Abstract
Perineural growth is a unique route of tumor metastasis that is associated with poor prognosis in several solid malignancies. It is diagnosed by the presence of tumor cells inside the neural space seen on histological or imaging evaluations. Little is known about molecular mechanisms involved in the growth and spread of tumor cells in neural spaces. The poor prognosis associated with perineural growth and lack of targeted approaches necessitates the study of molecular factors involved in communication between tumor and neural cells. Perineural growth rates, shown to be as high as 63% in head and neck squamous cell carcinoma (HNSCC), correlate with increased local recurrence and decreased disease-free survival. Here we describe the literature on perineural growth in HNSCC. In addition, we discuss factors implicated in perineural growth of cancer. These factors include brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotropin-3 and -4, glial cell-line derived neurotrophic factor (GDNF), the neural cell adhesion molecule (NCAM), substance P (SP), and chemokines. We also explore the literature on membrane receptors, including the Trk family and the low-affinity nerve growth factor receptor. This review highlights areas for further study of the mechanisms of perineural invasion which may facilitate the identification of therapeutic targets in HNSCC.
Keywords: Perineural growth, perineural invasion, squamous cell carcinoma, neurotrophic factors, brain-derived neurotropic factor, glia-derived neurotropic factor, neurotropin, chemokines
Introduction
Head and neck cancer accounts for approximately 650,000, or nearly 6%, of new cancer cases worldwide each year, as well as nearly 350,000 deaths. Head and neck cancer is a diverse group of malignancies arising in the oral cavity, oropharynx, larynx and hypopharynx. A variety of benign and malignant neoplasms present in the head and neck including those arising from salivary glands, skin, nerves, blood vessels, muscles, and mucous membranes. Approximately 95% of head and neck cancer cases are diagnosed as head and neck squamous cell carcinomas (HNSCC). Cutaneous and salivary gland squamous cell carcinoma (SCC) together form the next most frequent type of head and neck cancer [1] HNSCC differs in etiology and biology from cutaneous and salivary gland SCC. Alcohol and tobacco use is highly correlated with HNSCC, and human papillomavirus (HPV) has been implicated in oropharyngeal carcinoma progression. [2-4] The five-year survival rates for HNSCC patients continue to be below 50%. Locoregional failure accounts for the vast majority of deaths from HNSCC. The majority of patients with regional recurrence or metastases qualify for palliative care. One of the factors implicated in local recurrence of HNSCC is the presence of perineural tumor growth.
Perineural tumor growth is a route for cancer extension described in many cancers including pancreatic, prostate, colorectal and head and neck. [5-12] Perineural growth is correlated with a decreased rate of disease-free survival, decreased quality of life due to symptoms caused by nerve bundle disruption, and an increase in nociception and locoregional recurrence. [13-15] Tumor growth within the nerve is not simply a path of least resistance. [16] Cancers have a tendency to spread centripetally (i.e. toward the central nervous system) along the nerve, and they also are known to form skip lesions in which the malignant cells do not undergo continuous growth but rather “jump” a section of the nerve and begin spreading farther away. [16-20] The mechanisms of perineural growth are not completely understood.
There are two classifications of perineural growth of cancer: perineural invasion (PNI) and perineural spread (PNS).PNI is the movement of cancer cells into the neural space, usually associated with smaller (unnamed) nerves. Its presence is generally established by histological evaluation of tissue sections and impossible to detect via full-body imaging (Figure 1). [19] PNS is the more gross extension of the tumor along a nerve, with no consensus as to whether or not this must involve a large or named nerve. [18, 19, 21] In general, magnetic resonance imaging (MRI) is used to detect PNS of HNSCC (Figure 2). Both PNI and PNS have been described further based on their involvement and pattern of growth within the neural space. Such patterns of growth include onion bulb formation, circular cell formation, crescent formation, and intraneural invasion. These have been reviewed previously in detail. [14, 22] We will refer broadly to both classifications as perineural growth in this paper.
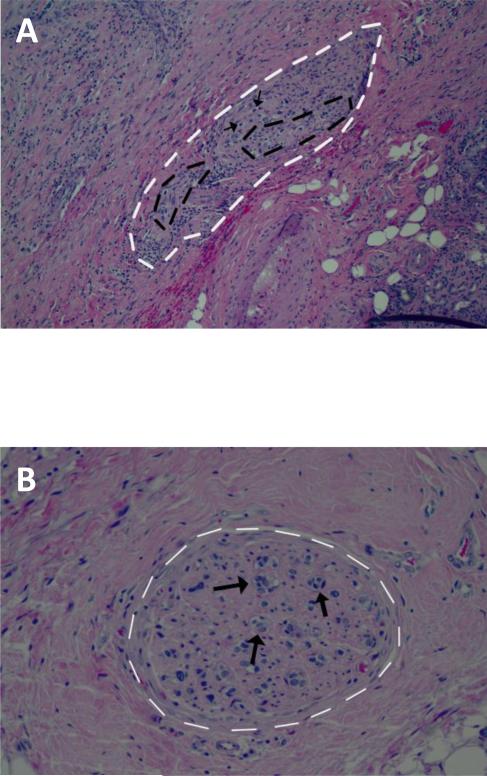
Figure 1. Histological analyses of perineural invasion of HNSCC.
Photomicrographs with representative examples of perineural (A), and intraneural invasion (B) by squamous cell carcinoma of the head and neck (Hematoxylin and Eosin, magnification 100 and 200x, respectively). White dotted lines indicate the nerve, black dotted lines indicate PNI, and black arrows indicated intraneural invasion.
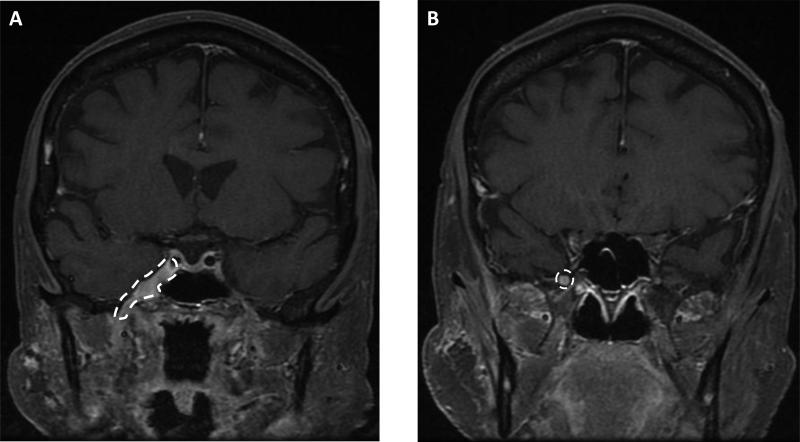
Figure 2. Magnetic resonance images indicate perineural spread of head and neck cancer.
MRI of carcinoma in a 67-year old female in the (A) cavernous sinus which houses several cranial nerves, and the foramen ovale where the V3 branch of the trigeminal nerve emerges from the brain, and (B) foramen rotundum where the V2 branch of the trigeminal nerve emerges from the brain. White dotted lines indicate perineural spread.
Rates of Perineural Growth
The rates of perineural growth in HNSCC are reported with a fair amount of variability. The incidence rates range from 14% to 63.2% (See Table 1). Sample sizes for these studies are also varied. However, as sample size increased across the studies, the rate of perineural growth did not tend to normalize around a common rate. Salivary gland SCCs, and in particular adenocystic carcinoma (ACC), are reported to have approximately 50% incidence rates of perineural growth. [23]
Table 1.
Rates of perineural invasion (PNI) in Non-Cutaneous head and neck squamous cell carcinoma
| TYPE OF PERINEURAL GROWTH | DETECTION METHOD | SITE | SAMPLE SIZE | RATE | REFERENCE |
|---|---|---|---|---|---|
| PNI of small peripheral nerves (<1mm); cancer touching or invading a nerve | microscopic evaluation | oral cavity, oropharynx, hypopharynx, larynx | 142 | 52% | [14] |
| undefined PNI | pathology report | oral cavity | 57 | 63.2% | [24] |
| tumor cells in the perineural space | microscopic evaluation | oral cavity | 307 | 17.1% and 36.6% for T1 and T2 OSCC, respectively | [15] |
| undefined PNI | histological examination of fixed tissue | oral tongue | 113 | 14.2% | [25] |
| undefined PNI | pathology report | tongue, floor of mouth, and other sites | 95 | 25% | [26] |
The reason for discrepancies in the incidence of perineural growth in HNSCC is not apparent. The uncertainty about what constitutes “true” perineural growth may be a factor. [16] While PNI and PNS definitions can be standardized, as noted above, histological definitions may not have been the same across the studies. In certain studies, perineural growth may not have been diagnosed if the nerve involved was unnamed or smaller than 1mm in diameter. [22] Some studies did define criteria for perineural growth [14, 15], while others gave no working definition of perineural involvement (see Table 1). [24-26] Different histological criteria, as well as differences in sample preparation and amount of resected lesion examined, may have played a role in the variation in reported rates of perineural growth in HNSCC. [27]
Detection of Perineural Growth
Perineural growth may first be discovered on pathologic examination of the resected lesion, though this is dependent on the extent and resultant microscopic visibility of the perineural growth. [28] MRI can be used accurately to find the presence of PNS in 95% of cases, though mapping the entirety of the PNS can only be done accurately in 63% of cases. [19] Conversely, there are no effective non-invasive methods to detect PNI, which is rarely evident on MRI studies. [19] Given the poor prognostic implications of perineural growth, it is important to be certain of the aggressiveness of treatment needed. One study suggests that even microscopic PNI warrants further exploration of nerve involvement in order to determine boundaries for post-operative radiation therapy in salivary gland ACC; this is likely a good consideration for HNSCC, too.[23]
Though imaging may not be detailed enough to detect the entire extent of PNI, it can detect PNI and therefore still may be useful in preoperative planning. [29] As the accuracy of mapping tumor extent via imaging increases, treatments may be better assigned to patients, increasing positive outcomes. [23] Importantly, even patients with advanced disease (PNS, for example) might be cured with aggressive treatment, and it may be important to treat any kind of perineural growth in an aggressive manner. [30]
Another issue in detecting perineural growth is that patients with such growth may initially be asymptomatic. Only 30% to 40% of patients with perineural growth may present with symptoms. [28] However, these numbers may be misleading, as case studies suggest that clinical symptoms, including dysesthesia (distorted sense of touch) and numbness, are subtle and often missed by the physician. [30, 31] These subtle symptoms may be present more often than noted, and their presence can even be an early indicator of recurrence. [13]. While symptoms of perineural growth can sometimes be subtle, they can also cause a more dramatic decrease in the quality of life of the patient, eventually causing symptoms indicative of more advanced disease such as paralysis. [13, 18, 23, 30-33]
Prognostic Implications of Perineural Growth
Regardless of incidence rate differences, perineural growth in HNSCC is correlated with increased loco-regional recurrence and decreased disease-free survival. [14, 15, 24, 26] A variety of studies have found that patients with perineural growth have a local recurrence rate from 23-36% compared to 9-5% of patients without perineural growth. [14, 15, 24] In these studies, disease-specific survival rates dramatically decreased for those patients with perineural growth. [14, 15] Similarly, Sinha et al. found that the presence of PNI was a significant indicator for both disease-specific and recurrence-free survival. [26] While studies have found different rates of recurrence and survival in patients with perineural growth, the presence of such growth is agreed to be a poor prognostic indicator in HNSCC.
Nerves of the Head and Neck Involved in Perineural Growth
Perineural growth in HNSCC is often found in the fifth and seventh cranial nerves (the trigeminal nerve and facial nerve, respectively) and their branches. [18-20, 30, 32, 34] These two nerves innervate much of the face: the trigeminal nerve supplies most of the sensory innervation to the face while the facial nerve provides motor innervation. The trigeminal and facial nerves meet in at least 3 different locations: at the sphenopalatine ganglion, at the junction of the chorda tympani and the lingual nerve, and at the parotid gland along the auriculotemporal branch of the mandibular nerve. [33, 35-38] These meeting points may provide routes for the cancer to spread from one nerve to the other. There does not seem to be an affinity for invasion of particular nerves in HNSCC. These noted nerves may play a large role in perineural growth in HNSCC due to their widespread innervation of the head and neck. [13]
Mechanisms of Perineural Growth
To date, relatively little work has been done to understand the mechanisms of perineural growth, especially in HNSCC. The poor prognostic implications coupled with the tendency for the cancer to spread centripetally and often with skip lesions indicate there are likely interactions between the cancer cells and the nerve microenvironment that lead to a more aggressive type of cancer. Indeed, cancer cells in a nerve environment not only display an increase in proliferation but also a decrease in apoptosis by upregulating genes associated with a nuclear factor κB (NF-κB) pathway and its downstream targets. [6] Tumor cells have also been shown to secrete molecules which can increase neurite outgrowth from the nerve toward the tumor. [39-43] Tumor cells in a nerve environment, therefore, exhibit markedly different proliferative and survival behavior and can also interact with the nerve.
A variety of molecules may be found in the nerve microenvironment, and it is likely that one or more of these factors play a role in the poor prognosis of patients with perineural growth. [44] These factors, secreted either by the nerves or the cancer cells, include brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin-3 (NT-3), neurotrophin-4 (NT-4), glial cell-line derived neurotrophic factor (GDNF) and family, the neural cell adhesion molecule (NCAM), substance P (SP), chemokines, and other factors (Figure 3). [12, 39-43, 45] These interactions and the cancers in which these factors have been studied are noted in Table 2. Such factors bind to a variety of different receptors with different affinities, many of them binding to the tyrosine kinase (Trk) family of receptors. Neurotrophins generally promote the development of both the central and peripheral nervous systems and are important for axonal outgrowth. [46, 47] They can also activate complex signaling cascades within cells. [48]
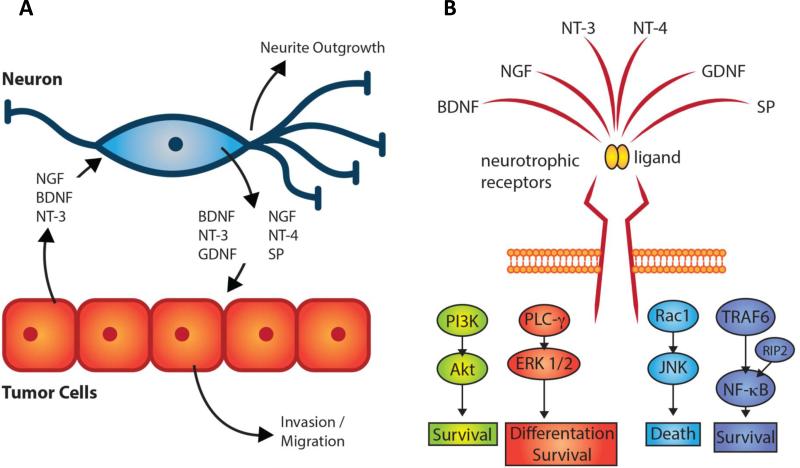
Figure 3. Critical signaling molecules with potential relevance in HNSCC neuron-tumor cell interaction.
(A) HNSCC tumor cells can secrete molecules including NGF, BDNF and NT-3 which lead to neurite outgrowth toward the cancer cells. The nerves also secrete molecules which can cause complex signaling cascades in the cancer cells facilitating tumor invasion and migration. [39-43, 45](B) Major cytoplasmic signaling cascades activated downstream of neurotrophic receptors induce a variety of cellular responses including cell survival, differentiation or death.
Table 2.
Factors/Receptors Implicated in Perineural Cancer Growth
| FACTOR | PRIMARY RECEPTOR | OTHER RECEPTORS | IMPLICATED CANCER(S) | REFERENCE |
|---|---|---|---|---|
| Brain-derived neurotrophic factor (BDNF) | TrkB | Nerve growth factor receptor (NGFR) | ACC, pancreatic, gastric, SCC | [49-52, 62] |
| Nerve growth factor (NGF) | TrkA | NGFR | Pancreatic, breast prostate, SCC, ACC, lung, breast, esophageal | [42, 56-58, 106, 107] |
| Neurotrophin-3 (NT-3) | TrkC | TrkA, TrkB, NGFR | Pancreatic | [62] |
| Neurotrophin-4 (NT-4) | TrkB | NGFR | Pancreatic | [50] |
| Glial cell-line derived neurotrophic factor (GDNF) family, including neurturin, artemin, and persephin | GFRα1, GFRα2, GFRα3, GFRα4, RET | Pancreatic, bile duct carcinoma | [12, 64, 66, 68-74, 108] | |
| Neural cell adhesion molecule (NCAM) | NCAM | Pancreatic, basal cell carcinoma, cutaneous SCC, ACC | [12, 61, 79, 109] | |
| Substance P (SP) | Tachykinin receptor l (TACR/NK-1R) | Pancreatic | [45] | |
| Chemokines | Head and neck, pancreatic, prostate, breast, and more | [12, 93, 97, 101, 102] |
ACC: salivary gland adenocystic carcinoma SCC: Squamous cell carcinoma
Little is known about how such molecules might mediate perineural growth in HNSCC. Still, these mediators have been examined in other cancers, and it is likely that examining the molecules involved in perineural growth in other cancers will be helpful in further guiding HNSCC-specific research. It is worth noting that although many of these studies used artificial models, this does not discount that work nor make it irrelevant to HNSCC. Instead, these models and lessons can be applied to research on perineural growth in HNSCC. Here we outline some of the factors that merit further study pertaining to their role in perineural growth of HNSCC.
Brain-Derived Neurotrophic Factor
The role of brain-derived neurotrophic factor (BDNF) in perineural progression has been examined in ACC, pancreatic and gastric cancers. [49-51] In salivary gland ACC, all tissue samples tested in one study stained positive for BDNF at different levels of intensity, although there was no correlation with perineural growth. [49] However, a previous study did find that culturing select lines of pancreatic adenocarcinoma with low concentrations of BDNF significantly increased invasiveness of the cancer cells through Matrigel matrix. [50] Most recently, in gastric cancer, Okugawa et al reported that BDNF was elevated at the invasive front of tumors and was correlated with poor prognostic factors. [51] In mice models, too, tumor size was significantly lower in those mice injected with a BDNF/TrkB positive cancer line and treated with a Trk antagonist compared to those that were not treated with the antagonist. [51] Given these recent results, and given that both salivary gland ACC and pancreatic adenocarcinomas are highly neurotropic, further studies on BDNF are warranted in HNSCC as well.
Specifically in HNSCC, TrkB, the high-affinity receptor for both BDNF and NT-4, was more highly expressed in the cancer than in the surrounding mucosa, and a small-molecule blockade of the TrkB receptor suppressed the growth of these cancer lines in vitro. [52] Although BDNF and NT-4 have not been reported to be directly involved in perineural growth, they can stimulate tumor cell invasion. The TrkB receptor has also been shown to play an important role in HNSCC progression. [53-55] Further studies on the role of this signaling axis in perineural growth in HNSCC are warranted. Regardless of the role of TrkB, BDNF, and NT-4 in HNSCC neurotropism, the TrkB receptor has shown potential utility for improving chemotherapy regimens. By utilizing a small molecule inhibitor against TrkB, HNSCC cell lines were shown to have increased sensitivity to chemotherapy. [52] This perhaps indicates a promising line of inquiry into the treatment of chemotherapy-resistant cancers via the targeting of neurotrophin receptors.
Nerve Growth Factor
Nerve growth factor (NGF) and its high-affinity receptor TrkA have been implicated in perineural growth of several cancers including breast, prostate, and pancreas. [42, 56-58] In one study, immunohistochemistry was performed on 42 T1/T2 oral tongue SCC specimens, half of which expressed histological evidence of PNI, while the other half did not. [59] In specimens positive for PNI, strong staining for NGF and TrkA was detected. The staining was significantly higher for PNI-positive SCCs as compared to those which were PNI-negative, though NGF and TrkA were still weakly detected in these. [59] Another study confirmed that NGF is expressed at higher levels in HNSCC. [60] Further, NGF blockade decreased pain receptors and therefore nociception, weight loss, and tumor proliferation in mouse models. [60] Yet another report demonstrated higher levels of TrkA, B, and C in PNI-positive SCC tumors as compared to those that were negative for PNI. Further, the majority of tumors expressed NGF. [61] Thus, NGF and its primary receptor, TrkA, may play an important role in perineural growth and aggression in HNSCC.
Neurotropin-3 and Neurotropin-4
Both NT-3 and NT-4 have received relatively little attention in regards to the role they might play in perineural growth of cancers. NT-4 binds with high affinity to TrkB, and NT-3 binds with high affinity to TrkC. Ketterer et al. found that in pancreatic ductal adenocarcinoma (PDAC) NT-3 and NT-4 were both overexpressed compared to normal pancreas tissue samples, and these neurotrophins and their associated receptors were more highly expressed in nerves in the tumor mass. [62] Prior studies have also shown that the presence of neurotrophins in PDAC cultures increased the invasiveness of the cancer. [50] Furthermore, in neuroblastomas, NT-3 is upregulated and blocks TrkC-induced apoptosis. [63] Given that PNI-positive cutaneous SCCs were found to more highly express the Trk family of receptors compared to PNI-negative samples, NT-3 and NT-4 warrant further investigation in perineural growth of mucosal HNSCC. [61]
Glial Cell-Line Derived Neurotrophic Factor and Family
Glial cell-line derived neurotrophic factor (GDNF) has been shown in other cancers to play a role in cell proliferation and migration. [64-66] In pancreatic cancer, GDNF receptor units were shown to be present and, when activated, to increase invasiveness and adhesiveness. [66] In a mouse dorsal root ganglion (DRG) model using human pancreatic cancer cells, invasion of the DRG was shown to be somewhat dependent on the DRG's secretion of GDNF which was modulated via radiation, indicating that cancer cell viability is not the only factor in invasion and metastasis following radiation treatment. [67] Furthermore, in bile duct carcinoma, GDNF is correlated with perineural invasion. [68]
GDNF, its receptors, and its mechanisms have been most thoroughly explored in pancreatic cancer, which is highly neurotrophic. GDNF has been found to be highly present in pancreatic cancer as compared to benign pancreatic tumors, and it is found more highly in those patients with PNI as compared to those without. [69] Most recently, presence of GFRα1 was found to increase cancer cell movement toward GDNF, and it also increased RET phosphorylation and MAP kinase activity. [70] These newer studies, coupled with those which have previously reviewed GDNF involvement in pancreatic cancer and RET signaling in cancer more broadly, indicate a complex and elegant system which seems to play a major role in pancreatic cancer progression especially as related to perineural growth. [71, 72] More widely, the GDNF family also includes the ligands neurturin, artemin, and persephin. Neurturin and persephin were expressed at low levels, while GDNF and artemin were strongly expressed in pancreatic cancer. [73] Further work has shown that artemin increases the invasiveness of tumor along pancreatic nerves. [74]
GDNF has also recently been shown to induce a higher expression of matrix metalloproteinases, specifically MMP-9 and MMP-13, in human oral SCCs, increasing the invasiveness of the cancer cells. [75] Given that GDNF activates MAP kinase pathways in human gliomas, and given that higher expression of MMP-9 and MMP-13 is correlated with metastasis in HNSCCs, GDNF and its family of ligands may play a particularly important role in increasing tumor aggression in the perineural space. [75-78] These studies provide excellent rationale for the investigation of the role of GDNF signaling axis in HNSCC.
Neural Cell Adhesion Molecule
The neural cell adhesion molecule (NCAM) has also been explored as a potential factor in head and neck cancer perineural tumor growth. Using immunohistochemical staining, one study reported that six of eight basal cell carcinomas (BCC) and two of eight cutaneous SCCs were positive for NCAM. [61] Furthermore, in salivary gland ACC, PNI-positive tissues expressed significantly higher levels of NCAM as compared to those that were PNI-negative. [79] Vural et al., reported that in HNSCC, 93% of tumors with PNS expressed NCAM, while only 36% of those without PNS did. [80] More recently, in cutaneous HNSCC, Solares et al. found that NCAM did not predict neurotropism. [81] Importantly, the sample size in the Vural et al. study was nearly five times that of the Solares et al. study, and it was not restricted to the use of cutaneous HNSCC samples. This may help to account for the different findings in the two studies.
Despite the greater comparative rigor of the Vural et al. study above, other studies concluded that NCAM likely does not play any role in perineural growth. [61, 82] They base this in part on prior studies which showed that the presence of NCAM actually decreased tumors’ aggressiveness and invasiveness. [61] Further, BCCs tend to express NCAM and tend not to metastasize easily while cutaneous SCCs express low levels of NCAM but tend to be metastatic. There have been similar findings of the effects of NCAM in a rat glioma and in a human breast cancer line. [82, 83] NCAM may play little to no role in head and neck cancers, perhaps even decreasing a tumor's aggressiveness and increasing the rate of cell loss. [82] NCAM may only begin to play a role in perineural growth in more advanced growth, when tumors begin to exhibit PNS, or only at particular organ sites. [80] Thus NCAM's role in perineural growth remains unclear, and despite findings in other cancers, NCAM should still be explored more thoroughly in HNSCC.
Substance P
Recently, Substance P (SP), a member of the tachykinin family of peptides, has been implicated in perineural growth in pancreatic cancer. [45] Li et al. found that neurite outgrowth from mouse DRG exhibited higher levels of SP, and the pancreatic cancer lines tested also exhibited higher levels of the receptor for SP, neurokinin 1 receptor (NK-1R). When cocultured, the pancreatic cancer cells were more proliferative and invasive, and SP induced expression of MMP-2. NK-1R antagonists blocked all of these effects. [45] SP may play an important role in perineural metastasis in HNSCC, too, though this has not yet been studied. Even so, SP and NK-1R have been shown to be highly expressed in oral SCC, supporting the idea that SP and NK-1R may play a proliferative role in oral SCC in general and in perineural growth. [84]
Nerve Growth Factor Receptor
The low-affinity nerve growth receptor (NGFR), also known as p75NTR, may play an important role in perineural growth of HNSCC given that it can bind with low affinity to a wide variety of ligands. In desmoplastic melanomas, a cancer that is highly neurotropic, NGFR is highly expressed. [85] All spindle cell melanomas in one study stained expressed NGFR in at least 10% of cells, while most expressed it in more than 50% of cells. Melanomas which did not express the neurotropic phenotype all had between 0% and 10% of cells which expressed NGFR. [85] Similarly, NGFR has been found to potentially have a mechanistic role in PNI in malignant melanomas, though NGFR was not found in tested SCCs, whether perineural growth was present or not. [86] Another study found stronger staining for NGFR in four of five PNI-positive cutaneous SCCs perineurally as compared to the rest of the tumor. [61] In pancreatic cancer, too, clarity on the role of NGFR has not been reached, with different studies reporting positive or negative correlations with PNI. [12]
Still, NGFR has complex signaling cascades which can activate both survival and death pathways. [87-89] Different cancers may utilize the receptor in different ways leading to the different phenotypes noted above. Furthermore, NGFR can act as a Trk co-receptor, increasing the affinity for the receptors’ associated ligands. [48] As a result of NGFR being co-expressed with different receptors, different pathways may be activated when binding neurotrophins. [88, 90-92] As described above with other factors, the evidence for mechanisms of NGRF relating to perineural growth in HNSCC remain unclear, and further research into NGFR and its potential role is necessary.
Chemokines
Chemokines are small molecules that, along with their receptors, help to control cell migration. A variety of these chemokine/receptor pairs have been implicated in tumor cell migration. [93] The receptor CXCR4 and its ligand CXCL12 may increase malignancies’ invasiveness, and blockade of CXCR4 may inhibit this invasiveness. [94-96] Katayama et al. found that CXCR4 was significantly correlated with distant metastases in HNSCC patients, and that inhibiting the receptor decreased the cancer cells’ migration and proliferation. [97]
Given the proven relevance of this chemokine/receptor pair to HNSCC, other chemokine/receptor pairs warrant further investigation, too. [98] Indeed, the CX3CR1 receptor has importance in neural cancer adhesive properties, and it has also been found to be elevated in cancers including breast and prostate. [99] In pancreatic cancer, the CX3CR1 receptor is involved in PNI. [99, 100] This receptor has been found in some HNSCC cancer lines, and it may therefore be a promising line of inquiry. [101] Furthermore, HNSCC has been found to express many other chemokines and receptors, particularly the ligand CXCL8 and the receptors CXCR1 and CXCR2 which have been implicated in progression in colon cancer. [101] Even other molecules including SEMA3A, PLXNA1, and NRP1 have been found to be elevated in PDAC, though their role in perineural growth remains unclear. [12, 102] Thus, while there is solid groundwork on which to explore chemokines’ roles in perineural growth of HNSCC, much work remains.
Other Factors
There may also be other factors and receptors which contribute to perineural growth in HNSCC. MMPs other than those mentioned above, for example, may contribute to perineural growth in other cancers and should similarly be explored in HNSCC. [12] Myelin-associated glycoprotein (MAG) and mucin 1 (MUC1) increase metastatic behavior in pancreatic cancer, and binding interactions between the two proteins have been shown to play a significant role in PNI of PDAC. [103, 104] L1 cell adhesion molecule (L1-CAM) is associated with PNI and a poorer prognosis in PDAC, and L1-CAM may also, therefore, warrant exploration in HNSCC. [105] Though other above-mentioned factors and receptors might have greater initial research behind them, these other factors should also be considered when looking at perineural growth in HNSCC, especially given the likely complexity of the interactions involved in such growth.
Conclusion
Perineural growth in HNSCC is indicative of a poorer outcome in patients. It may be that perineural growth itself is simply difficult to treat, that perineural growth is indicative of a more aggressive cancer, or both. This particular type of metastasis is unique and not a path of least resistance. There is evidence that cancer cells can both influence and be influenced by the nerve and its environment through secreted factors. Here, we reviewed several factors that have been explored in perineural cancer growth. More specifically, we looked at if and how these factors have been examined in relation to perineural growth in HNSCC. While many of these factors have not yet been examined with respect to HNSCC, the results, methods, and models discussed above serve as valuable groundwork for further study of these factors in perineural growth in HNSCC.
Much work remains to be done to elucidate the mechanisms of perineural growth in HNSCC. While the evidence remains inconclusive for the factors explored in this paper, the Trk family of receptors and their associated ligands as well as the low-affinity NGFR, are potentially the most promising lines of research.
Highlights.
Tumor growth within the neural space of nerves is called perineural growth
Perineural growth is associated with poor prognosis in HNSCC patients
The mechanisms of HNSCC perineural growth is not well understood
Key molecules associated with perineural growth
Acknowledgement
Department of Otolaryngology, University of Kansas Medical Center and Kansas Intellectual and Developmental Disabilities Center (NICHD HD00258) were the funding sources. Authors acknowledge Mr. Phil Shafer for generating the schematic diagram.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None declared
References
- 1.Skarsgard DP, Groome PA, Mackillop WJ, Zhou S, Rothwell D, Dixon PF, et al. Cancers of the upper aerodigestive tract in Ontario, Canada, and the United States. Cancer. 2000;88:1728–38. doi: 10.1002/(sici)1097-0142(20000401)88:7<1728::aid-cncr29>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–7. [PubMed] [Google Scholar]
- 3.Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96:99–106. doi: 10.1093/jnci/djh014. [DOI] [PubMed] [Google Scholar]
- 4.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 5.Ceyhan GO, Demir IE, Altintas B, Rauch U, Thiel G, Muller MW, et al. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochem Biophys Res Commun. 2008;374:442–7. doi: 10.1016/j.bbrc.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Ayala GE, Dai H, Ittmann M, Li R, Powell M, Frolov A, et al. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;64:6082–90. doi: 10.1158/0008-5472.CAN-04-0838. [DOI] [PubMed] [Google Scholar]
- 7.Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27:5131–7. doi: 10.1200/JCO.2009.22.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirai I, Kimura W, Ozawa K, Kudo S, Suto K, Kuzu H, et al. Perineural invasion in pancreatic cancer. Pancreas. 2002;24:15–25. doi: 10.1097/00006676-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Lu KY. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1:469–76. [PubMed] [Google Scholar]
- 10.Huh JW, Kim HR, Kim YJ. Prognostic value of perineural invasion in patients with stage II colorectal cancer. Ann Surg Oncol. 2010;17:2066–72. doi: 10.1245/s10434-010-0982-7. [DOI] [PubMed] [Google Scholar]
- 11.Horn A, Dahl O, Morild I. Venous and neural invasion as predictors of recurrence in rectal adenocarcinoma. Dis Colon Rectum. 1991;34:798–804. doi: 10.1007/BF02051074. [DOI] [PubMed] [Google Scholar]
- 12.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 13.Boerman RH, Maassen EM, Joosten J, Kaanders HA, Marres HA, van Overbeeke J, et al. Trigeminal neuropathy secondary to perineural invasion of head and neck carcinomas. Neurology. 1999;53:213–6. doi: 10.1212/wnl.53.1.213. [DOI] [PubMed] [Google Scholar]
- 14.Fagan JJ, Collins B, Barnes L, D'Amico F, Myers EN, Johnson JT. Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:637–40. doi: 10.1001/archotol.124.6.637. [DOI] [PubMed] [Google Scholar]
- 15.Tai SK, Li WY, Yang MH, Chu PY, Wang YF. Perineural invasion in T1 oral squamous cell carcinoma indicates the need for aggressive elective neck dissection. Am J Surg Pathol. 2013;37:1164–72. doi: 10.1097/PAS.0b013e318285f684. [DOI] [PubMed] [Google Scholar]
- 16.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–91. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 17.Ong CK, Chong VF. Imaging of perineural spread in head and neck tumours. Cancer Imaging. 2010;10(Spec no A):S92–8. doi: 10.1102/1470-7330.2010.9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panizza B, Warren TA, Solares CA, Boyle GM, Lambie D, Brown I. Histopathological features of clinical perineural invasion of cutaneous squamous cell carcinoma of the head and neck and the potential implications for treatment. Head Neck. 2013 doi: 10.1002/hed.23509. doi: 10.1002/hed.23509 [Epub] [DOI] [PubMed] [Google Scholar]
- 19.Nemzek WR, Hecht S, Gandour-Edwards R, Donald P, McKennan K. Perineural spread of head and neck tumors: how accurate is MR imaging? AJNR Am J Neuroradiol. 1998;19:701–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Yousem DM, Gad K, Tufano RP. Resectability issues with head and neck cancer. AJNR Am J Neuroradiol. 2006;27:2024–36. [PMC free article] [PubMed] [Google Scholar]
- 21.Ginsberg LE. Imaging of perineural tumor spread in head and neck cancer. Semin Ultrasound CT MR. 1999;20:175–86. doi: 10.1016/s0887-2171(99)90018-5. [DOI] [PubMed] [Google Scholar]
- 22.Teymoortash A, Zieger L, Hoch S, Pagenstecher A, Hofer MJ. Distinct microscopic features of perineural invasion in adenoid cystic carcinoma of the head and neck. Histopathology. 2014;64:1037–9. doi: 10.1111/his.12210. [DOI] [PubMed] [Google Scholar]
- 23.Barrett AW, Speight PM. Perineural invasion in adenoid cystic carcinoma of the salivary glands: a valid prognostic indicator? Oral Oncol. 2009;45:936–40. doi: 10.1016/j.oraloncology.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Pinto FR, de Matos LL, Palermo FC, Kulcsar MA, Cavalheiro BG, de Mello ES, et al. Tumor thickness as an independent risk factor of early recurrence in oral cavity squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2014;271:1747–54. doi: 10.1007/s00405-013-2704-9. [DOI] [PubMed] [Google Scholar]
- 25.Hilly O, Shkedy Y, Hod R, Soudry E, Mizrachi A, Hamzany Y, et al. Carcinoma of the oral tongue in patients younger than 30 years: comparison with patients older than 60 years. Oral Oncol. 2013;49:987–90. doi: 10.1016/j.oraloncology.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Sinha P, Hackman T, Nussenbaum B, Wu N, Lewis JS, Jr., Haughey BH. Transoral laser microsurgery for oral squamous cell carcinoma: oncologic outcomes and prognostic factors. Head Neck. 2014;36:340–51. doi: 10.1002/hed.23293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binmadi NO, Basile JR. Perineural invasion in oral squamous cell carcinoma: a discussion of significance and review of the literature. Oral Oncol. 2011;47:1005–10. doi: 10.1016/j.oraloncology.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Mendenhall WM, Amdur RJ, Williams LS, Mancuso AA, Stringer SP, Price Mendenhall N. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 2002;24:78–83. doi: 10.1002/hed.10025. [DOI] [PubMed] [Google Scholar]
- 29.Jennings L, Schmults CD. Management of high-risk cutaneous squamous cell carcinoma. J Clin Aesthet Dermatol. 2010;3:39–48. [PMC free article] [PubMed] [Google Scholar]
- 30.Galloway TJ, Morris CG, Mancuso AA, Amdur RJ, Mendenhall WM. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103:1254–7. doi: 10.1002/cncr.20913. [DOI] [PubMed] [Google Scholar]
- 31.Warden KF, Parmar H, Trobe JD. Perineural spread of cancer along the three trigeminal divisions. J Neuroophthalmol. 2009;29:300–7. doi: 10.1097/WNO.0b013e3181b1b39a. [DOI] [PubMed] [Google Scholar]
- 32.Catalano PJ, Sen C, Biller HF. Cranial neuropathy secondary to perineural spread of cutaneous malignancies. Am J Otol. 1995;16:772–7. [PubMed] [Google Scholar]
- 33.Schmalfuss IM, Tart RP, Mukherji S, Mancuso AA. Perineural tumor spread along the auriculotemporal nerve. AJNR Am J Neuroradiol. 2002;23:303–11. [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Serra A, Hinerman RW, Mendenhall WM, Amdur RJ, Morris CG, Williams LS, et al. Carcinoma of the skin with perineural invasion. Head Neck. 2003;25:1027–33. doi: 10.1002/hed.10334. [DOI] [PubMed] [Google Scholar]
- 35.Daniels DL, Mark LP, Ulmer JL, Mafee MF, McDaniel J, Shah NC, et al. Osseous anatomy of the pterygopalatine fossa. AJNR Am J Neuroradiol. 1998;19:1423–32. [PMC free article] [PubMed] [Google Scholar]
- 36.Dodd GD, Dolan PA, Ballantyne AJ, Ibanez ML, Chau P. The dissemination of tumors of the head and neck via the cranial nerves. Radiol Clin North Am. 1970;8:445–61. [PubMed] [Google Scholar]
- 37.Ginsberg LE, De Monte F, Gillenwater AM. Greater superficial petrosal nerve: anatomy and MR findings in perineural tumor spread. AJNR Am J Neuroradiol. 1996;17:389–93. [PMC free article] [PubMed] [Google Scholar]
- 38.Gandhi D, Gujar S, Mukherji SK. Magnetic resonance imaging of perineural spread of head and neck malignancies. Top Magn Reson Imaging. 2004;15:79–85. doi: 10.1097/01.rmr.0000130601.57619.bd. [DOI] [PubMed] [Google Scholar]
- 39.Mancino M, Ametller E, Gascon P, Almendro V. The neuronal influence on tumor progression. Biochim Biophys Acta. 2011;1816:105–18. doi: 10.1016/j.bbcan.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Dolle L, El Yazidi-Belkoura I, Adriaenssens E, Nurcombe V, Hondermarck H. Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene. 2003;22:5592–601. doi: 10.1038/sj.onc.1206805. [DOI] [PubMed] [Google Scholar]
- 41.Chedotal A, Kerjan G, Moreau-Fauvarque C. The brain within the tumor: new roles for axon guidance molecules in cancers. Cell Death Differ. 2005;12:1044–56. doi: 10.1038/sj.cdd.4401707. [DOI] [PubMed] [Google Scholar]
- 42.Geldof AA, Van Haarst EP, Newling DW. Neurotrophic factors in prostate and prostatic cancer. Prostate Cancer Prostatic Dis. 1998;1:236–41. doi: 10.1038/sj.pcan.4500247. [DOI] [PubMed] [Google Scholar]
- 43.Ricci A, Greco S, Mariotta S, Felici L, Bronzetti E, Cavazzana A, et al. Neurotrophins and neurotrophin receptors in human lung cancer. Am J Respir Cell Mol Biol. 2001;25:439–46. doi: 10.1165/ajrcmb.25.4.4470. [DOI] [PubMed] [Google Scholar]
- 44.Li S, Sun Y, Gao D. Role of the nervous system in cancer metastasis. Oncol Lett. 2013;5:1101–11. doi: 10.3892/ol.2013.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Ma G, Ma Q, Li W, Liu J, Han L, et al. Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Mol Cancer Res. 2013;11:294–302. doi: 10.1158/1541-7786.MCR-12-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- 47.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–62. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 48.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 49.Kowalski PJ, Paulino AF. Perineural invasion in adenoid cystic carcinoma: Its causation/promotion by brain-derived neurotrophic factor. Hum Pathol. 2002;33:933–6. doi: 10.1053/hupa.2002.128249. [DOI] [PubMed] [Google Scholar]
- 50.Miknyoczki SJ, Lang D, Huang L, Klein-Szanto AJ, Dionne CA, Ruggeri BA. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int J Cancer. 1999;81:417–27. doi: 10.1002/(sici)1097-0215(19990505)81:3<417::aid-ijc16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Okugawa Y, Tanaka K, Inoue Y, Kawamura M, Kawamoto A, Hiro J, et al. Brain-derived neurotrophic factor/tropomyosin-related kinase B pathway in gastric cancer. Br J Cancer. 2013;108:121–30. doi: 10.1038/bjc.2012.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yilmaz T, Jiffar T, de la Garza G, Lin H, Milas Z, Takahashi Y, et al. Theraputic targeting of Trk supresses tumor proliferation and enhances cisplatin activity in HNSCC. Cancer Biol Ther. 2010;10:644–53. doi: 10.4161/cbt.10.6.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dudas J, Bitsche M, Schartinger V, Falkeis C, Sprinzl GM, Riechelmann H. Fibroblasts produce brain-derived neurotrophic factor and induce mesenchymal transition of oral tumor cells. Oral Oncol. 2011;47:98–103. doi: 10.1016/j.oraloncology.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kupferman ME, Jiffar T, El-Naggar A, Yilmaz T, Zhou G, Xie T, et al. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene. 2010;29:2047–59. doi: 10.1038/onc.2009.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu L, Werner JA, Mandic R. Implications of tropomyosin-related kinase B (TrkB) in head and neck cancer. Anticancer Res. 2007;27:3121–6. [PubMed] [Google Scholar]
- 56.Descamps S, Pawlowski V, Revillion F, Hornez L, Hebbar M, Boilly B, et al. Expression of nerve growth factor receptors and their prognostic value in human breast cancer. Cancer Res. 2001;61:4337–40. [PubMed] [Google Scholar]
- 57.Descamps S, Toillon RA, Adriaenssens E, Pawlowski V, Cool SM, Nurcombe V, et al. Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J Biol Chem. 2001;276:17864–70. doi: 10.1074/jbc.M010499200. [DOI] [PubMed] [Google Scholar]
- 58.Zhu Z, Friess H, diMola FF, Zimmermann A, Graber HU, Korc M, et al. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol. 1999;17:2419–28. doi: 10.1200/JCO.1999.17.8.2419. [DOI] [PubMed] [Google Scholar]
- 59.Kolokythas A, Cox DP, Dekker N, Schmidt BL. Nerve growth factor and tyrosine kinase A receptor in oral squamous cell carcinoma: is there an association with perineural invasion? J Oral Maxillofac Surg. 2010;68:1290–5. doi: 10.1016/j.joms.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Ye Y, Dang D, Zhang J, Viet CT, Lam DK, Dolan JC, et al. Nerve growth factor links oral cancer progression, pain, and cachexia. Mol Cancer Ther. 2011;10:1667–76. doi: 10.1158/1535-7163.MCT-11-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen-Tsai CP, Colome-Grimmer M, Wagner RF., Jr. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 2004;30:1009–16. doi: 10.1111/j.1524-4725.2004.30306.x. [DOI] [PubMed] [Google Scholar]
- 62.Ketterer K, Rao S, Friess H, Weiss J, Buchler MW, Korc M. Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin Cancer Res. 2003;9:5127–36. [PubMed] [Google Scholar]
- 63.Bouzas-Rodriguez J, Cabrera JR, Delloye-Bourgeois C, Ichim G, Delcros JG, Raquin MA, et al. Neurotrophin-3 production promotes human neuroblastoma cell survival by inhibiting TrkC-induced apoptosis. J Clin Invest. 2010;120:850–8. doi: 10.1172/JCI41013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okada Y, Takeyama H, Sato M, Morikawa M, Sobue K, Asai K, et al. Experimental implication of celiac ganglionotropic invasion of pancreatic-cancer cells bearing c-ret proto oncogene with reference to glial-cell-line-derived neurotrophic factor (GDNF). Int J Cancer. 1999;81:67–73. doi: 10.1002/(sici)1097-0215(19990331)81:1<67::aid-ijc13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 65.Wiesenhofer B, Stockhammer G, Kostron H, Maier H, Hinterhuber H, Humpel C. Glial cell line-derived neurotrophic factor (GDNF) and its receptor (GFR-alpha 1) are strongly expressed in human gliomas. Acta Neuropathol. 2000;99:131–7. doi: 10.1007/pl00007416. [DOI] [PubMed] [Google Scholar]
- 66.Funahashi H, Okada Y, Sawai H, Takahashi H, Matsuo Y, Takeyama H, et al. The role of glial cell line-derived neurotrophic factor (GDNF) and integrins for invasion and metastasis in human pancreatic cancer cells. J Surg Oncol. 2005;91:77–83. doi: 10.1002/jso.20277. [DOI] [PubMed] [Google Scholar]
- 67.Bakst RL, Lee N, He S, Chernichenko N, Chen CH, Linkov G, et al. Radiation impairs perineural invasion by modulating the nerve microenvironment. PLoS One. 2012;7:e39925. doi: 10.1371/journal.pone.0039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iwahashi N, Nagasaka T, Tezel G, Iwashita T, Asai N, Murakumo Y, et al. Expression of glial cell line-derived neurotrophic factor correlates with perineural invasion of bile duct carcinoma. Cancer. 2002;94:167–74. doi: 10.1002/cncr.10169. [DOI] [PubMed] [Google Scholar]
- 69.Zeng Q, Cheng Y, Zhu Q, Yu Z, Wu X, Huang K, et al. The Relationship between Over-expression of Glial Cell-derived Neurotrophic Factor and Its RET Receptor with Progression and Prognosis of Human Pancreatic Cancer. Journal of International Medical Research. 2008;36:656–64. doi: 10.1177/147323000803600406. [DOI] [PubMed] [Google Scholar]
- 70.He S, Chen CH, Chernichenko N, He S, Bakst RL, Barajas F, et al. GFRalpha1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling. Proc Natl Acad Sci U S A. 2014;111:E2008–17. doi: 10.1073/pnas.1402944111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu H, Li X, Xu Q, Lv S, Li J, Ma Q. Role of glial cell line-derived neurotrophic factor in perineural invasion of pancreatic cancer. Biochim Biophys Acta. 2012;1826:112–20. doi: 10.1016/j.bbcan.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 72.Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005;16:441–67. doi: 10.1016/j.cytogfr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 73.Ito Y, Okada Y, Sato M, Sawai H, Funahashi H, Murase T, et al. Expression of glial cell line-derived neurotrophic factor family members and their receptors in pancreatic cancers. Surgery. 2005;138:788–94. doi: 10.1016/j.surg.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 74.Ceyhan GO, Giese NA, Erkan M, Kerscher AG, Wente MN, Giese T, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244:274–81. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chuang JY, Tsai CF, Chang SW, Chiang IP, Huang SM, Lin HY, et al. Glial cell line-derived neurotrophic factor induces cell migration in human oral squamous cell carcinoma. Oral Oncol. 2013;49:1103–12. doi: 10.1016/j.oraloncology.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 76.Song H, Moon A. Glial cell-derived neurotrophic factor (GDNF) promotes low-grade Hs683 glioma cell migration through JNK, ERK-1/2 and p38 MAPK signaling pathways. Neurosci Res. 2006;56:29–38. doi: 10.1016/j.neures.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 77.Fan HX, Li HX, Chen D, Gao ZX, Zheng JH. Changes in the expression of MMP2, MMP9, and ColIV in stromal cells in oral squamous tongue cell carcinoma: relationships and prognostic implications. J Exp Clin Cancer Res. 2012;31:90. doi: 10.1186/1756-9966-31-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luukkaa M, Vihinen P, Kronqvist P, Vahlberg T, Pyrhonen S, Kahari VM, et al. Association between high collagenase-3 expression levels and poor prognosis in patients with head and neck cancer. Head Neck. 2006;28:225–34. doi: 10.1002/hed.20322. [DOI] [PubMed] [Google Scholar]
- 79.Shang J, Sheng L, Wang K, Shui Y, Wei Q. Expression of neural cell adhesion molecule in salivary adenoid cystic carcinoma and its correlation with perineural invasion. Oncol Rep. 2007;18:1413–6. [PubMed] [Google Scholar]
- 80.Vural E, Hutcheson J, Korourian S, Kechelava S, Hanna E. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000;122:717–20. doi: 10.1016/S0194-5998(00)70203-8. [DOI] [PubMed] [Google Scholar]
- 81.Solares CA, Brown I, Boyle GM, Parsons PG, Panizza B. Neural cell adhesion molecule expression: no correlation with perineural invasion in cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2009;31:802–6. doi: 10.1002/hed.21037. [DOI] [PubMed] [Google Scholar]
- 82.Edvardsen K, Bock E, Jirus S, Frandsen TL, Holst-Hansen C, Moser C, et al. Effect of NCAM-transfection on growth and invasion of a human cancer cell line. APMIS. 1997;105:919–30. doi: 10.1111/j.1699-0463.1997.tb05103.x. [DOI] [PubMed] [Google Scholar]
- 83.Owens GC, Orr EA, DeMasters BK, Muschel RJ, Berens ME, Kruse CA. Overexpression of a transmembrane isoform of neural cell adhesion molecule alters the invasiveness of rat CNS-1 glioma. Cancer Res. 1998;58:2020–8. [PubMed] [Google Scholar]
- 84.Brener S, Gonzalez-Moles MA, Tostes D, Esteban F, Gil-Montoya JA, Ruiz-Avila I, et al. A role for the substance P/NK-1 receptor complex in cell proliferation in oral squamous cell carcinoma. Anticancer Res. 2009;29:2323–9. [PubMed] [Google Scholar]
- 85.Iwamoto S, Odland PB, Piepkorn M, Bothwell M. Evidence that the p75 neurotrophin receptor mediates perineural spread of desmoplastic melanoma. J Am Acad Dermatol. 1996;35:725–31. doi: 10.1016/s0190-9622(96)90728-8. [DOI] [PubMed] [Google Scholar]
- 86.Chan MM, Tahan SR. Low-affinity nerve growth factor receptor (P75 NGFR) as a marker of perineural invasion in malignant melanomas. J Cutan Pathol. 2010;37:336–43. doi: 10.1111/j.1600-0560.2009.01349.x. [DOI] [PubMed] [Google Scholar]
- 87.Nykjaer A, Willnow TE, Petersen CM. p75NTR--live or let die. Curr Opin Neurobiol. 2005;15:49–57. doi: 10.1016/j.conb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 88.Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42:529–33. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 89.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–33. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 90.Kume T, Nishikawa H, Tomioka H, Katsuki H, Akaike A, Kaneko S, et al. p75-mediated neuroprotection by NGF against glutamate cytotoxicity in cortical cultures. Brain Res. 2000;852:279–89. doi: 10.1016/s0006-8993(99)02226-x. [DOI] [PubMed] [Google Scholar]
- 91.Yan C, Liang Y, Nylander KD, Wong J, Rudavsky RM, Saragovi HU, et al. p75-nerve growth factor as an antiapoptotic complex: independence versus cooperativity in protection from enediyne chemotherapeutic agents. Mol Pharmacol. 2002;61:710–9. doi: 10.1124/mol.61.4.710. [DOI] [PubMed] [Google Scholar]
- 92.Friedman WJ, Greene LA. Neurotrophin signaling via Trks and p75. Exp Cell Res. 1999;253:131–42. doi: 10.1006/excr.1999.4705. [DOI] [PubMed] [Google Scholar]
- 93.Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–9. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- 94.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–9. [PubMed] [Google Scholar]
- 95.Chen Y, Stamatoyannopoulos G, Song CZ. Down-regulation of CXCR4 by inducible small interfering RNA inhibits breast cancer cell invasion in vitro. Cancer Res. 2003;63:4801–4. [PubMed] [Google Scholar]
- 96.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–9. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 97.Katayama A, Ogino T, Bandoh N, Nonaka S, Harabuchi Y. Expression of CXCR4 and its down-regulation by IFN-gamma in head and neck squamous cell carcinoma. Clin Cancer Res. 2005;11:2937–46. doi: 10.1158/1078-0432.CCR-04-1470. [DOI] [PubMed] [Google Scholar]
- 98.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 99.Marchesi F, Locatelli M, Solinas G, Erreni M, Allavena P, Mantovani A. Role of CX3CR1/CX3CL1 axis in primary and secondary involvement of the nervous system by cancer. J Neuroimmunol. 2010;224:39–44. doi: 10.1016/j.jneuroim.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 100.Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21:77–82. doi: 10.1016/j.cytogfr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 101.Muller A, Sonkoly E, Eulert C, Gerber PA, Kubitza R, Schirlau K, et al. Chemokine receptors in head and neck cancer: association with metastatic spread and regulation during chemotherapy. Int J Cancer. 2006;118:2147–57. doi: 10.1002/ijc.21514. [DOI] [PubMed] [Google Scholar]
- 102.Muller MW, Giese NA, Swiercz JM, Ceyhan GO, Esposito I, Hinz U, et al. Association of axon guidance factor semaphorin 3A with poor outcome in pancreatic cancer. Int J Cancer. 2007;121:2421–33. doi: 10.1002/ijc.22949. [DOI] [PubMed] [Google Scholar]
- 103.Trapp BD, Andrews SB, Cootauco C, Quarles R. The myelin-associated glycoprotein is enriched in multivesicular bodies and periaxonal membranes of actively myelinating oligodendrocytes. The Journal of Cell Biology. 1989;109:2417–26. doi: 10.1083/jcb.109.5.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, Hollingsworth MA. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67:10222–9. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- 105.Ben QW, Wang JC, Liu J, Zhu Y, Yuan F, Yao WY, et al. Positive expression of L1-CAM is associated with perineural invasion and poor outcome in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2010;17:2213–21. doi: 10.1245/s10434-010-0955-x. [DOI] [PubMed] [Google Scholar]
- 106.Dang C, Zhang Y, Ma Q, Shimahara Y. Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J Gastroenterol Hepatol. 2006;21:850–8. doi: 10.1111/j.1440-1746.2006.04074.x. [DOI] [PubMed] [Google Scholar]
- 107.Wang L, Sun M, Jiang Y, Yang L, Lei D, Lu C, et al. Nerve growth factor and tyrosine kinase A in human salivary adenoid cystic carcinoma: expression patterns and effects on in vitro invasive behavior. J Oral Maxillofac Surg. 2006;64:636–41. doi: 10.1016/j.joms.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 108.Gil Z, Cavel O, Kelly K, Brader P, Rein A, Gao SP, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102:107–18. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu H, Ma Q, Xu Q, Lei J, Li X, Wang Z, et al. Therapeutic potential of perineural invasion, hypoxia and desmoplasia in pancreatic cancer. Curr Pharm Des. 2012;18:2395–403. doi: 10.2174/13816128112092395. [DOI] [PMC free article] [PubMed] [Google Scholar]