Abstract
Introduction
Posttraumatic stress disorder (PTSD) is a prevalent, chronic, and disabling anxiety disorder that may develop following exposure to a traumatic event. Despite the public health significance of PTSD, relatively little is known about the etiology or pathophysiology of this disorder, and pharmacotherapy development to date has been largely opportunistic instead of mechanism-based. Recently, an accumulating body of evidence has implicated the endocannabinoid system in the etiology of PTSD, and targets within this system are believed to be suitable for treatment development.
Methods
Herein, we describe evidence from translational studies arguing for the relevance of the endocannabinoid system in the etiology of PTSD. We also show mechanisms relevant for treatment development.
Results
There is convincing evidence from multiple studies for reduced endocannabinoid availability in PTSD. Brain imaging studies show molecular adaptations with elevated cannabinoid type 1 (CB1) receptor availability in PTSD which is linked to abnormal threat processing and anxious arousal symptoms.
Conclusion
Of particular relevance is evidence showing reduced levels of the endocannabinoid anandamide and compensatory increase of CB1 receptor availability in PTSD, and an association between increased CB1 receptor availability in the amygdala and abnormal threat processing, as well as increased severity of hyperarousal, but not dysphoric symptomatology, in trauma survivors. Given that hyperarousal symptoms are the key drivers of more disabling aspects of PTSD such as emotional numbing or suicidality, novel, mechanism-based pharmacotherapies that target this particular symptom cluster in patients with PTSD may have utility in mitigating the chronicity and morbidity of the disorder.
Keywords: PTSD, endocannabinoids, 5-factor PTSD model, anxious arousal, treatment
Introduction
Posttraumatic stress disorder (PTSD) related to non-combat trauma is a major public health concern. According to U.S. population-based studies such as the National Comorbidity Survey (NCS) (Kessler et al., 1995), NCS-Replication (Kessler et al., 2005), and the National Epidemiologic Survey on Alcohol and Related Conditions (Pietrzak et al., 2011), the lifetime prevalence of PTSD ranges from 6.4% to 7.8%. PTSD is a chronic disorder, with population-based studies indicating that it can persist for up to 10 years, especially if left untreated (Kessler et al., 1995, Pietrzak et al., 2011). Conversely, PTSD is one of the most prevalent, chronic, and disabling psychiatric disorders in solders exposed to war. For example, a study of 18,305 U.S. Army personnel found that 23.6% of active component soldiers and 30.5% of National Guard soldiers screened positive for DSM-IV PTSD 12 months after returning from deployment to Iraq (Thomas et al., 2010). Despite the high prevalence of PTSD in combat veterans, however, most veterans with PTSD do not seek treatment or receive inadequate or inappropriate treatment (Hoge et al., 2008).
One of the biggest challenges to novel pharmacotherapy development in PTSD is that studies have largely failed to develop mechanism-based treatments that target heterogeneous aspects of this disorder. While PTSD is often characterized as a unitary disorder, a large body of evidence suggests that it is comprised of heterogeneous symptom clusters that have unique neurobiological correlates and may be differentially sensitive to treatment (Pietrzak et al., 2014, Pietrzak et al., 2013a, Pietrzak et al., 2013b). For example, several confirmatory factor analytic studies in both veterans (Tsai et al., 2012, Pietrzak et al., 2012, Harpaz-Rotem et al., 2014) and civilians (Armour et al., 2012, Elhai et al., 2011, Contractor et al., 2014) have revealed that PTSD is best characterized as being comprised of five distinct symptom clusters—re-experiencing, avoidance, emotional numbing, dysphoric arousal (e.g., sleep difficulties, concentration problems, anger/irritability), and anxious arousal (i.e., hypervigilance, exaggerated startle); this same symptom structure was recently confirmed using DSM-5 data (Tsai et al., in press). Our research team aimed to evaluate neurobiological and functional endophenotypic correlates of this novel dimensional model of PTSD (Pietrzak et al., 2014, Pietrzak et al., 2013a, Pietrzak et al., 2013b).
Besides psychotherapeutic interventions, there are only a few available pharmacotherapies for PTSD. These include selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs), both of which the Food and Drug Administration (FDA) has approved for the treatment of PTSD and which have been demonstrated to provide some benefit in the management of PTSD symptoms (Brady et al., 2000, Marshall et al., 2001, Davidson et al., 2001, Davidson et al., 2006a, Davidson et al., 2006b, Ipser and Stein, 2012) However, meta-analyses (Stein et al., 2006) have concluded that effect sizes of these pharmacotherapies are small (i.e., mean total CAPS score for the medication group was 5.76 points lower than that observed for the placebo group); and that there may be relatively less benefit for subgroups of individuals with PTSD, such as those with complicated PTSD (Friedman et al., 2007). Commonly utilized pharmacotherapeutic augmentation strategies such as second-generation antipsychotic medications (e.g., risperidone), were also recently shown to be ineffective in treating PTSD (Krystal et al.). Neurobiological mechanisms relevant to etiology of PTSD and key aspects of the PTSD phenotype are linked to the mechanism of action of endocannabinoids and may provide a basis for novel treatment development, specifically for PTSD.
Is There a Role for Endocannabinoids in the Etiology of PTSD?
A number of translational approaches have yielded insights into model mechanisms of PTSD. Amongst those, fear conditioning experiments highlight the role of an amygdala-hippocampalcortico-striatal circuit as a key brain circuit responsible for processing and storing fear-related memories and for coordinating fear-related behaviors (Rogan et al., 1997, LeDoux, 2000, Rodrigues et al., 2004), leading to the hypothesis that PTSD is characterized by amygdala over-activity or hyper-responsiveness to threatening stimuli in humans (Grillon et al., 1996, Phelps and LeDoux, 2005, Rauch et al., 2006). Indeed, a convergence of findings from functional neuroimaging investigations in clinical populations supports a neurocircuitry model of PTSD characterized by abnormally elevated amygdala activity (subserving exaggerated acquisition of fear associations and expression of fear responses) coupled with deficient regulation by prefrontal cortical structures (mediating deficits in extinction and the capacity to suppress attention/response to trauma-related stimuli), as well as abnormal hippocampus (mediating deficits in appreciation of safe contexts and explicit learning/memory) and basal ganglia functions (moderating stimulant and conditioned reinforcement, affective and motor habits). Whereas the neurocircuitry of PTSD is well established and has been consistently reported in the literature, the neurochemistry that moderates the function of this circuit and its alterations relevant to the development of the PTSD phenotype remains incompletely understood.
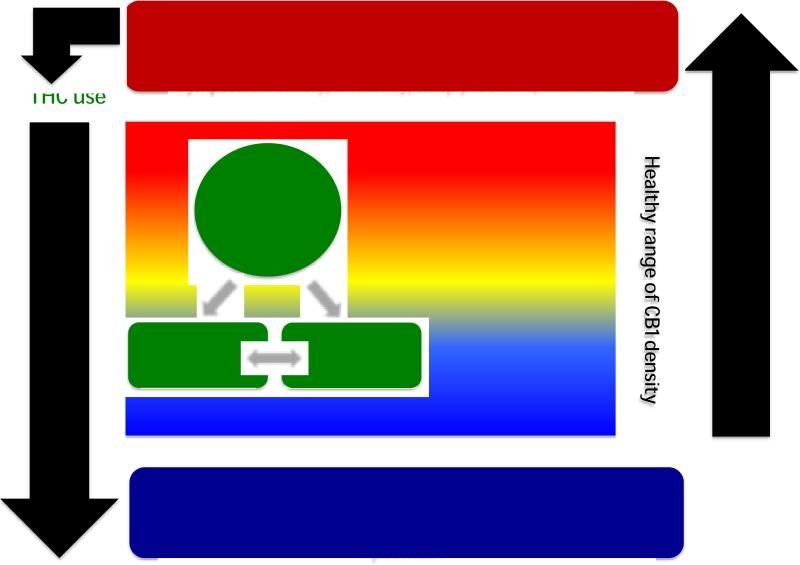
Various lines of evidence suggest that the endocannabinoids, anandamide (AEA) and 2-arachidonolyflycerol (2-AG) which exert much of their actions through the two known CB receptors (CB1, CB2), play an important role in the development (Krebs-Kraft et al.) and function of the PTSD circuit, specifically in stress responses (Hill et al., Rademacher et al., 2008, Reich et al., 2009, Gorzalka et al., 2008, Hill et al., 2009, Hill et al., 2008b). CB1 receptors are the most abundant G-protein-coupled receptors in the central nervous system (Glass et al., 1997, Herkenham et al., 1990) and are found in high concentrations in the aforementioned PTSD circuit. Genetic or pharmacological disruption of CB1 receptor signaling results in anxiety phenotypes (Haller et al., 2002, Haller et al., 2004). Moreover, brain CB1 receptor signaling controls extinction of aversive memories (Marsicano et al., 2002, Varvel et al., 2007, Chhatwal et al., 2005) and deficits in the learned inhibition of fear characterize patients with PTSD. Therefore, impaired CB1 receptor function is a potentially important mechanism in the etiology of PTSD (Figure 1.).
Figure 1.
Model of the role of endocannabinoids in the etiology of posttraumatic stress disorder (PTSD). The availability of anandamide (AEA) regulates the expression of cannabinoid (CB) type 1 (CB1) receptors. Male gender and age have been reported to be associated with lower CB1 receptor density in the human brain. While there is a range of healthy CB1 receptor availability, female gender and trauma exposure result in reduced AEA availability and consequent CB1 receptor upregulation, associated with increasing stress reactivity. Once a critical treshhold of CB1 receptor upregulation is reached, symptoms of PTSD emerge. Patients with PTSD show high rates of Δ(9)-tetrahydrocannabinol (THC) which results in a downregulation of CB1 receptors. Chronic THC exposure and low CB1 receptor availability cause anxiety, irritability, sleep problems and abuse liability.
Recent animal studies (Hill et al., 2005) show that chronic stress is associated with significantly decreased AEA levels in the amygdala-hippocampal-cortico-striatal ciruit. Notably, this finding complements results from human studies in depression (Hill et al., 2008b) reporting that female outpatients with major depressive disorder have lower serum 2-AG levels than controls. The magnitude of the decrease was associated with the length and severity of the depressive episode. While AEA was not associated with major depression per se, an inverse relationship was found between serum AEA content and Hamilton anxiety ratings, suggesting that AEA tone may relate to the anxiety component of the depression phenotype. Interestingly, there is now substantial evidence from independent PTSD cohorts for lower endocannabinoid concentrations in human PTSD (Hill et al., 2013, Neumeister, 2013, Pietrzak et al., 2014), associated with upregulation of brain CB1 receptors (Neumeister, 2013) as a molecular adaption to this reduced synaptic availability (Pietrzak et al., 2014). These data add to more recent studies demonstrating an association between trauma-related disorders such as PTSD, MDD, and GAD, and abnormal threat processing (Sveen et al., 2009, Lindstrom et al., 2011, Fani et al., 2012) by implicating the CB1 receptor system as a key neurobiological mechanism of this endophenotype and its concomitant phenotypic expression of trauma-related threat symptomatology, particularly hyperarousal symptoms.
These results are consistent with the high rates of cannabis abuse among PTSD patients (Vetter et al., 2008, Cornelius et al.). They authenticate, at least in part, emerging evidence that synthetic cannabinoid receptor agonists (Fraser, 2009) or plant-derived cannabinoids such as marijuana (Passie et al., 2012) may possess some benefits in individuals with PTSD by helping relieve haunting nightmares and other threat-related symptoms of PTSD. However, such data do not allow the conclusion that self-medication with cannabis with its primary psychoactive constituent tetrahydrocannabinol should be recommended for the treatment of PTSD, as direct activation of CB1 receptors with plant-derived cannabinoids over an extended period of time leads to down-regulation of CB1 receptors (Hirvonen et al., Leweke and Koethe, 2008), which may in turn facilitate the emergence of a depression-like phenotype in certain individuals (Beyer et al., 2010) and increase risk of addiction (Klugmann et al.).
What is the Evidence for Gender Disparity of CB1 Receptor Functions in PTSD?
Important sex-related differences exist in cannabinoid pharmacology (for review (Fattore and Fratta)) mediating the profound sex differences in the stress response on a systemic level (Hill et al., 2005). These studies directly implicate the CB1 receptor (Reich et al., 2009) in modulating the functions of the brain PTSD circuit. Rodent studies report basal (non-stress) sex differences in hippocampal CB1 receptor levels with males having higher CB1 levels than females, but no differences in fatty acid amide hydrolase (FAAH), the main degradative enzyme for circulating endocannabinoids (Hill et al., 2005, Reich et al., 2009). Chronic stress produced an upregulation of FAAH levels, regardless of sex. Gender disparities in CB1 receptor regulation were observed at this step in the stress response such that chronic stress reliably produced a downregulation of CB1 receptors in male animals (Hill et al., 2008a, Hill et al., 2005, Reich et al., 2009) and a robust upregulation of CB1 receptors in female animals (Reich et al., 2009) which was associated with impaired CB1 receptor-mediated eCB signaling (Suarez et al., 2009). Whereas there is evidence for developmental influence of sex hormones on eCB function (Hill et al., 2007), the observed CB1 receptor changes were found in both intact and gonadectomized animals and are therefore not a result of circulating sex hormones or glucocorticoids (Reich et al., 2009).
These sex-related results from animal work accord with our recent brain imaging data using the CB1 receptor radiotracer [11 C]OMAR and positron emission tomography demonstrating sex differences in CB1 receptor regulation, with up-regulation of CB1 receptors observed predominantly in women, particularly those with PTSD (Neumeister, 2013).
Such data can contribute to models aiming to understand why women are at greater risk for developing PTSD following exposure to various types of trauma than men even when sexual trauma—which is more common in women—is accounted for (Stein et al., 2000), and why women with PTSD have a higher disability burden and reduced quality of life compared to men (Dell'osso et al., Freedy et al., Irish et al., Luxton et al., Ditlevsen and Elklit, Bowler et al., Galovski et al., Breslau and Anthony, 2007, Breslau, 2002, McLean et al., Schnurr and Lunney, 2008). In addition, evidence for the influence of gender on endocannabinoid functioning should be considered in the correct interpretation of study results and the planning of treatment intervention trials involving both, men and women.
Potential Relevance of Fatty Acid Amid Hydrolase (FAAH) Inhibitors in the Treatment of PTSD
Although several psychotherapies are available to treat PTSD, novel pharmacotherapy development has lagged, and only recently have intensive pre-clinical and clinical studies yielded novel, potentially breakthrough discoveries that have the potential to introduce a new era of pharmacotherapies for PTSD. One noteworthy development in recent years has been the identification of the fatty acid amide hydrolase (FAAH) enzyme as a critical mediator of endocannabinoid metabolism and thus a potential target for novel pharmacotherapy development for PTSD and related disorders (Petrosino and Di Marzo, 2010, Gunduz-Cinar et al., 2013a).
FAAH inhibitors may help mitigate PTSD symptoms via multiple mechanisms: (1) restoring back to normal the low levels of anandamide (possibly through increased FAAH activity), which represent a vulnerability factor to developing PTSD and were found to be associated with increased anxiety and hyperarousal symptoms (Neumeister et al., 2013, Pietrzak et al., 2014, Hill et al., 2013); (2) suppression of amygdala hyperreactivity, thereby facilitating the mitigation of anxious arousal and more rapid habituation to threat (Gunduz-Cinar et al., 2013b); (3) restoration of the PTSD-characteristic hypothalamic-pituitary-adrenal (HPA)-axis dysregulation (Roberts et al., 2014); (4) promotion of sleep and suppression of rapid eye movement (REM) sleep (Garcia-Garcia et al., 2009), which can increase re-experiencing and hyperconsolidation of traumatic memories during sleep; (5) reduction of hyperarousal and sympathetic tone via activation of CB1 receptors on noradrenergic nerve terminals (Kirilly et al., 2013); and/or (6) by modulating other eCBs, such as palmitoylethanolamide or oleoylethanolamide, which are anti-inflammatory and analgesic, and regulate satiety, respectively. In addition to FAAH inhibitors potentially reducing PTSD symptoms, particularly hyperarousal, as well as depression symtpoms, they may also help mitigate altered pain sensitivity (Geuze et al., 2007), as well as low-grade inflammation (Lindqvist et al., 2014), which have been documented in PTSD. This broad spectrum of action could further enhance their utility in treating PTSD.
Although there is now preliminary evidence that orally absorbable Δ(9)-tetrahydrocannabinol (THC) may ameliorate symptoms of chronic PTSD (Roitman et al., 2014), several key questions remain unanswered: it is unclear what dose of THC may provide optimal treatment effects; what targets in the brain are engaged by THC besides direct CB1 receptor activation; does THC affect specific PTSD circuits in the brain or are effects observed brain-wide and unspecific; and finally, the important question of abuse liability needs to be addressed before THC could become a relevant compound for treatment development.
In contrast, several lines of evidence demonstrate the superiority of a FAAH inhibitor over direct CB1 receptor agonists for the treatment of PTSD. These include: (1) anandamide is a weak, partial CB1 agonist, unlike Δ9-THC, and has no reinforcing effects; (2) there are no cognitive impairments; (3) cardiovascular liabilities (tachycardia, orthostasis, syncope); or (4) hyperphagia are associated with FAAH inhibitor treatment; (5) the pharmacology of FAAH inhibitors can be localized to active pathways; and (6) FAAH inhibitors exhibit polypharmacology by influencing multiple endocannabinoids in their metabolism, possibly resulting in complementary positive effects in mitigating anxiety, as well as pain and inflammatory responses.
Conclusion
In conclusion, unlike prior pharmacotherapy studies in PTSD that have been largely opportunistic in nature, the upcoming generation of clinical trials to explore the utility of endocannabinoid-related compounds is based on a substantial body of preclinical and translational data that have directly implicated the endocannabinoid system in the pathophysiology of PTSD. We suggest that these trials employ a novel, theory-driven and empirically supported five-factor phenotypic model of PTSD symptomatology in evaluating treatment response. Unlike DSM-based approaches to classifying PTSD symptom dimensionality, this novel model, which suggests that the PTSD phenotype is best represented by five symptom dimensions—re-experiencing, avoidance, numbing, dysphoric arousal (e.g., sleep disturbance), and anxious arousal (e.g., exaggerated startle response)—has received extensive empirical support from a large body of confirmatory factor analytic studies of various trauma-exposed populations, including Veterans(Harpaz-Rotem et al., 2014). Accordingly, this approach will provide greater specificity in understanding how the novel compounds differentially modulate unique dimensions of the multi-faceted PTSD phenotype. This increased specificity in assessing treatment response in individuals with PTSD is particularly relevant to the mechanism of action of endocannabinoid drugs, i.e. FAAH inhibition, which act primarily to modulate anxious arousal symptoms such as exaggerated startle and hypervigilance. Thus, we anticipate that results of these trials, while focused on evaluating the efficacy of endocannabinoid function modulating compounds in treating PTSD, will have potential relevance to a broad, transdiagnostic range of psychiatric syndromes characterized by elevated hyperarousal symptoms, such as generalized anxiety disorder, panic disorder, and major depressive disorder.
Highlights.
Endocannabinoids are involved in the etiology of PTSD
They are linked to anxious arousal symptoms
Enhancing endocannabinois function may specifically treat this symptom complex of PTSD
Acknowledgments
Funding Information:
This project was supported by the National Institutes of Health (NIH) through the following awards: R21MH096105, R21MH085627, RO1MH096876 and RO1MH102566; the Office of the Assistant Secretary of Defense for Health Affairs under Award No. W81XWH-14-1-0084. The Clinical Neurosciences Division of the United States Department of Veterans Affairs National Center for Posttraumatic Stress Disorder. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense, the NIH or VA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors declare no conflict of interest with the work presented in this manuscript.
Dr. Neumeister has received consulting fees from Pfizer, Inc. This activity is unrelated to the present publication. Dr. Neumeister has received grant support from Lilly, Inc. which is unrelated to the present publication. Dr. Pietrzak is a scientific consultant to Cogstate, Ltd. and this activity is unrelated to the present report. No other potential conflicts of interest relevant to this article were reported by the other authors.
References
- ARMOUR C, CARRAGHER N, ELHAI JD. Assessing the fit of the Dysphoric Arousal model across two nationally representative epidemiological surveys: The Australian NSMHWB and the United States NESARC. J Anxiety Disord. 2012;27:109–115. doi: 10.1016/j.janxdis.2012.10.006. [DOI] [PubMed] [Google Scholar]
- BEYER CE, DWYER JM, PIESLA MJ, PLATT BJ, SHEN R, RAHMAN Z, CHAN K, MANNERS MT, SAMAD TA, KENNEDY JD, BINGHAM B, WHITESIDE GT. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol Dis. 2010;39:148–55. doi: 10.1016/j.nbd.2010.03.020. [DOI] [PubMed] [Google Scholar]
- BOWLER RM, HAN H, GOCHEVA V, NAKAGAWA S, ALPER H, DIGRANDE L, CONE JE. Gender differences in probable posttraumatic stress disorder among police responders to the 2001 World Trade Center terrorist attack. Am J Ind Med. 53:1186–96. doi: 10.1002/ajim.20876. [DOI] [PubMed] [Google Scholar]
- BRADY K, PEARLSTEIN T, ASNIS GM, BAKER D, ROTHBAUM B, SIKES CR, FARFEL GM. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283:1837–44. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- BRESLAU N. Gender differences in trauma and posttraumatic stress disorder. J Gend Specif Med. 2002;5:34–40. [PubMed] [Google Scholar]
- BRESLAU N, ANTHONY JC. Gender differences in the sensitivity to posttraumatic stress disorder: An epidemiological study of urban young adults. J Abnorm Psychol. 2007;116:607–11. doi: 10.1037/0021-843X.116.3.607. [DOI] [PubMed] [Google Scholar]
- CHHATWAL JP, DAVIS M, MAGUSCHAK KA, RESSLER KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–24. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- CONTRACTOR AA, DURHAM TA, BRENNAN JA, ARMOUR C, WUTRICK HR, FRUEH BC, ELHAI JD. DSM-5 PTSD's symptom dimensions and relations with major depression's symptom dimensions in a primary care sample. Psychiatry Res. 2014;215:146–53. doi: 10.1016/j.psychres.2013.10.015. [DOI] [PubMed] [Google Scholar]
- CORNELIUS JR, KIRISCI L, REYNOLDS M, CLARK DB, HAYES J, TARTER R. PTSD contributes to teen and young adult cannabis use disorders. Addict Behav. 35:91–4. doi: 10.1016/j.addbeh.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON J, BALDWIN D, STEIN DJ, KUPER E, BENATTIA I, AHMED S, PEDERSEN R, MUSGNUNG J. Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry. 2006a;63:1158–65. doi: 10.1001/archpsyc.63.10.1158. [DOI] [PubMed] [Google Scholar]
- DAVIDSON J, ROTHBAUM BO, TUCKER P, ASNIS G, BENATTIA I, MUSGNUNG JJ. Venlafaxine extended release in posttraumatic stress disorder: a sertraline- and placebo-controlled study. J Clin Psychopharmacol. 2006b;26:259–67. doi: 10.1097/01.jcp.0000222514.71390.c1. [DOI] [PubMed] [Google Scholar]
- DAVIDSON JR, ROTHBAUM BO, VAN DER KOLK BA, SIKES CR, FARFEL GM. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry. 2001;58:485–92. doi: 10.1001/archpsyc.58.5.485. [DOI] [PubMed] [Google Scholar]
- DELL'OSSO L, CARMASSI C, MASSIMETTI G, DANELUZZO E, DI TOMMASO S, ROSSI A. Full and partial PTSD among young adult survivors 10months after the L'Aquila 2009 earthquake: Gender differences. J Affect Disord. doi: 10.1016/j.jad.2010.11.023. [DOI] [PubMed] [Google Scholar]
- DITLEVSEN DN, ELKLIT A. The combined effect of gender and age on post traumatic stress disorder: do men and women show differences in the lifespan distribution of the disorder? Ann Gen Psychiatry. 9:32. doi: 10.1186/1744-859X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELHAI JD, BIEHN TL, ARMOUR C, KLOPPER JJ, FRUEH BC, PALMIERI PA. Evidence for a unique PTSD construct represented by PTSD's D1-D3 symptoms. J Anxiety Disord. 2011;25:340–5. doi: 10.1016/j.janxdis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- FANI N, TONE EB, PHIFER J, NORRHOLM SD, BRADLEY B, RESSLER KJ, KAMKWALALA A, JOVANOVIC T. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med. 2012;42:533–43. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATTORE L, FRATTA W. How important are sex differences in cannabinoid action? Br J Pharmacol. 160:544–8. doi: 10.1111/j.1476-5381.2010.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15:84–8. doi: 10.1111/j.1755-5949.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEDY JR, MAGRUDER KM, MAINOUS AG, FRUEH BC, GEESEY ME, CARNEMOLLA M. Gender differences in traumatic event exposure and mental health among veteran primary care patients. Mil Med. 175:750–8. doi: 10.7205/milmed-d-10-00123. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN MJ, MARMAR CR, BAKER DG, SIKES CR, FARFEL GM. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68:711–20. doi: 10.4088/jcp.v68n0508. [DOI] [PubMed] [Google Scholar]
- GALOVSKI TE, MOTT J, YOUNG-XU Y, RESICK PA. Gender differences in the clinical presentation of PTSD and its concomitants in survivors of interpersonal assault. J Interpers Violence. 26:789–806. doi: 10.1177/0886260510365865. [DOI] [PubMed] [Google Scholar]
- GARCIA-GARCIA F, ACOSTA-PENA E, VENEBRA-MUNOZ A, MURILLO-RODRIGUEZ E. Sleep-inducing factors. CNS Neurol Disord Drug Targets. 2009;8:235–44. doi: 10.2174/187152709788921672. [DOI] [PubMed] [Google Scholar]
- GEUZE E, WESTENBERG HG, JOCHIMS A, DE KLOET CS, BOHUS M, VERMETTEN E, SCHMAHL C. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry. 2007;64:76–85. doi: 10.1001/archpsyc.64.1.76. [DOI] [PubMed] [Google Scholar]
- GLASS M, DRAGUNOW M, FAULL RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- GORZALKA BB, HILL MN, HILLARD CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–60. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- GRILLON C, SOUTHWICK SM, CHARNEY DS. The psychobiological basis of posttraumatic stress disorder. Mol Psychiatry. 1996;1:278–97. [PubMed] [Google Scholar]
- GUNDUZ-CINAR O, HILL MN, MCEWEN BS, HOLMES A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci. 2013a;34:637–44. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNDUZ-CINAR O, MACPHERSON KP, CINAR R, GAMBLE-GEORGE J, SUGDEN K, WILLIAMS B, GODLEWSKI G, RAMIKIE TS, GORKA AX, ALAPAFUJA SO, NIKAS SP, MAKRIYANNIS A, POULTON R, PATEL S, HARIRI AR, CASPI A, MOFFITT TE, KUNOS G, HOLMES A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013b;18:813–23. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLER J, BAKOS N, SZIRMAY M, LEDENT C, FREUND TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–8. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- HALLER J, VARGA B, LEDENT C, FREUND TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- HARPAZ-ROTEM I, TSAI J, PIETRZAK RH, HOFF R. The dimensional structure of posttraumatic stress symptomatology in 323,903 U.S. veterans. J Psychiatr Res. 2014;49:31–6. doi: 10.1016/j.jpsychires.2013.10.020. [DOI] [PubMed] [Google Scholar]
- HERKENHAM M, LYNN AB, LITTLE MD, JOHNSON MR, MELVIN LS, DE COSTA BR, RICE KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL MN, BIERER LM, MAKOTKINE I, GOLIER JA, GALEA S, MCEWEN BS, HILLARD CJ, YEHUDA R. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the world trade center attacks. Psychoneuroendocrinology. 2013;38:2952–61. doi: 10.1016/j.psyneuen.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL MN, CARRIER EJ, MCLAUGHLIN RJ, MORRISH AC, MEIER SE, HILLARD CJ, GORZALKA BB. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem. 2008a;106:2322–36. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL MN, KARACABEYLI ES, GORZALKA BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32:350–7. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- HILL MN, MCLAUGHLIN RJ, BINGHAM B, SHRESTHA L, LEE TT, GRAY JM, HILLARD CJ, GORZALKA BB, VIAU V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A. 107:9406–11. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL MN, MILLER GE, CARRIER EJ, GORZALKA BB, HILLARD CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34:1257–62. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL MN, MILLER GE, HO WS, GORZALKA BB, HILLARD CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008b;41:48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL MN, PATEL S, CARRIER EJ, RADEMACHER DJ, ORMEROD BK, HILLARD CJ, GORZALKA BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–15. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- HIRVONEN J, GOODWIN RS, LI CT, TERRY GE, ZOGHBI SS, MORSE C, PIKE VW, VOLKOW ND, HUESTIS MA, INNIS RB. Reversible and regionally selective downregulation of brain cannabinoid CB(1) receptors in chronic daily cannabis smokers. Mol Psychiatry. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGE CW, CASTRO CA, MESSER SC, MCGURK D, COTTING DI, KOFFMAN RL. Combat duty in Iraq and Afghanistan, mental health problems and barriers to care. US Army Med Dep J. 2008:7–17. [PubMed] [Google Scholar]
- IPSER JC, STEIN DJ. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD). Int J Neuropsychopharmacol. 2012;15:825–40. doi: 10.1017/S1461145711001209. [DOI] [PubMed] [Google Scholar]
- IRISH LA, FISCHER B, FALLON W, SPOONSTER E, SLEDJESKI EM, DELAHANTY DL. Gender differences in PTSD symptoms: an exploration of peritraumatic mechanisms. J Anxiety Disord. 25:209–16. doi: 10.1016/j.janxdis.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER RC, CHIU WT, DEMLER O, MERIKANGAS KR, WALTERS EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER RC, SONNEGA A, BROMET E, HUGHES M, NELSON CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- KIRILLY E, HUNYADY L, BAGDY G. Opposing local effects of endocannabinoids on the activity of noradrenergic neurons and release of noradrenaline: relevance for their role in depression and in the actions of CB(1) receptor antagonists. J Neural Transm. 2013;120:177–86. doi: 10.1007/s00702-012-0900-1. [DOI] [PubMed] [Google Scholar]
- KLUGMANN M, KLIPPENSTEIN V, LEWEKE FM, SPANAGEL R, SCHNEIDER M. Cannabinoid exposure in pubertal rats increases spontaneous ethanol consumption and NMDA receptor associated protein levels. Int J Neuropsychopharmacol. 14:505–17. doi: 10.1017/S1461145710001562. [DOI] [PubMed] [Google Scholar]
- KREBS-KRAFT DL, HILL MN, HILLARD CJ, MCCARTHY MM. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc Natl Acad Sci U S A. 107:20535–40. doi: 10.1073/pnas.1005003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRYSTAL JH, ROSENHECK RA, CRAMER JA, VESSICCHIO JC, JONES KM, VERTREES JE, HORNEY RA, HUANG GD, STOCK C. Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related PTSD: a randomized trial. JAMA. 2011;306:493–502. doi: 10.1001/jama.2011.1080. [DOI] [PubMed] [Google Scholar]
- LEDOUX JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LEWEKE FM, KOETHE D. Cannabis and psychiatric disorders: it is not only addiction. Addict Biol. 2008;13:264–75. doi: 10.1111/j.1369-1600.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- LINDQVIST D, WOLKOWITZ OM, MELLON S, YEHUDA R, FLORY JD, HENN HAASE C, BIERER LM, ABU-AMARA D, COY M, NEYLAN TC, MAKOTKINE I, REUS VI, YAN X, TAYLOR NM, MARMAR CR, DHABHAR FS. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- LINDSTROM KM, MANDELL DJ, MUSA GJ, BRITTON JC, SANKIN LS, MOGG K, BRADLEY BP, ERNST M, DOAN T, BAR-HAIM Y, LEIBENLUFT E, PINE DS, HOVEN CW. Attention orientation in parents exposed to the 9 /11 terrorist attacks and their children. Psychiatry Res. 2011;187:261–6. doi: 10.1016/j.psychres.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUXTON DD, SKOPP NA, MAGUEN S. Gender differences in depression and PTSD symptoms following combat exposure. Depress Anxiety. 27:1027–33. doi: 10.1002/da.20730. [DOI] [PubMed] [Google Scholar]
- MARSHALL RD, BEEBE KL, OLDHAM M, ZANINELLI R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry. 2001;158:1982–8. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- MARSICANO G, WOTJAK CT, AZAD SC, BISOGNO T, RAMMES G, CASCIO MG, HERMANN H, TANG J, HOFMANN C, ZIEGLGANSBERGER W, DI MARZO V, LUTZ B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- MCLEAN CP, ASNAANI A, LITZ BT, HOFMANN SG. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUMEISTER A. The endocannabinoid system provides an avenue for evidence-based treatment development for PTSD. Depress Anxiety. 2013;30:93–6. doi: 10.1002/da.22031. [DOI] [PubMed] [Google Scholar]
- NEUMEISTER A, NORMANDIN MD, PIETRZAK RH, PIOMELLI D, ZHENG MQ, GUJARRO-ANTON A, POTENZA MN, BAILEY CR, LIN SF, NAJAFZADEH S, ROPCHAN J, HENRY S, CORSI-TRAVALI S, CARSON RE, HUANG Y. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry. 2013;18:1034–40. doi: 10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASSIE T, EMRICH HM, KARST M, BRANDT SD, HALPERN JH. Mitigation of post-traumatic stress symptoms by Cannabis resin: a review of the clinical and neurobiological evidence. Drug Test Anal. 2012;4:649–59. doi: 10.1002/dta.1377. [DOI] [PubMed] [Google Scholar]
- PETROSINO S, DI MARZO V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr Opin Investig Drugs. 2010;11:51–62. [PubMed] [Google Scholar]
- PHELPS EA, LEDOUX JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- PIETRZAK RH, GALLEZOT JD, DING YS, HENRY S, POTENZA MN, SOUTHWICK SM, KRYSTAL JH, CARSON RE, NEUMEISTER A. Association of posttraumatic stress disorder with reduced in vivo norepinephrine transporter availability in the locus coeruleus. JAMA Psychiatry. 2013a;70:1199–205. doi: 10.1001/jamapsychiatry.2013.399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- PIETRZAK RH, GOLDSTEIN RB, SOUTHWICK SM, GRANT BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25:456–65. doi: 10.1016/j.janxdis.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIETRZAK RH, HENRY S, SOUTHWICK SM, KRYSTAL JH, NEUMEISTER A. Linking in vivo brain serotonin type 1B receptor density to phenotypic heterogeneity of posttraumatic stress symptomatology. Mol Psychiatry. 2013 b;18:399–401. doi: 10.1038/mp.2012.60. [DOI] [PubMed] [Google Scholar]
- PIETRZAK RH, HUANG Y, CORSI-TRAVALI S, ZHENG MQ, LIN SF, HENRY S, POTENZA MN, PIOMELLI D, CARSON RE, NEUMEISTER A. Cannabinoid Type 1 Receptor Availability in the Amygdala Mediates Threat Processing in Trauma Survivors. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- PIETRZAK RH, TSAI J, HARPAZ-ROTEM I, WHEALIN JM, SOUTHWICK SM. Support for a novel five-factor model of posttraumatic stress symptoms in three independent samples of Iraq/Afghanistan veterans: a confirmatory factor analytic study. J Psychiatr Res. 2012;46:317–22. doi: 10.1016/j.jpsychires.2011.11.013. [DOI] [PubMed] [Google Scholar]
- RADEMACHER DJ, MEIER SE, SHI L, HO WS, JARRAHIAN A, HILLARD CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–16. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- RAUCH SL, SHIN LM, PHELPS EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- REICH CG, TAYLOR ME, MCCARTHY MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–9. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS CJ, STUHR KL, HUTZ MJ, RAFF H, HILLARD CJ. Endocannabinoid signaling in hypothalamic-pituitary-adrenocortical axis recovery following stress: effects of indirect agonists and comparison of male and female mice. Pharmacol Biochem Behav. 2014;117:17–24. doi: 10.1016/j.pbb.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUES SM, SCHAFE GE, LEDOUX JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- ROGAN MT, STAUBLI UV, LEDOUX JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–7. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- ROITMAN P, MECHOULAM R, COOPER-KAZAZ R, SHALEV A. Preliminary, open-label, pilot study of add-on oral Delta9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin Drug Investig. 2014;34:587–91. doi: 10.1007/s40261-014-0212-3. [DOI] [PubMed] [Google Scholar]
- SCHNURR PP, LUNNEY CA. Exploration of gender differences in how quality of life relates to posttraumatic stress disorder in male and female veterans. J Rehabil Res Dev. 2008;45:383–93. doi: 10.1682/jrrd.2007.06.0099. [DOI] [PubMed] [Google Scholar]
- STEIN DJ, IPSER JC, SEEDAT S. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006:CD002795. doi: 10.1002/14651858.CD002795.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN MB, WALKER JR, FORDE DR. Gender differences in susceptibility to posttraumatic stress disorder. Behav Res Ther. 2000;38:619–28. doi: 10.1016/s0005-7967(99)00098-4. [DOI] [PubMed] [Google Scholar]
- SUAREZ J, LLORENTE R, ROMERO-ZERBO SY, MATEOS B, BERMUDEZ-SILVA FJ, DE FONSECA FR, VIVEROS MP. Early maternal deprivation induces gender-dependent changes on the expression of hippocampal CB(1) and CB(2) cannabinoid receptors of neonatal rats. Hippocampus. 2009;19:623–32. doi: 10.1002/hipo.20537. [DOI] [PubMed] [Google Scholar]
- SVEEN J, DYSTER-AAS J, WILLEBRAND M. Attentional bias and symptoms of posttraumatic stress disorder one year after burn injury. J Nerv Ment Dis. 2009;197:850–5. doi: 10.1097/NMD.0b013e3181bea555. [DOI] [PubMed] [Google Scholar]
- THOMAS JL, WILK JE, RIVIERE LA, MCGURK D, CASTRO CA, HOGE CW. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67:614–23. doi: 10.1001/archgenpsychiatry.2010.54. [DOI] [PubMed] [Google Scholar]
- TSAI J, HARPAZ-ROTEM I, ARMOUR C, SOUTHWICK SM, KRYSTAL JH, PIETRZAK RH. Dimensional structure of DSM-5 posttraumatic stress symptoms: Results from the National Health and Resilience in Veterans Study. Journal of Clinical Psychiatry. doi: 10.4088/JCP.14m09091. in press. [DOI] [PubMed] [Google Scholar]
- TSAI J, WHEALIN JM, SCOTT JC, HARPAZ-ROTEM I, PIETRZAK RH. Examining the relation between combat-related concussion, a novel 5-factor model of posttraumatic stress symptoms, and health-related quality of life in Iraq and Afghanistan veterans. J Clin Psychiatry. 2012;73:1110–8. doi: 10.4088/JCP.11m07587. [DOI] [PubMed] [Google Scholar]
- VARVEL SA, WISE LE, NIYUHIRE F, CRAVATT BF, LICHTMAN AH. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32:1032–41. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- VETTER S, ROSSEGGER A, ROSSLER W, BISSON JI, ENDRASS J. Exposure to the tsunami disaster, PTSD symptoms and increased substance use-an Internet based survey of male and female residents of Switzerland. BMC Public Health. 2008;8:92. doi: 10.1186/1471-2458-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]