [34]. Visceral fat mass increases by 11–23% within 1 year of initiating PD, probably due to the metabolic consequences of intraperitoneal administration of glucose-containing PD solutions [35]–[37]. In contrast, increases in visceral fat mass are generally not observed in HD patients [38], [39]. Importantly, our study demonstrated that the average BMI at 1 year of follow-up was higher than the baseline value in PD patients, but lower in HD patients. We did not observe an increased mortality risk in PD patients with time-varying BMI >40 kg/m2, possibly due to small patient numbers (95 patients).
A major limitation of our study was that BMI does not distinguish fat mass from lean mass, especially in patients with muscle wasting [40]. Furthermore, BMI does not reflect body-fat distribution. Given that fat distribution varies substantially across various ethnic backgrounds at the same level of BMI, BMI is not an ideal surrogate of fat mass [41]. The ANZDATA Registry does not collect data on other anthropometric measures, body composition, laboratory indices of nutrition and inflammation, hospitalizations or cardiovascular events. The accuracy of reported data on dry-weight could not be validated as data on the assessment of volume status are not collected. One common limitation with survival regression models incorporating time-varying covariates is the difficulty to identify the non-linearity of the time-varying covariates. However, given the observed BMI trajectory, the hazard estimates obtained through the weighted Cox regression models should be sufficiently robust. Other limitations of our study include adjustment for a limited number of baseline variables and variable frequency of weight reporting. Patients with less than 6 months follow-up on dialysis were excluded, potentially leading to bias. Since our study predominantly included Caucasian patients, the results of this study may not be generalizable to patient populations with different ethnic compositions.
Considering the obesity epidemic in ESKD, the results of our study have important clinical and research implications, despite its limitations. The finding of a non-linear change in BMI, especially in the 1st year of starting dialysis, suggests that particular attention should be paid by clinicians to optimising nutritional intake in this early period in the hope of preventing early weight loss and heightened early mortality. Prospective studies are required to understand the dynamic association between BMI and abdominal obesity in dialysis patients and its variation according to dialysis modality. Randomised clinical trials are required to study the effect of nutritional interventions on BMI and important clinical outcomes, such as quality of life and mortality. Finally, research evaluating the effects of glucose-sparing PD regimens on visceral fat mass and patient outcomes is needed.
In conclusion, BMI changed in a non-linear fashion in incident dialysis patients. Time-varying measures of BMI were significantly associated with all-cause mortality risk. Lower time-varying BMI categories were associated with increased mortality risk in both HD and PD patients. Higher time-varying BMI categories were associated with decreased mortality in HD patients, but not in PD patients.
Supporting Information
Supporting tables and figure. Table S1. Distributions of BMI groups according to ethnicity and dialysis modality. Table S2. Number of weight measurements per patient. Table S3. Causes of death. Table S4: Hazard ratio (95% CI) for mortality associated with individual covariates from the multivariate Cox-regression model with time-varying BMI as covariate. Figure S1. Flow diagram describing patient selection in the study.
(DOCX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. The primary dataset for this manuscript was generated and made available to the authors by the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry, Adelaide, Australia. The ANZDATA Data Use Agreement between the ANZDATA Registry and the authors does not allow the authors to make the data publicly available. The authors confirm that all data underlying the findings can be obtained without restriction from the ANZDATA Registry. The interested researchers are advised to contact the ANZDATA Registry independently (email address requests@anzdata.org.au).
Funding Statement
The authors have no funding or support to report.
References
- 1. Flegal KM, Carroll MD, Ogden CL, Curtin LR (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303:235–241. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. [DOI] [PMC free article] [PubMed]
- 3. Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS (2006) Body mass index and risk for end-stage renal disease. Ann Intern Med 144:21–28. [DOI] [PubMed] [Google Scholar]
- 4. Kramer HJ, Saranathan A, Luke A, Durazo-Arvizu RA, Guichan C, et al. (2006) Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol 17:1453–1459. [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System, USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. (2011) Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
- 6. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, et al. (2010) Body-mass index and mortality among 1.46 million white adults. N Engl J Med 363:2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. (2009) Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. (1998) Bethesda, MD: National Heart, Lung, and Blood Institute.
- 9.Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults. (2003) National Health & Medical Research Council, Department of Health and Aging, Commonwealth of Australia.
- 10. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH (2001) A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38:1251–1263. [DOI] [PubMed] [Google Scholar]
- 11. Abbott KC, Glanton CW, Trespalacios FC, Oliver DK, Ortiz MI, et al. (2004) Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int 65:597–605. [DOI] [PubMed] [Google Scholar]
- 12. Molnar MZ, Streja E, Kovesdy CP, Bunnapradist S, Sampaio MS, et al. (2011) Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. Am J Transplant 11:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johansen KL, Young B, Kaysen GA, Chertow GM (2004) Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr 80:324–332. [DOI] [PubMed] [Google Scholar]
- 14. Stack AG, Murthy BV, Molony DA (2004) Survival differences between peritoneal dialysis and hemodialysis among “large” ESRD patients in the United States. Kidney Int 65:2398–2408. [DOI] [PubMed] [Google Scholar]
- 15. Vashistha T, Mehrotra R, Park J, Streja E, Dukkipati R, et al. (2014) Effect of age and dialysis vintage on obesity paradox in long-term hemodialysis patients. Am J Kidney Dis 63:612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson DW, Herzig KA, Purdie DM, Chang W, Brown AM, et al. (2000) Is obesity a favorable prognostic factor in peritoneal dialysis patients? Perit Dial Int 20:715–721. [PubMed] [Google Scholar]
- 17. Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ (2003) Body size and outcomes on peritoneal dialysis in the United States. Kidney Int 64:1838–1844. [DOI] [PubMed] [Google Scholar]
- 18. Pliakogiannis T, Trpeski L, Taskapan H, Shah H, Ahmad M, et al. (2007) Reverse epidemiology in peritoneal dialysis patients: the Canadian experience and review of the literature. Int Urol Nephrol 39:281–288. [DOI] [PubMed] [Google Scholar]
- 19. de Mutsert R, Grootendorst DC, Boeschoten EW, Dekker FW, Krediet RT (2009) Is obesity associated with a survival advantage in patients starting peritoneal dialysis? Contrib Nephrol 163:124–131. [DOI] [PubMed] [Google Scholar]
- 20. McDonald SP, Collins JF, Johnson DW (2003) Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J Am Soc Nephrol 14:2894–2901. [DOI] [PubMed] [Google Scholar]
- 21. Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, et al. (2005) Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis 46:489–500. [DOI] [PubMed] [Google Scholar]
- 22. Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, et al. (2010) The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc 85:991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campbell KL, MacLaughlin HL (2010) Unintentional weight loss is an independent predictor of mortality in a hemodialysis population. J Ren Nutr 20:414–418. [DOI] [PubMed] [Google Scholar]
- 24. Chazot C, Gassia JP, Di Benedetto A, Cesare S, Ponce P, et al. (2009) Is there any survival advantage of obesity in Southern European haemodialysis patients? Nephrol Dial Transplant 24:2871–2876. [DOI] [PubMed] [Google Scholar]
- 25. Kalantar-Zadeh K, Streja E, Molnar MZ, Lukowsky LR, Krishnan M, et al. (2012) Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol 175:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kotanko P, Thijssen S, Usvyat L, Tashman A, Kruse A, et al. (2009) Temporal evolution of clinical parameters before death in dialysis patients: a new concept. Blood Purif 27:38–47. [DOI] [PubMed] [Google Scholar]
- 27. Lievense H, Kalantar-Zadeh K, Lukowsky LR, Molnar MZ, Duong U, et al. (2012) Relationship of body size and initial dialysis modality on subsequent transplantation, mortality and weight gain of ESRD patients. Nephrol Dial Transplant 27:3631–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, et al. (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147:573–577. [DOI] [PubMed] [Google Scholar]
- 29. McDonald SP, Craig JC, Australian, New Zealand Paediatric Nephrology A (2004) Long-term survival of children with end-stage renal disease. N Engl J Med 350:2654–2662. [DOI] [PubMed] [Google Scholar]
- 30. Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, et al. (2006) Cancer incidence before and after kidney transplantation. JAMA 296:2823–2831. [DOI] [PubMed] [Google Scholar]
- 31. Delgado-Rodriguez M, Llorca J (2004) Bias. J Epidemiol Community Health 58:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bland JM, Altman DG (1994) Some examples of regression towards the mean. BMJ 309:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, et al. (2007) Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2:89–99. [DOI] [PubMed] [Google Scholar]
- 34. Postorino M, Marino C, Tripepi G, Zoccali C (2009) Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol 53:1265–1272. [DOI] [PubMed] [Google Scholar]
- 35. Stenvinkel P, Lindholm B, Lonnqvist F, Katzarski K, Heimburger O (2000) Increases in serum leptin levels during peritoneal dialysis are associated with inflammation and a decrease in lean body mass. J Am Soc Nephrol 11:1303–1309. [DOI] [PubMed] [Google Scholar]
- 36. Fernstrom A, Hylander B, Moritz A, Jacobsson H, Rossner S (1998) Increase of intra-abdominal fat in patients treated with continuous ambulatory peritoneal dialysis. Perit Dial Int 18:166–171. [PubMed] [Google Scholar]
- 37. Cho KH, Do JY, Park JW, Yoon KW (2010) Effect of icodextrin dialysis solution on body weight and fat accumulation over time in CAPD patients. Nephrol Dial Transplant 25:593–599. [DOI] [PubMed] [Google Scholar]
- 38. Rodriguez Ayala E, Pecoits-Filho R, Heimburger O, Lindholm B, Nordfors L, et al. (2004) Associations between plasma ghrelin levels and body composition in end-stage renal disease: a longitudinal study. Nephrol Dial Transplant 19:421–426. [DOI] [PubMed] [Google Scholar]
- 39. Pellicano R, Strauss BJ, Polkinghorne KR, Kerr PG (2011) Longitudinal body composition changes due to dialysis. Clin J Am Soc Nephrol 6:1668–1675. [DOI] [PubMed] [Google Scholar]
- 40. Beddhu S, Pappas LM, Ramkumar N, Samore M (2003) Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol 14:2366–2372. [DOI] [PubMed] [Google Scholar]
- 41. Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P (2000) The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes Relat Metab Disord 24:1011–1017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting tables and figure. Table S1. Distributions of BMI groups according to ethnicity and dialysis modality. Table S2. Number of weight measurements per patient. Table S3. Causes of death. Table S4: Hazard ratio (95% CI) for mortality associated with individual covariates from the multivariate Cox-regression model with time-varying BMI as covariate. Figure S1. Flow diagram describing patient selection in the study.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. The primary dataset for this manuscript was generated and made available to the authors by the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry, Adelaide, Australia. The ANZDATA Data Use Agreement between the ANZDATA Registry and the authors does not allow the authors to make the data publicly available. The authors confirm that all data underlying the findings can be obtained without restriction from the ANZDATA Registry. The interested researchers are advised to contact the ANZDATA Registry independently (email address requests@anzdata.org.au).