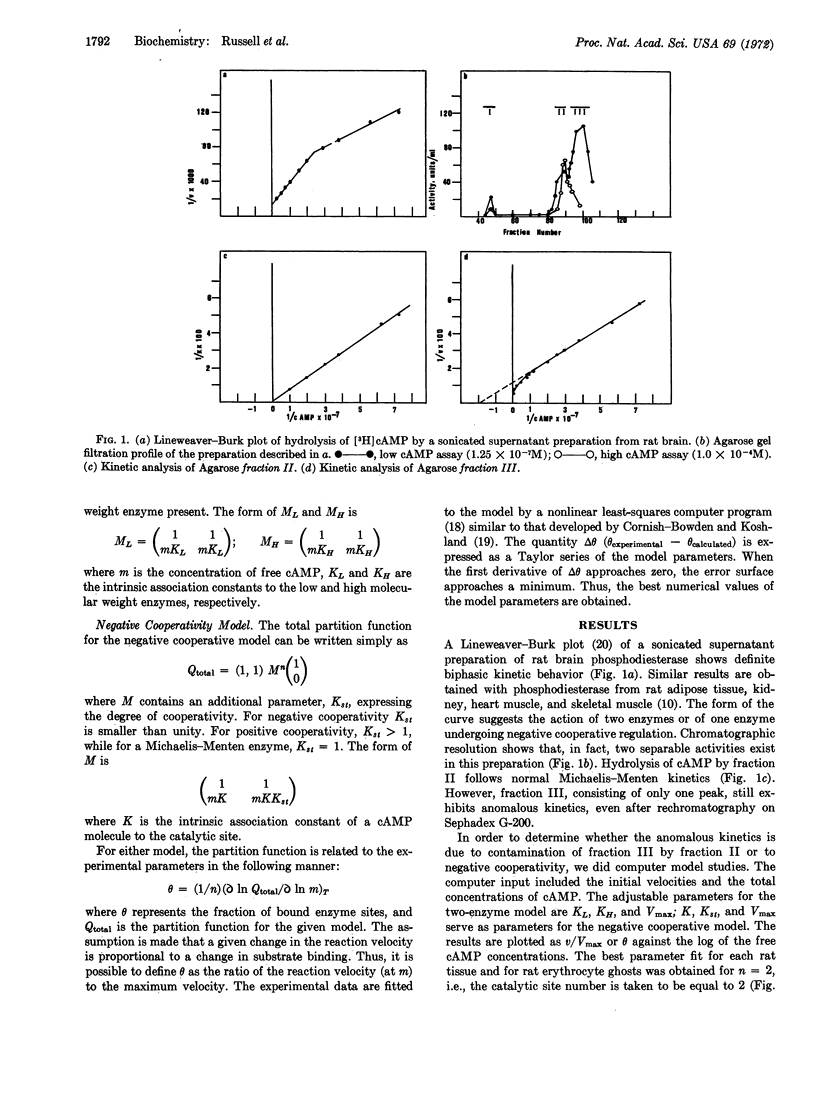
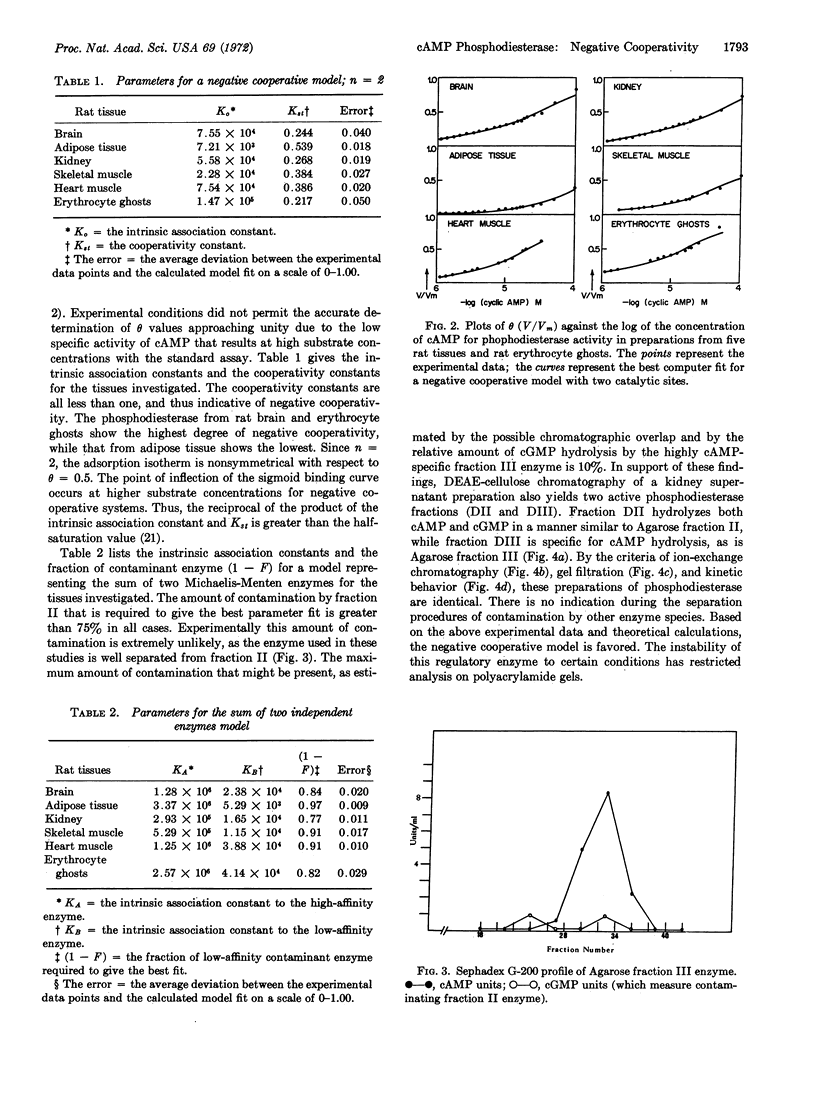
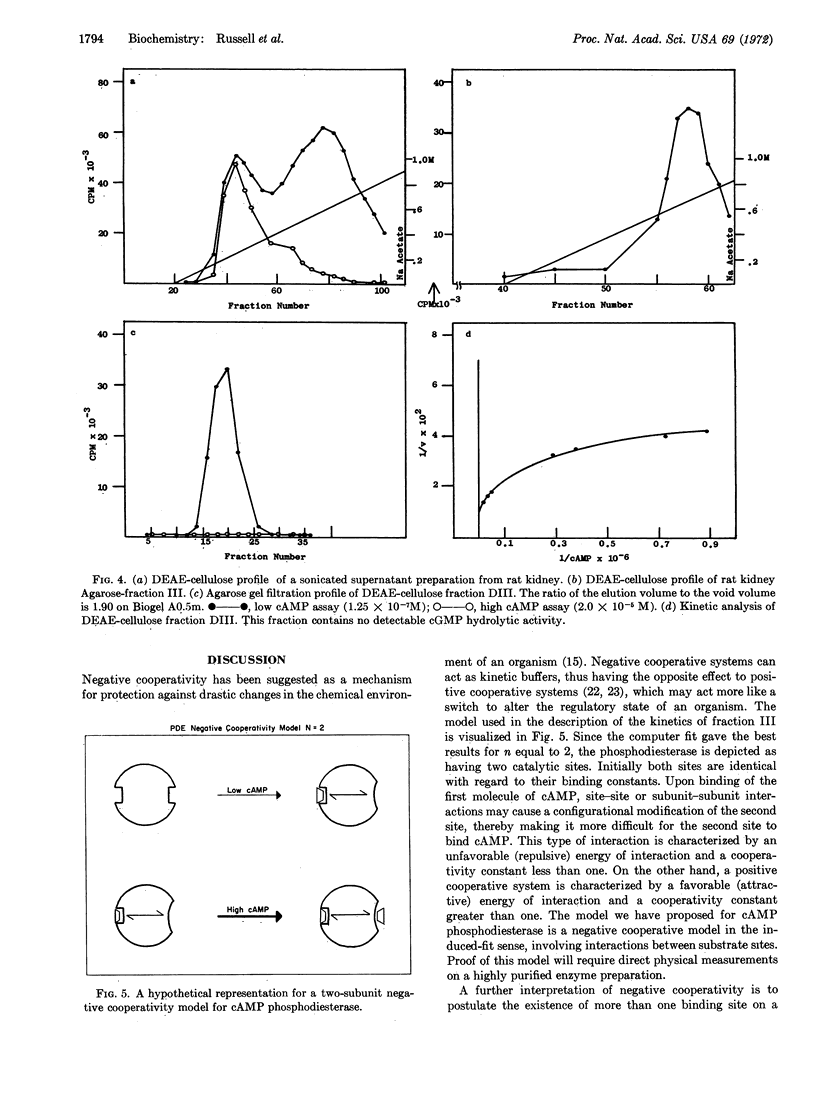
Abstract
Kinetic and chromatographic analysis of cyclic nucleotide phosphodiesterase (EC 3.1.4.c) obtained from rat tissue has revealed that this enzyme exists in at least two molecular forms. After chromatographic separation, one form (cyclic AMP phosphodiesterase) still exhibits kinetics suggestive of the action of either two enzymes or one enzyme under negative cooperative regulation. Computer model studies were undertaken to distinguish between these two possibilities. The matrix method was used to generate the partition functions for (a) the sum of two independent enzymes and (b) one enzyme exhibiting negative cooperative kinetics. The experimental data were fitted to the theoretical models by a nonlinear least-squares computer program. The results show that, while both models can fit the data, the two-enzyme model would require contamination far in excess of what is detectable physically or by activity measurements. Thus, the negative cooperative model seems the more appropriate theoretical explanation of the observed kinetic behavior of this enzyme. The implication of negative cooperativity with respect to the regulation of cyclic AMP concentrations in physiological systems is discussed.
Keywords: kinetics, models, computer analysis, enzyme regulation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Beavo J. A., Hardman J. G., Sutherland E. W. Hydrolysis of cyclic guanosine and adenosine 3',5'-monophosphates by rat and bovine tissues. J Biol Chem. 1970 Nov 10;245(21):5649–5655. [PubMed] [Google Scholar]
- Brooker G., Thomas L. J., Jr, Appleman M. M. The assay of adenosine 3',5'-cyclic monophosphate and guanosine 3',5'-cyclic monophosphate in biological materials by enzymatic radioisotopic displacement. Biochemistry. 1968 Dec;7(12):4177–4181. doi: 10.1021/bi00852a006. [DOI] [PubMed] [Google Scholar]
- Chang Y. Y. Cyclic 3',5'-adenosine monophosphate phosphodiesterase produced by the slime mold Dictyostelium discoideum. Science. 1968 Jul 5;161(3836):57–59. doi: 10.1126/science.161.3836.57. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Properties of cyclic 3',5'-nucleotide phosphodiesterase from rat brain. Biochemistry. 1967 Apr;6(4):1079–1087. doi: 10.1021/bi00856a017. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Koshland D. E., Jr A general method for the quantitative determination of saturation curves for multisubunit proteins. Biochemistry. 1970 Aug 18;9(17):3325–3336. doi: 10.1021/bi00819a006. [DOI] [PubMed] [Google Scholar]
- DRUMMOND G. I., PERROTT-YEE S. Enzymatic hydrolysis of adenosine 3',5'-phosphoric acid. J Biol Chem. 1961 Apr;236:1126–1129. [PubMed] [Google Scholar]
- Jard S., Bernard M. Presence of two 3'-5'-cyclic AMP phosphodiesterases in rat kidney and frog bladder epithelial cells extracts. Biochem Biophys Res Commun. 1970 Nov 9;41(3):781–788. doi: 10.1016/0006-291x(70)90081-1. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R., Teshima Y. Cyclic 3',5'-nucleotide phosphodiesterase, IV. Two enzymes with different properties from brain. Biochem Biophys Res Commun. 1971 Mar 5;42(5):968–974. doi: 10.1016/0006-291x(71)90525-0. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Monard D., Janecek J., Rickenberg H. V. The enzymic degradation of 3',5' cyclic AMP in strains of E. Coli sensitive and resistant to catobolite repression. Biochem Biophys Res Commun. 1969 May 22;35(4):584–591. doi: 10.1016/0006-291x(69)90388-x. [DOI] [PubMed] [Google Scholar]
- Nair K. G. Purification and properties of 3',5'-cyclic nucleotide phosphodiesterase from dog heart. Biochemistry. 1966 Jan;5(1):150–157. doi: 10.1021/bi00865a020. [DOI] [PubMed] [Google Scholar]
- Rosen O. M. Preparation and properties of a cyclic 3',5'-nucleotide phosphodiesterase isolated from frog erythrocytes. Arch Biochem Biophys. 1970 Apr;137(2):435–441. doi: 10.1016/0003-9861(70)90460-1. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958 Jun;232(2):1077–1091. [PubMed] [Google Scholar]
- Schneider F. W., Russell T. R., Rawlings P. K. Co-operative subunit protein models and the matrix method. J Mol Biol. 1970 Feb 28;48(1):103–107. doi: 10.1016/0022-2836(70)90221-4. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Characterization of cyclic nucleotide phosphodiesterases of rat tissues. J Biol Chem. 1971 May 25;246(10):3145–3150. [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]


