Abstract
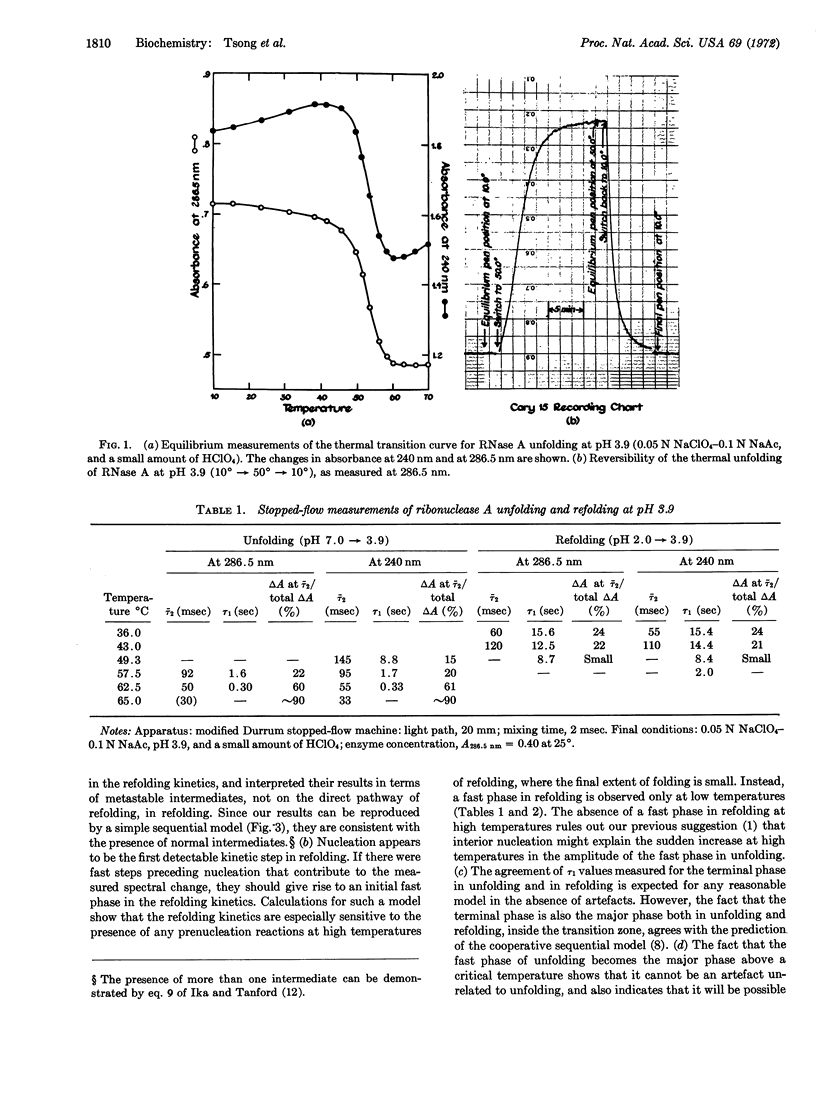
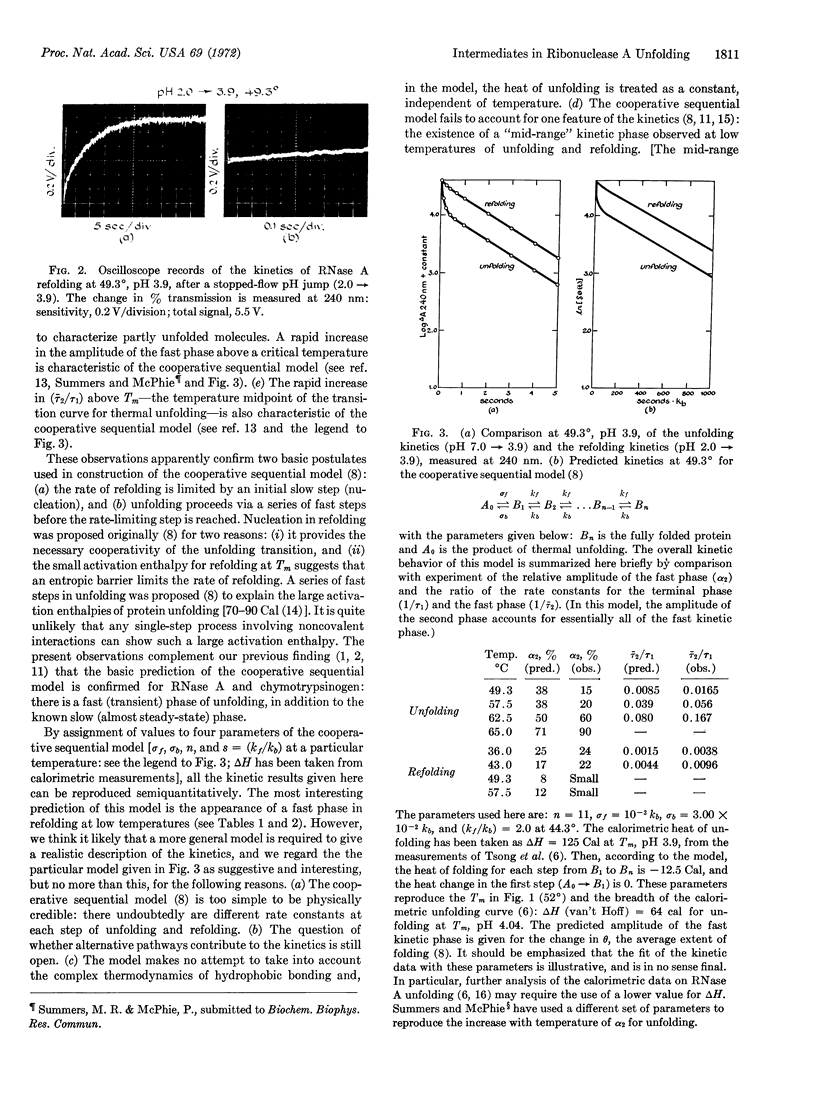
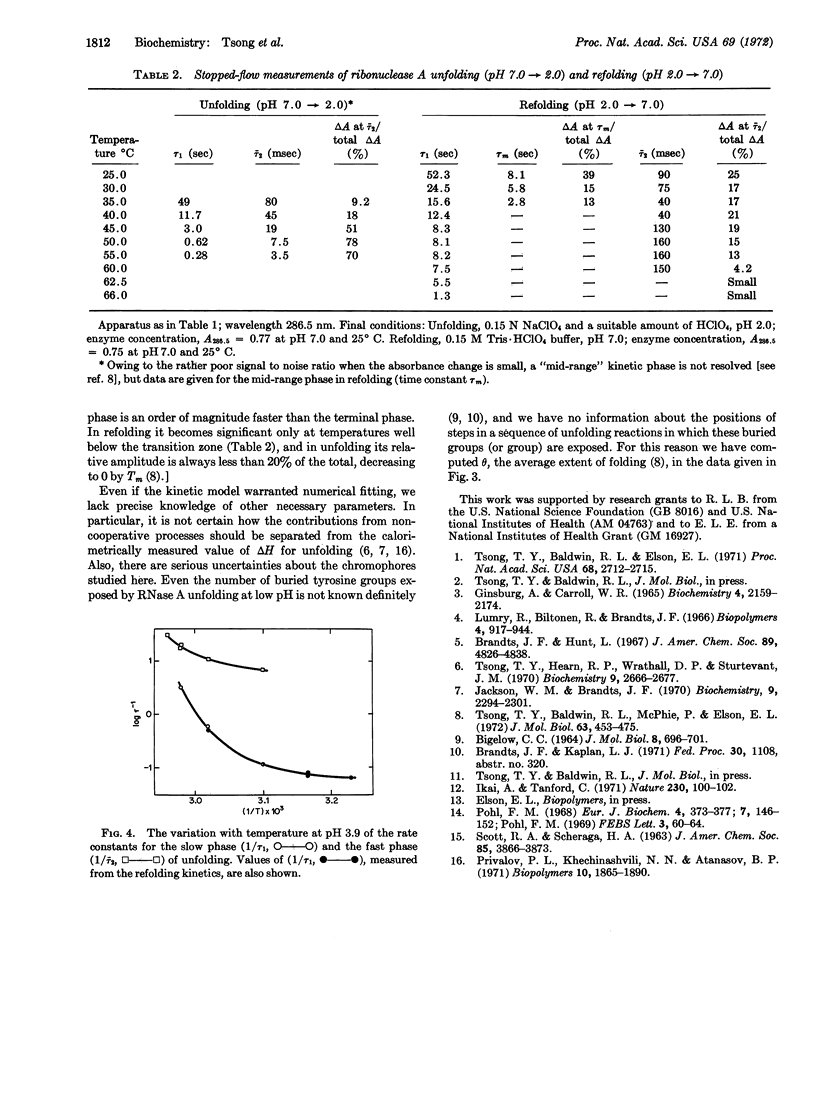
Both the refolding kinetics and unfolding kinetics of ribonuclease A have been measured at the same final conditions, as a function of temperature at pH 3.9, by stopped-flow (pH-jump) experiments; absorbance changes at 240 and 286.5 nm were measured. Refolding follows first-order kinetics in the upper two-thirds of the thermal transition zone. Under the same conditions, the unfolding kinetics are biphasic; the terminal phase has the same rate constant as refolding. The biphasic kinetics of unfolding demonstrate the presence of intermediate states. Since both the refolding and unfolding kinetics are consistent with a simple sequential model, the intermediates satisfy kinetic criteria for being on the direct pathway of unfolding. At temperatures just above the transition zone, the fast phase of unfolding becomes the major kinetic phase. The rate of the slow unfolding reaction increases rapidly with temperature, and approaches the average rate of the fast phase at temperatures just above the transition zone. The entire set of kinetic results can be reproduced semiquantitatively by assignment of values to four parameters in a cooperative sequential model. However, reasons are given for the belief that this simple model will have to be generalized before it can give a realistic description of the kinetics of the unfolding reaction.
Keywords: kinetic intermediates, protein unfolding, nucleation
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGELOW C. C. THE DENATURED STATES OF RIBONUCLEASE. J Mol Biol. 1964 May;8:696–701. doi: 10.1016/s0022-2836(64)80118-2. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Hunt L. The thermodynamics of protein denaturation. 3. The denaturation of ribonuclease in water and in aqueous urea and aqueous ethanol mixtures. J Am Chem Soc. 1967 Sep 13;89(19):4826–4838. doi: 10.1021/ja00995a002. [DOI] [PubMed] [Google Scholar]
- Ikai A., Tanford C. Kinetic evidence for incorrectly folded intermediate states in the refolding of denatured proteins. Nature. 1971 Mar 12;230(5289):100–102. doi: 10.1038/230100a0. [DOI] [PubMed] [Google Scholar]
- Jackson W. M., Brandts J. F. Thermodynamics of protein denaturation. A calorimetric study of the reversible denaturation of chymotrypsinogen and conclusions regarding the accuracy of the two-state approximation. Biochemistry. 1970 May 26;9(11):2294–2301. doi: 10.1021/bi00813a011. [DOI] [PubMed] [Google Scholar]
- Lumry R., Biltonen R. Validity of the "two-state" hypothesis for conformational transitions of proteins. Biopolymers. 1966 Sep;4(8):917–944. doi: 10.1002/bip.1966.360040808. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Einfache Temperatursprung-Methode im Sekunden-bis Stundenbereich und die reversible denaturierung von Chymotrypsin. Eur J Biochem. 1968 Apr;4(3):373–377. doi: 10.1111/j.1432-1033.1968.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. On the kinetics of structural transition I of some pancreatic proteins. FEBS Lett. 1969 Apr;3(1):60–64. doi: 10.1016/0014-5793(69)80097-9. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Khechinashvili N. N., Atanasov B. P. Thermodynamic analysis of thermal transitions in globular proteins. I. Calorimetric study of chymotrypsinogen, ribonuclease and myoglobin. Biopolymers. 1971 Oct;10(10):1865–1890. doi: 10.1002/bip.360101009. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Baldwin R. L. A sequential model of nucleation-dependent protein folding: kinetic studies of ribonuclease A. J Mol Biol. 1972 Feb 14;63(3):453–469. doi: 10.1016/0022-2836(72)90440-8. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Baldwin R. L., Elson E. L. The sequential unfolding of ribonuclease A: detection of a fast initial phase in the kinetics of unfolding. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2712–2715. doi: 10.1073/pnas.68.11.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y., Hearn R. P., Wrathall D. P., Sturtevant J. M. A calorimetric study of thermally induced conformational transitions of ribonuclease A and certain of its derivatives. Biochemistry. 1970 Jun 23;9(13):2666–2677. doi: 10.1021/bi00815a015. [DOI] [PubMed] [Google Scholar]



