Abstract
Dynamic changes in the brain’s lateral ventricles on MRI are powerful biomarkers of disease progression in mild cognitive impairment (MCI) and Alzheimer’s disease (AD). Ventricular measures can represent accumulation of diffuse brain atrophy with very high effect sizes. Despite having no direct role in cognition, ventricular expansion co-occurs with volumetric loss in gray and white matter structures. To better understand relationships between ventricular and cortical changes over time, we related ventricular expansion to atrophy in cognitively-relevant cortical gray matter surfaces, which are more challenging to segment. In ADNI participants, percent change in ventricular volumes at one- (N=677) and two-year (N=536) intervals was significantly associated with baseline cortical thickness and volume in the full sample controlling for age, sex, and diagnosis, and in MCI separately. Ventricular expansion in MCI was associated with thinner GM in frontal, temporal, and parietal regions affected by AD. Ventricular expansion reflects cortical atrophy in early AD, offering a useful biomarker for clinical trials of interventions to slow AD progression.
Keywords: biomarkers, Alzheimer’s disease, mild cognitive impairment, brain imaging, magnetic resonance imaging (MRI), cortical, gray matter, atrophy, thickness, volume, surface area, brain structure, longitudinal
Introduction
As brain tissue is lost in normal aging and dementia, the volume of cerebral spinal fluid (CSF) in the lateral ventricles and surrounding the brain expands to fill the space, within the fixed volume of the skull (Ferrarini et al., 2008, Nestor et al., 2008, Sullivan et al., 2002, Walhovd et al., 2005). The clear tissue contrast between CSF and brain tissues makes it easier to reliably segment and measure the lateral ventricles in standard T1-weighted anatomical MRI scans, even in populations that present challenges for segmentation of other brain structures (Ferrarini et al., 2008, Qiu et al., 2009). The lateral ventricles can be reliably segmented with semi- or fully-automated methods that measure their overall volume (Jack et al., 2004, Nestor et al., 2008, Resnick et al., 2003), shape (Chou, 2007, Ferrarini et al., 2006, Gong, 2011), radial width (Apostolova et al., 2012, Frisoni et al., 2002, Thompson et al., 2004), or boundary shift integral (Ridha et al., 2008, Schott et al., 2005). By comparison, accurate and reliable segmentation of the cortical gray matter surface is somewhat challenging in the brains of older adults, as gray and white matter (GM, WM) contrast decreases with age and the cortical surface may also become increasingly complex and irregular in shape as more brain tissue is lost. Time-consuming manual editing is often required even with the most sophisticated, widely-used cortical GM segmentation packages (Fischl and Dale, 2000, Sanchez-Benavides et al., 2010). Some of these issues can be alleviated by collecting scans with specialized protocols to increase the signal to noise ratio at the cortical boundary. Some researchers advocate averaging two or more MRI scans within participant to improve the accuracy of cortical segmentation, although collecting several scans is not always feasible (Perlman, 2007). Relating expansion of the lateral ventricles to detailed 3D maps of cortical GM thinning takes advantage of a subcortical brain structure that is very easily segmented in standard MRI data from older adults, while also allowing interpretations of the likely cortical changes, which have more direct clinical relevance.
Ventricular measures achieve some of the highest possible effect sizes for tracking longitudinal changes in the human brain. Ventricular volume is a powerful MRI biomarker that has been widely-used in studies of normal aging, mild cognitive impairment (MCI), and Alzheimer’s disease (AD) (Apostolova et al., 2012, Chou, 2007, Ferrarini et al., 2006, Fleisher et al., 2008, Jack et al., 2008, Jack et al., 2004, Jack et al., 2005, Nestor et al., 2008, Qiu et al., 2009, Thompson et al., 2004, Wang et al., 2002). In comparative studies, fewer participants may be needed1 (Hua et al., 2013) to detect statistical effects of disease-modifying interventions in clinical trials using ventricular biomarkers compared to using many other neuroimaging measures. One study demonstrated, for example, that approximately 60 participants are needed to detect a fixed percentage of slowing of ventricular expansion versus 90 participants needed to detect slowing in hippocampal atrophy and 300 participants needed to detect the same proportional slowing of the rate of decline on neuropsychological tests (Ridha et al., 2008).
Larger or expanding ventricles are linked with a broad range of brain-related health factors in older adults, including current cognitive status and future memory decline (Coffey et al., 2001, Murphy et al., 2010), the brain reserve or general resiliency against neurodegeneration (Cavedo et al., 2012), depression, language scores, CSF measures of amyloid beta, APOE genotype (Chou et al., 2010), poorer cardiovascular health (Isaac et al., 2011), vitamin D deficiency (Annweiler et al., 2013), elevated homocysteine levels (Feng et al., 2013), post-operative cognitive dysfunction (Bourne et al., 2012, Kline et al., 2012), decreased survival in dementia (Olesen et al., 2011), and conversion to MCI and AD (Carmichael et al., 2007b, Fleisher et al., 2008, Jack et al., 2004, Nestor et al., 2008).
Although the ventricles provide several practically useful MRI biomarkers, the structure does not play a direct role in cognition. Therefore it is vital to determine how the changes in brain regions of functional and cognitive significance in AD relate to expansion in lateral ventricles. Lateral ventricle expansion co-occurs with degeneration of gray and white matter globally and nearby subcortical regions (Ferrarini et al., 2008, Qiu et al., 2009). By associating ventricular expansion with detailed profiles and patterns in cortical GM thickness, we can make good use of the reliability and ease of ventricular segmentation, relating changes to likely differences in cortical structures that are somewhat more difficult or time-consuming to segment, but which are more directly susceptible to AD-related pathologies.
2. Methods
2.1. Study population
We analyzed participants that underwent high-resolution, T1-weighted structural MRI scanning of the brain, as part of phase 1 of the Alzheimer’s Disease Neuroimaging Initiative (ADNI1). Our sample included only participants listed in the standard set of N=817 baseline, N=685 one-year follow-up, and N=544 two-year follow-up scans obtaining during the ADNI1 phase of data collection that was created to promote rigor and more meaningful comparability across ADNI studies (Wyman et al., 2012). In the ventricle analysis, two baseline scans and one scan from the one-year follow-up time-point were not included, even though they are currently listed in the standard set, because they were added to the list after we had competed data processing. In the cortical GM analysis, all standard participants were processed.
ADNI was launched in 2004 by the National Institutes of Health, the Food and Drug Administration, private pharmaceutical companies, and non-profit organizations to identify and evaluate biomarkers of AD for use in multisite studies. All ADNI data are publicly available at adni.loni.usc.edu. All ADNI studies are conducted in compliance with the Good Clinical Practice guidelines, the Declaration of Helsinki, and the US 21 CFR Part 50–Protection of Human Subjects, and Part 56–Institutional Review Boards. Written informed consent was obtained from all ADNI participants prior to the study. ADNI is a multi-site, longitudinal study of patients with Alzheimer’s disease (AD), mild cognitive impairment (MCI) and healthy older adult controls (HC). Standardized protocols maximize consistency across scan sites.
All individuals received a thorough clinical and cognitive evaluation near the time of their scan. The examination included the mini-mental state examination (MMSE, a standardized and widely-used 30 point questionnaire, with scores of 24–30 typically indicating normal cognition for participants without memory complaints, scores of 24–30 indicating probable MCI for participants with objective memory loss, and scores of 20–26 indicating probable AD (Folstein et al., 1975)) and diagnosis of probable AD, MCI, or cognitively normal. Inclusion and exclusion criteria are detailed in the ADNI protocol (Mueller et al., 2005) and is available online at http://adni.loni.usc.edu/wp-content/uploads/2010/09/ADNI_GeneralProceduresManual.pdf.
2.2. Image acquisition
High-resolution structural MRI scans of the brain were acquired for participants included in the standardized ADNI list (Wyman et al., 2012) on 1.5T scanners from General Electric (Milwaukee, Wisconsin, USA), Siemens (Germany), or Philips (The Netherlands) using a standardized MRI protocol for three-dimensional sagittal magnetization-prepared rapid gradient-echo sequences (Jack et al., 2008).
For lateral ventricle segmentation, we analyzed baseline (N=834), one-year (N=677), and two-year (N=536) follow-up brain MRI scans (1.5-Tesla, T1-weighted 3D MP-RAGE, TR/TE = 2400/1000 ms, flip angle = 8°, slice thickness = 1.2 mm, final voxel resolution = 0.9375 × 0.9375 × 1.2 mm3). Raw MRI scans were pre-processed to reduce signal inhomogeneity and linearly registered to the ICBM template (Mazziotta et al., 2001) (using 9 parameter registration). Percent change was calculated from both follow-ups compared to baseline, resulting in one-year (N=677) and two-year (N=536) percent change in ventricular volume.
For cortical GM segmentation, we analyzed baseline (N=677), one-year (N=646), and two-year (N=507) follow-up brain MRI scans (1.5-Tesla, T1-weighted 3D MP-RAGE, TR/TE = 2400/1000 ms, flip angle = 8°, slice thickness = 1.2 mm, 24-cm field of view, a 192×192×166 acquisition matrix, final voxel resolution = 1.25×1.25×1.2 mm3, later reconstructed to 1 mm isotropic voxels).
The samples analyzed in exploratory general linear models (GLMs) included all participants with the applicable data, resulting in slightly different sample sizes for GLMs using ventricular expansion and clinical variables only compared to the GLMs run on cortical surface maps. The sample analyzed in GLMs on cortical surface maps included participants who had usable data for both cortical GM and percent change in ventricular volume at the appropriate time points (baseline GM: N=677 for associations with one-year percent change in ventricular volume, N=536 for associations with two-year percent change in ventricular volume; one-year follow-up cortical GM: N=646 for associations with one-year percent change in ventricular volume). QC procedures are described below.
2.3. Segmentation of the lateral ventricles
Prior methods for ventricular segmentation have used semi-automated, automated (Chou et al., 2008), and single-atlas or multi-atlas methods (Chou et al., 2009). Here we chose to segment the ventricles with a modified multi-atlas approach described previously (Gutman et al., 2013), which builds on two validated methods developed in our lab (Chou et al., 2008, Leow et al., 2007). The method was tested in a preliminary analysis (Madsen et al., 2013) that we have expanded upon here. Our segmentation approach uses group-wise registration of manually delineated surface templates for point-to-point surface correspondence, and surface-based template blending to yield more accurate results. Similar to the validated method of (Chou et al., 2008), ventricular surfaces were deformed onto each new participant image using an inverse-consistent fluid registration with a mutual information fidelity term. (Leow et al., 2007). The template surfaces were registered as a group using medial-spherical registration and the template that best fits the new boundary was selected for each individual participant independently at each surface point (Gutman et al., 2013), allowing more flexible segmentation, particularly for outliers. All segmentations are represented as one continuous piece connecting the body and horns of the lateral ventricles. In cases where a fissure separates a horn from the main body of the ventricle, as described in (Djamanakova et al., 2013), the method is susceptible to excluding the portion of the lateral ventricle that is visibly separated from the main body in the MR image. Few of our participants would be expect to exhibit this morphology because ventricular expansion would only accentuate the connection to the occipital horn of the lateral ventricle, which should be visible in our participants at the T1 resolution of 1 mm. Therefore, we did not exclude these cases, even though this may result in slightly lower estimates of ventricle volume for those participants. Segmentations were assessed visually for defects from multiple views. All participants passed QC for ventricular segmentations.
2.3. Segmentation of cortical GM
Cortical reconstructions and segmentations were obtained to measure cortical GM thickness and volume at each surface point, using the FreeSurfer image analysis package (v5.0.0), which is documented and freely downloadable online (http://surfer.nmr.mgh.harvard.edu/). Technical details have been described previously (Dale et al., 1999, Fischl and Dale, 2000, Fischl et al., 2002, Fischl et al., 1999a, Fischl et al., 1999b, Fischl et al., 2004, Han et al., 2006). Briefly, the processing pipeline involves removing non-brain tissue, intensity normalization, tessellation of the cortical GM/WM boundary, automated topology correction and surface deformation along intensity gradients to optimally define the cortical surface, alignment of cortical anatomy across individuals via registration to a spherical atlas using individual cortical folding patterns, segmentation of total cortical GM volume for the left and right hemispheres, and creation of 3D maps of GM thickness and volume at each cortical surface point in the left and right hemispheres for each participant. Processed images are in an isotropic space of 256 voxels along each axis (x, y, and z) with a final voxel size of 1 mm3. Six participants at baseline and 35 participants at one-year follow-up were excluded during QC of cortical GM surfaces.
2.4. Statistical analysis: associations with total cortical gray matter volumes
Our main interest was to examine how ventricle expansion related to local differences in cortical GM thickness and volume, which is novel. To confirm the expected relationship between ventricular expansion and overall (global) measures of cortical GM in our data, we also chose to analyze total GM volume - a single value that describes the total amount of cortical GM in each hemisphere. This seemed more appropriate than average cortical GM thickness across each hemisphere. General linear models (GLMs) associating left and right ventricular volumes with total cortical GM volumes were conducted in R (http://www.r-project.org/, R Development Core Team, 2008).
To analyze our cortical surface data, we used a series of GLMs, which have received growing acceptance for the statistical analysis of longitudinal brain imaging data (Bernal-Rusiel et al., 2013). We tested GLMs with outcome variables of either ventricular volume at baseline (N=834), percent change in ventricular volume (relative to baseline volume) after one year (N=677), percent change in ventricular volume after two years (N=536), or total baseline cortical GM volume (N=677) in the left and right hemispheres separately.
Dependent variables included age, sex, and diagnosis (i.e., healthy older adults, MCI, or AD). We also tested GLMs with outcome variables of total and percent change in cortical GM volume at one-year (N=646) and two-year (N=507) follow-ups, using dependent variables of baseline ventricular volumes, age, sex, and diagnosis. Diagnosis was coded using indicator variables for each of the AD and MCI groups, with healthy controls as the reference group. We also ran similar GLMs in each diagnostic group separately and tested for associations between total baseline cortical GM volumes and ventricular volume measures, controlling for the appropriate covariates in the left and right hemispheres. Education was not a significant predictor of ventricular or cortical GM measures in these models, so it was not included as a covariate in subsequent analyses.
Post hoc determination of clinical threshold in ventricular expansion to assist AD classification
Clinically, it would be useful to identify a threshold level of ventricular expansion that best predicts dementia status. Classification algorithms are highly nuanced – often including features from more than one imaging modality – and a full diagnostic classification analysis is beyond the scope of the current study. Instead, we wanted to determine if ventricle expansion rates alone could differentiate AD from controls, and how well. We calculated quantiles (every 10th percentile) for percent change in ventricle volume over both the one-year and two-year intervals, separately. Using these percentiles as classification thresholds, we calculated the sensitivity and specificity of each threshold value for classifying each participant as AD or not AD. For the purposes of the experiment, participants whose percent change in ventricle volume was greater than or equal to the cutoff threshold were classified as AD. The classifications were then compared to the actual AD diagnosis made by ADNI, to generate ROC plots.
2.5. Statistical Analysis: cortical surface maps
Statistical tests were conducted at each point on the left and right cortical surface using the general linear model (GLM) analysis tools in FreeSurfer (mri_glmfit, v5.0.0). Prior to analysis, a smoothing kernel of size 25 mm (full width at half maximum) was applied to each participant’s 3D cortical surface map for GM thickness and volume, separately. We chose a large smoothing kernel because the regions of brain atrophy seen in normal aging and in AD are relatively large (Buckner et al., 2005b, Serra et al., 2010). We did not perform exploratory analyses at different smoothing kernel sizes in this series of analyses, as we wanted to focus on testing our a priori hypotheses involving large regions of the cortical GM surface.
Based on the results of the GLMs using ventricular volume measures and total cortical GM volumes, we tested a series of GLMs for percent change in ventricular volume on baseline cortical GM thickness and volume after: (1) controlling for effects of sex, age, and diagnosis (AD, MCI, or healthy older adults) in all individuals (one-year interval: N=677; two-year interval: N=536), (2) controlling for sex and age in AD, MCI, and control groups, separately (one-year: AD N=142, MCI N=335, Control N=200; two-year: AD N=109, MCI N=251, Control N=176), and (3) controlling for sex and age, in matched groups of N=100 AD, MCI and controls, separately to equalize statistical power. We also tested one-year percent change in ventricular volume for relationships with cortical GM one year after baseline, after controlling for effects of sex, age, and diagnosis. Analyses were run separately for the left and right hemispheres, testing for associations of percent change in left lateral ventricles with left baseline cortical GM at each surface point; and similarly for the right ventricle and right cortical surface. To control the rate of false positives, we enforced a standard false discovery rate (FDR) correction (Benjamini and Hochberg, 1995) for multiple statistical comparisons across all surface vertices on the entire left and right cortical surfaces, using the conventionally accepted false positive rate of 5% (q=0.05).
To examine the magnitude of significant associations in these vertex-wise GLMs, we calculated mean cortical GM thickness or total cortical GM volume within significant regions and obtained beta coefficients using the GLM design setup described above.
3. Results
3.1 Study population characteristics
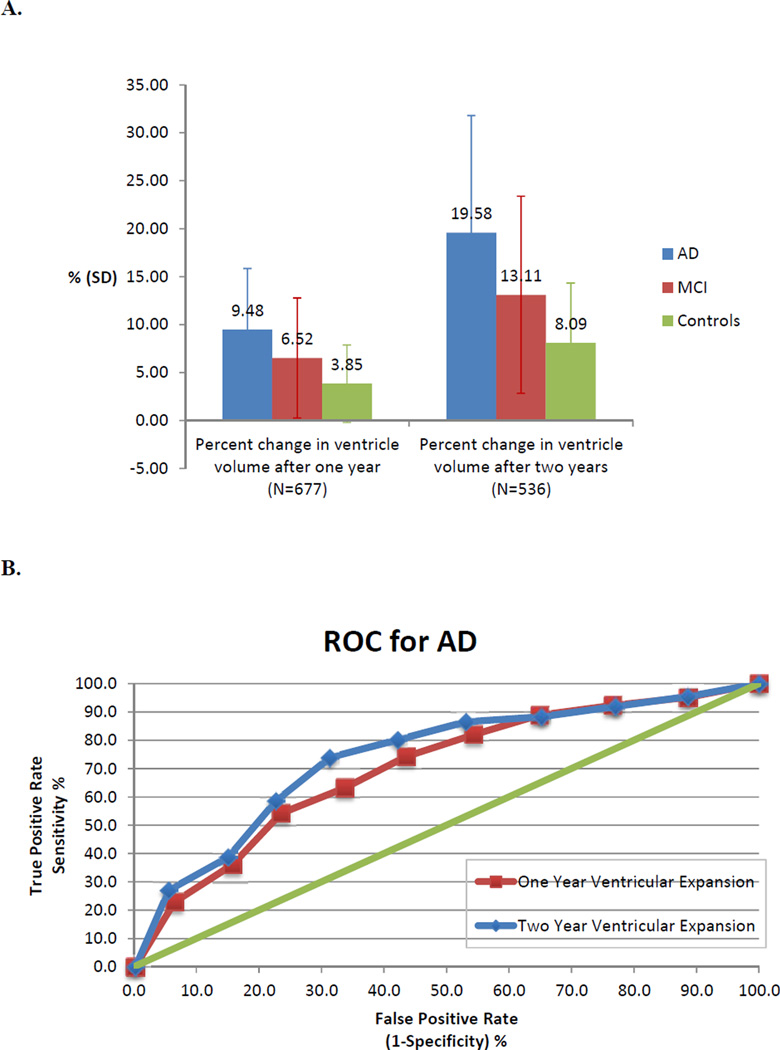
Characteristics of the study population are shown in Table 1. Total GM volumes at baseline were 7.41% lower in AD and 1.77% lower in MCI compared to controls, while ventricular volumes at baseline were 50.6% larger in AD and 18.5% larger in MCI compared to controls, after averaging the left and right hemisphere values. Rates of ventricular expansion also differed between groups, with average percent change between baseline and one-year and baseline and two-years showing a successive increase from controls to MCI to AD. As expected, the ventricles expanded by about twice as much over the two-year interval than they did over the one-year interval, for each of the AD, MCI, and control groups (Figure 1).
Table 1. Demographic characteristics of the N=677 individuals analyzed in the cortical surface maps.
Selected demographic information (mean ± standard deviation). We report the volumes to 3 significant figures, as it is not possible to measure them more accurately.
| AD | MCI | Controls | All Individuals | |
|---|---|---|---|---|
| Sample Size (n) | 142 | 335 | 200 | 677 |
| Sex (women/men) | 67/75 | 124/211 | 95/105 | 286/391 |
| Age (years) | 75 ± 7 | 75 ± 7 | 76 ± 5 | 75 ± 7 |
| MMSE (baseline) | 23 ± 2 | 27 ± 2 | 29 ± 1 | 27 ± 3 |
| Lateral ventricular volume (mm3) | ||||
| Left: | 28,600 ± 13,300 | 22,500 ± 12,300 | 18,800 ± 10,200 | 25,000 ± 12,200 |
| Right: | 26,500 ± 11,900 | 20,800 ± 11,000 | 17,719 ± 8,900 | 22,700 ± 11,000 |
Figure 1. Rates of ventricular expansion across groups and ROC curve for AD.
A. Percent change in ventricular volume compared to baseline after one- and two-year follow-up intervals averaged across left and right hemispheres for AD, MCI, and control groups. As expected, the amount of expansion (since baseline) roughly doubles from the one-year to two-year interval. Without controlling for confounding factors, and perhaps surprisingly, the standard deviations in these measures were large enough that neither the baseline nor percent change measures were significantly different between any pair of groups. Error bars are standard deviation. B. ROC plot is shown for classification of AD based on tenth percentiles of ventricle volume expansion rates over both one-year and two-year intervals.
Looking at the ROC curve for ventricular expansion rates and AD classification, the two-year interval data performs slightly better, achieving a higher true positive classification at any given level of false positives. This might be expected as the brain changes more substantially over a longer interval. From this data alone, it would be difficult to establish a single threshold for ventricular expansion to identify AD in a clinical setting.
3.2. Associations between total cortical GM volume, ventricular volumes, and dementia status
We found the expected relationships between cortical GM volume, ventricle volume, and dementia in our data.
The AD, MCI, and control groups differed significantly in total cortical GM volumes at baseline (AD: p<2×10−16; MCI: p<2×10−16, averaged for left and right with controls as the reference group) and in one-year and two-year percent difference in ventricular volumes (for one-year, AD: p<2×10−16; MCI: p=6.25×10−7; for two years, AD: p<2×10−16; MCI: p=5.17×10−7), after controlling for age and sex. It is noteworthy that the rate measures are much better at differentiating diagnostic groups than the baseline ventricular volumes, which depend on many factors unrelated to the disease (e.g., neurodevelopmental differences). The ROC plots showed that percent change in ventricle volume at both one-year and two-year intervals performed better than chance for all percentiles analyzed (Figure 1).
In these models, AD was associated, on average, with a 17,650 mm3 reduction in total cortical GM volume and amounts of ventricular expansion that were 5.55% greater over the one-year interval and 11.39% greater across the two-year interval compared to controls, on average. MCI was associated with an 8,580 mm3 average reduction in total cortical GM volume and amounts of ventricular expansion that were 2.53% greater for the one-year interval and 4.73% greater across the two-year interval compared to controls, on average.
Percent change in total GM volume is significantly associated with percent change in ventricular volume at one-year (p=2.44×10−9) and two-year (p=4.45×10−12) intervals, in the whole sample after controlling for age, sex, and diagnosis. Considering each group separately, in AD we found that lower total GM volumes at baseline were significantly associated with higher one-year (p=0.022) and two-year (p=0.043) percent expansion in lateral ventricular volume in the left, but not right hemisphere. In MCI, lower total GM volumes at baseline were significantly associated with higher ventricular expansion after two years (left: p=0.046, right: p=0.004), but not after one year. In controls, total GM volume at baseline was not associated with ventricular volume expansion in either interval or hemisphere.
3.2. Mapping ventricular expansion onto the cortical GM surface
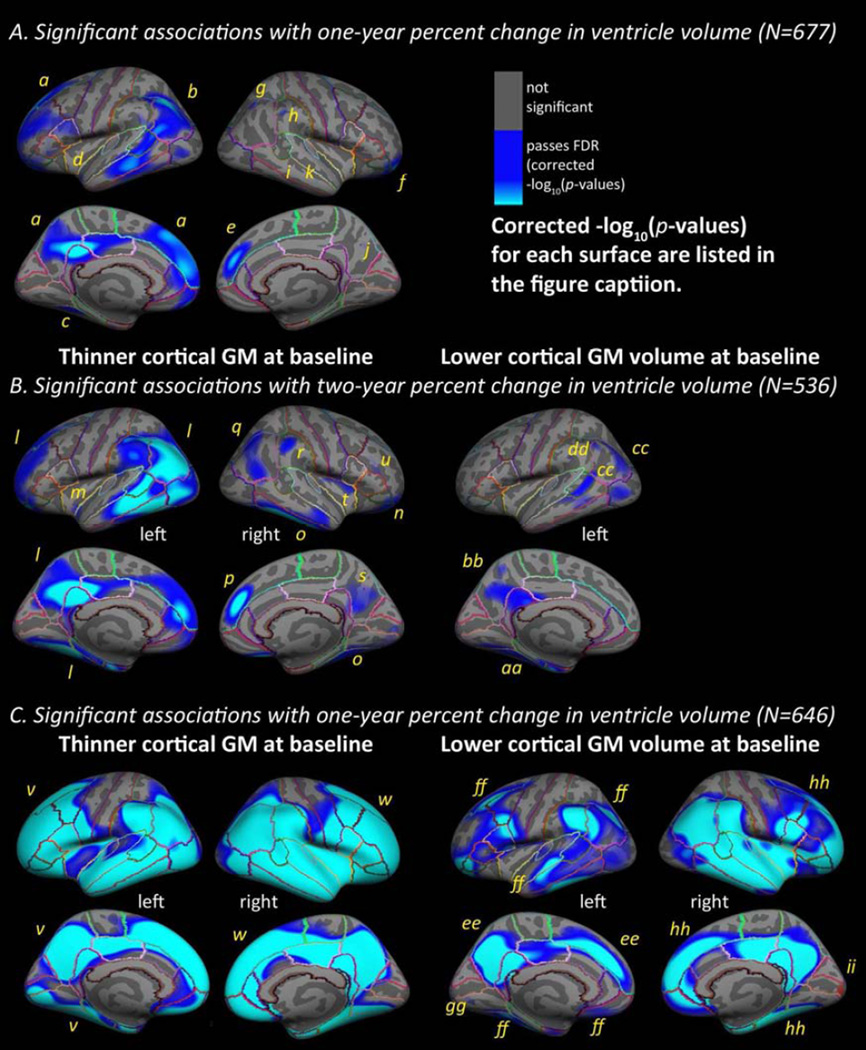
In maps of baseline cortical GM thickness in the full sample, (left panel of Figure 2A and 2B) percent change in ventricular volumes over one-year (N=677) and two-year (N=536) intervals was significantly associated with baseline cortical GM thickness in temporal, inferior and anterior frontal, inferior parietal, and lateral occipital regions, after controlling for age, sex, and diagnosis. The significant regions were somewhat more expansive, in the same areas, for the two-year percent change compared to the one-year percent change in ventricular volume. If we expect ventricular change to be roughly linear over time, these two sets of maps should be identical; however the map for change over a longer interval may have greater signal-to-noise-ratio, increasing the power to detect significant associations.
Figure 2. Ventricular expansion mapped onto cortical GM at baseline and follow-up.
Hemispheric 3D maps in the full sample (A, B) show significant negative associations of one-year (A, N=677) and two-year (B, N=536) percent change in ventricular volume with baseline cortical GM in the full sample (AD, MCI, and healthy older adults pooled) (A, baseline cortical GM thickness and one-year percent change, left: −log10(p-values)=1.77–4.03, right: −log10(p-values)=2.61–4.87; B, baseline cortical GM thickness and two-year change, left: −log10(p-values)=1.62–3.88, right: −log10(p-values)=1.98–4.24, and baseline cortical GM volume and two-year change, left: −log10(p-values)=2.61–4.42, corrected, controlling for age, sex, diagnosis). C shows associations between one-year percent change in ventricular volume (C, N=646) and cortical GM at one-year follow-up in the full sample (C, follow-up cortical GM thickness and one-year percent change, left: −log10(p-values)=1.41–3.67, right: −log10(p-values)=1.39–3.65, follow-up cortical GM volume and one-year percent change, left: −log10(p-values)=1.58–3.84, right: −log10(p-values)=1.48–3.73, corrected, controlling for age, sex, and diagnosis). To correct for multiple comparison, reported t-statistic values correspond to the significance threshold that controls the false discovery rate (FDR) at a q=0.05 threshold across the entire brain surface. Blue represents areas where corrected p-values passed the significance threshold for a negative relationship between longitudinal ventricular enlargement and cortical GM values (as hypothesized, higher rates of ventricular enlargement were associated with less cortical GM at baseline). Significant regions are labeled a-ii
In maps of baseline cortical GM volume in the full sample (right panel of Figure 2B), percent change in ventricular volumes over a two-year (N=536) interval were significantly associated with baseline cortical GM volume in the temporal, inferior parietal regions, and occipital regions, after controlling for age, sex, and diagnosis. No significant associations with baseline cortical GM volume were found for percent change in ventricular volume over a one-year interval (N=677) or in the right hemisphere for the two-year interval.
In follow-up maps of cortical GM thickness in the full sample, percent change in ventricular volumes over one-year (N=646) was significantly associated with cortical GM thickness at one-year follow-up (left panel of Figure 2C) in regions covering most of the cortical surface, except for the primary sensorimotor strip and medial occipital regions, after controlling for age, sex, and diagnosis. As expected, no areas of significant positive associations were found in any tests.
In follow-up maps of cortical GM volume in the full sample (right pane of Figure 2C), percent change in ventricular volumes over a one-year (N=646) interval was significantly associated with cortical GM volume at one-year follow-up in regions covering most of the cortical surface, although to a somewhat lesser extent than in the maps for cortical GM thickness, after controlling for age, sex, and diagnosis. One small region of significant positive association was found in the right occipital pole.
All results presented in this manuscript passed a hemispheric FDR correction at q=0.05. Table 2 lists beta coefficients for significant regions corresponding to Figure 2.
Table 2. Mean values and beta coefficients for regions with significant associations between ventricular expansion and cortical GM.
Cortical GM thickness in mm (mean ± standard deviation) or total cortical GM volume in mm3 (mean ± standard deviation), cluster area (mm2), and beta coefficients are listed for regions shown in Figure 2. The beta coefficient values represent the magnitude of cortical GM thinning (difference in thickness at baseline) associated with each one percent higher rate of ventricle expansion, after adjusting for effects of age, sex, and dementia status in our model.
| GM thickness (mean) |
GM thickness (SD) | Cluster Area (mm2) |
Beta Coefficient |
|
|---|---|---|---|---|
| a | 2.29 | 0.15 | 17480 | −0.0044 |
| b | 2.29 | 0.19 | 11306 | −0.0053 |
| c | 2.18 | 0.24 | 683 | −0.0058 |
| d | 3.16 | 0.40 | 349 | 0.0028 |
| e | 2.54 | 0.22 | 977 | −0.0070 |
| f | 2.39 | 0.20 | 2631 | −0.0059 |
| g | 2.14 | 0.29 | 408 | −0.0060 |
| h | 2.44 | 0.37 | 228 | −0.0073 |
| i | 2.78 | 0.39 | 213 | −0.0061 |
| j | 2.12 | 0.34 | 91 | −0.0048 |
| k | 2.79 | 0.42 | 61 | −0.0015 |
| l | 2.27 | 0.15 | 41533 | −0.0034 |
| m | 2.05 | 0.53 | 28 | −0.0033 |
| n | 2.41 | 0.20 | 2843 | −0.0040 |
| o | 2.33 | 0.17 | 8192 | −0.0030 |
| p | 2.55 | 0.22 | 1266 | −0.0044 |
| q | 2.19 | 0.21 | 2878 | 0.0001 |
| r | 2.36 | 0.26 | 917 | −0.0045 |
| s | 2.16 | 0.19 | 1495 | −0.0027 |
| t | 2.64 | 0.20 | 769 | −0.0032 |
| u | 2.05 | 0.22 | 399 | −0.0021 |
| v | 2.25 | 0.16 | 66530 | −0.0064 |
| w | 2.26 | 0.16 | 67514 | −0.0076 |
| Total GM volume (mean) |
Total GM volume (SD) |
Cluster Area (mm2) |
Beta Coefficient |
|
| aa | 13844 | 2044 | 4139 | −23 |
| bb | 4422 | 678 | 1987 | −13 |
| cc | 13357 | 2148 | 5012 | −45 |
| dd | 1473 | 415 | 497 | −7.0 |
| ee | 20120 | 2474 | 8173 | −69 |
| ff | 95654 | 12357 | 35980 | −37 |
| gg | 501 | 114 | 279 | −2.1 |
| hh | 148381 | 18135 | 54730 | −590 |
| ii | 737 | 64 | 485 | 2.30 |
3.3. Mapping ventricular expansion onto cortical GM in AD, MCI, and healthy older adults
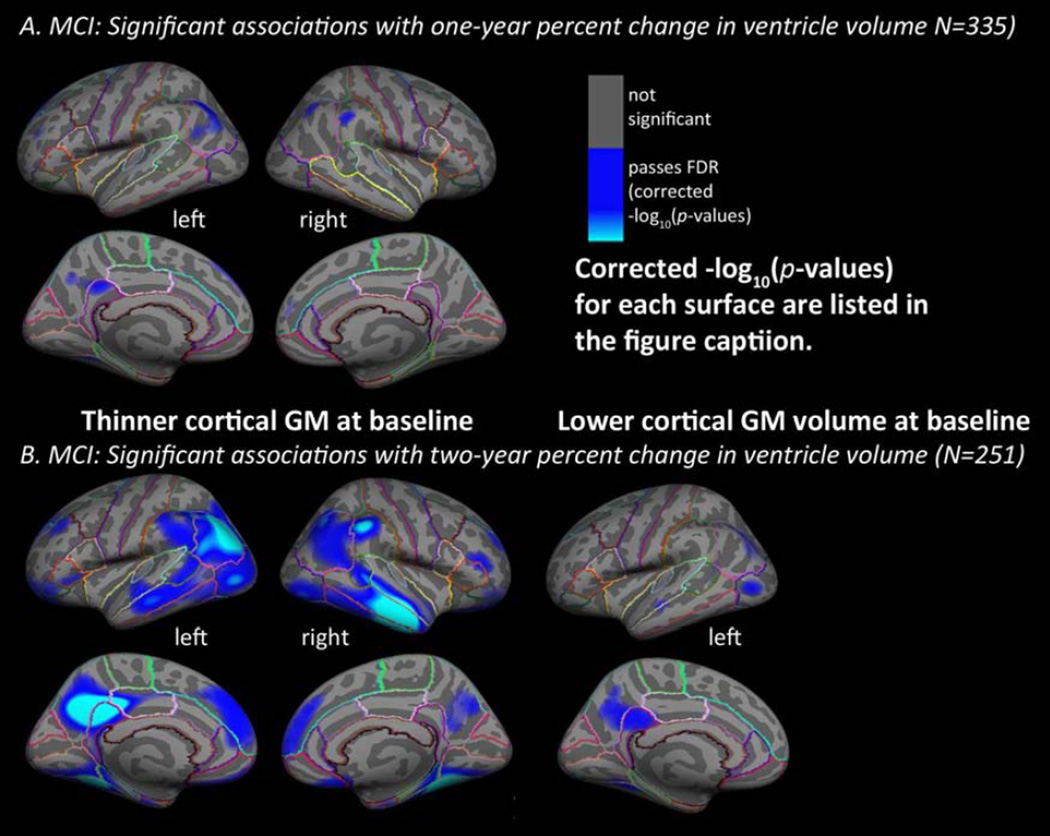
Considering each diagnostic group separately in maps of baseline cortical GM thickness, the one-year and two-year percent change in ventricular volume were significantly associated with baseline cortical GM thickness in MCI only (left pane of Figure 3), with no detectable associations in AD or in healthy older adults. In maps of baseline GM volume, two-year percent change in ventricular volume was significantly associated with baseline cortical GM volume in MCI only (right pane of Figure 3), with no detectable associations for one-year percent change in ventricular volume in MCI or any of the tests in AD or in healthy older adults.
Figure 3. Mapping ventricular expansion onto cortical GM thickness in MCI.
Hemispheric 3D maps show significant negative associations of one-year (A, N=335) and two-year (B, N=251) percent change in ventricular volume with baseline cortical GM in MCI, after controlling for age, sex (A. baseline cortical GM thickness and one-year percent change, left: −log10(p-values)=2.58–4.84, right: −log10(p-values)=3.15–5.41; baseline cortical GM volume and one-year percent change, left and right not significant; B. baseline cortical GM thickness and two-year percent change, left: −log10(p-values)=1.71–3.97, right: −log10(p-values)=1.79–4.04; baseline cortical GM volume and two-year percent change, left: −log10(p-values)=2.55–4.80, right: not significant, FDR corrected as described in Figure 2). No significant results were found for AD or healthy controls separately.
The MCI group has the largest sample size (N=335 for one-year and N=251 for two-year intervals) compared to the AD (N=142 for one-year and N=109 for two-year intervals) and control groups (N=200 for one-year and N=176 for two-year intervals), suggesting that the lower power could have contributed to the lack of results in AD and controls (this point is addressed in the section 3.4). In MCI, small regions of thinner baseline cortical GM - in the bilateral superior frontal and inferior parietal gyri, left precuneus and isthmus of the cingulate, and right supramarginal gyri - were significantly associated with one-year percent change in ventricular volume (left: −log10(p-values)=2.58–4.84, right: −log10(p-values)=3.15–5.41, corrected, controlling for age, sex). The maps associating baseline cortical GM thickness with two-year percent change in ventricular volume in MCI were much more expansive, with significant relationships detected in the bilateral superior, middle, and inferior temporal, lateral occipital, inferior and some superior parietal, supramarginal, rostral inferior, middle, and superior frontal gyri, the precuneus, posterior cingulate, medial orbitofrontal, entorhinal, parahippocampal, fusiform, and lingual gyri (left: −log10(p-values)=1.71–3.97, right: −log10(p-values)=1.79–4.04, corrected, controlling for age, sex), which matches the well-known pattern of AD-related pathology (Braak and Braak, 1991, Braskie et al., 2010, Thompson et al., 2003).No significant associations were found in the AD or healthy control groups. As expected, no areas of significant positive associations were found for any group.
We felt it was important to determine if significant results were found only in MCI, which had a larger N compared to the AD and control groups, due to differences in power related to the available sample sizes. After limiting the samples to equal-sized subsets of size N=100 (the approximate size of the smallest group) that were matched for age and sex, the data was re-analyzed for AD, MCI, and healthy older adults. For cortical thickness, significant negative associations were found in MCI for two-year percent change in left ventricular volume related to baseline cortical GM thickness in left superior temporal, supramarginal, inferior and superior parietal, lateral occipital, fusiform, precuneus, posterior cingulate, and small regions in the superior frontal gyri (left: −log10(p-values)=2.05–4.31, controlling for age, sex). No significant results were found for cortical thickness in AD or controls at either time interval or in the one-year interval for MCI using the smaller and equally sized samples. For cortical volume, no significant results were found in any group at either time interval. No significant positive associations were found.
4. Discussion
Our results add to the current literature that identifies changes in ventricular enlargement as a robust biomarker of AD. We make a novel contribution to the field, by showing how longitudinal changes in ventricular volume relate to specific patterns of thinner cortical GM in early stages of this neurodegenerative disorder. These results allow us to make connections between possible atrophy in functionally important cortical areas, and more obvious changes in ventricular segmentations. Ventricular measures are among the most reliable and robust MRI measures for tracking the progression of AD, but are naturally limited in terms of making specific inferences about brain structure and function. Since cortical regions can be challenging or time-consuming to segment in the brains of older adults, combining information about the cortical architecture and ventricular enlargement gives us a better understanding of early stages of neurodegenerative disease.
Interestingly, the pattern of significant associations in MCI matches the well-known pattern of progressive cortical atrophy in AD (see, e.g. Figure 6 in (Thompson et al., 2003)) and in MCI to AD conversion (see, e.g. Figures 2–4 in (Whitwell et al., 2007)), pathological amyloid plaque and tau neurofibrillary tangle deposition, metabolic disruption, and functional disconnectivity (see, e.g., Figure 6 in (Buckner et al., 2005a)) characteristic of AD progression. Primary sensorimotor areas that may not experience heavy disease burden, did not show significant associations in our MCI sample or in the whole sample. These are also relatively difficult cortical areas for measuring GM thickness as the extensive myelination can lead to poor tissue contrast on standard anatomical MRI. When diagnostic groups of the same sample size were studied, in an attempt to equalize statistical power to detect associations, longitudinal ventricular expansion was most strongly associated with thinner cortical GM in the early stages of disease progression, rather than in healthy older adults or in more severely impaired individuals.
In healthy older adults, the lateral ventricles expand approximately linearly with age, and the changes are accompanied by a progressive decrease in total brain volume (Blatter et al., 1995). Enlarged ventricles are commonly observed even in very healthy older adults (Longstreth, 1998). In cross-sectional studies, average ventricular volumes are largest in AD and MCI compared to healthy older adults (in AD, roughly 60% larger than in MCI and roughly 4 times larger than in controls) (Nestor et al., 2008). However, this measure is not ideal for diagnostic classification, as cross-sectional ventricular volumes overlap substantially between healthy older adults and AD participants (AD: 20–146 cm3, CON: 11–82 cm3) (Wang et al., 2002). This makes it vital to consider longitudinal patterns and rates of ventricular expansion. Interestingly, ventricular volumes are larger in AD compared to healthy centenarians, who did not differ from healthy 80–90 year old adults in one study (Gong, 2011). This suggests that the accelerated expansion of ventricular volumes in AD does eventually result in cross-sectional differences that are large enough to distinguish from normal aging.
Expansion of the lateral ventricles reflects the accumulation of brain tissue reductions globally, throughout multiple brain regions, in which rates of change may be too subtle or more challenging to detect directly. In normal aging, rates of ventricular enlargement accelerate around age sixty, then continue at a stable pace at least into the late nineties (Jernigan et al., 2001, Walhovd et al., 2005). In healthy older adults, the annual increase of 3–13% (approximately 1500 mm3) in ventricular volume represents a change of less than 0.5% in total brain volume and is much greater than the <1–3% volume reduction seen in the hippocampus (Fjell et al., 2009b, Resnick et al., 2000, Resnick et al., 2003, Ridha et al., 2008, Zhang et al., 2010). Perhaps because this measure is so powerful, many studies have concluded that ventricular measures are more strongly associated than temporal lobe structures with hallmark features of dementia such as baseline cognition, change in global clinical scores and dementia ratings over time, and plaque and tangle accumulation (Fjell et al., 2009a, Jack et al., 2004, Ridha et al., 2008, Silbert et al., 2003).
In our data we also found a greater number of significant associations and larger extent of cortical regions associated with ventricular volume expansion for cortical GM thickness compared to cortical GM volume. Lateral ventricle expansion may co-occur with patterns of local cortical GM thinning specifically, rather than the loss of cortical GM volume locally. Cortical GM thickness may be a more sensitive measure of global brain atrophy in older adults, compared to cortical GM volume. Cortical GM volume may be a noisier measure as it is calculated using cortical surface area, which is quite variable across individuals and may be less strongly related to disease- or age-related loss of brain tissue in older adults, in combination with cortical GM thickness, which may be less variable.
Due to the overlap in ventricular volumes among diagnostic groups, accelerated rates of brain atrophy may be better indicators of MCI and AD. Rates of ventricular enlargement distinguish AD from healthy older adults, even when cross-sectional ventricular volumes overlap between the two groups (Wang et al., 2002). The most extreme acceleration in the rate of ventricular expansion may occur in MCI and at the point of conversion from MCI to AD (Apostolova et al., 2012, Carmichael et al., 2007a, Jack et al., 2008, Ridha et al., 2008), while accumulated total volumes and expansion rates are most extreme later in the disease (Wahlund et al., 1993). Faster rates of ventricular expansion also independently predict cognitive decline (Adak et al., 2004), and are more predictive than other MRI measures in detecting early conversion to AD (Fleisher et al., 2008, Jack et al., 2005).
As far as we know, all previous joint studies of cortical gray matter and the lateral ventricles have been conducted using region of interest summary measures for volumes or thickness. Prior studies have reported the co-occurrence of significant associations between ventricle and cortical measures with factors such as age (Coffey et al., 1992, Fjell et al., 2009b, Long et al., 2012) and memory decline (Murphy et al., 2010) in healthy older adults, and with APOE genotype in MCI and AD (Liu et al., 2010). One study directly examined the correlation between whole brain mean cortical thickness and lateral ventricle “width” in normal aging, finding a significant and strong correlation of approximately r= −0.4 (Preul et al., 2006). It makes sense that lateral ventricle expansion might be associated with cortical thickness in older adults; we believe this is the first paper to report these associations using detailed 3D maps of the cortical surface.
One limitation of the current study is that we used an automated segmentation for cortical gray matter thickness and lateral ventricle volume. These automated methods have been validated, but there is some error involved in using any segmentation tool - in both manual and automated segmentations. We chose automated methods for practical reasons given the large sample size in our analyses. Also, we used ventricle volume measures rather than ventricle shape, which arguably may be a more sensitive biomarker even though it is perhaps not quite as intuitive (Thompson et al., 2004). It would also be interesting to apply the reverse approach - i.e., mapping cortical GM measures (average and change in GM thickness in regions of interest, for example) onto 3D ventricular shapes, to see exactly which areas of the ventricles are most strongly associated with cortical thickness changes, at different stages of the illness. In their study, Ferrarini et al., 2006, reported that 22% of the ventricular surface was “significantly different” between AD and healthy older adults, although this number will vary depending on sample size. Even so, the frontal and temporal horns are most sensitive to AD progression in this study and others (Apostolova et al., 2012, Thompson et al., 2004). If significant associations are found between cortical GM and ventricular shape at later stages of AD, we might expect to see expansion of the frontal horns of the lateral ventricle associated with thinner frontal GM, which is typically affected later in the disease.
Acknowledgments
This research was also supported by a National Defense Science and Engineering Graduate (NDSEG) Fellowship (32 CFR 168a), to S.K.M., from the DoD, and Air Force Office of Scientific Research. Algorithm development and image analysis for this study was funded, in part, by grants to PT from the NIBIB (R01 EB008281, R01 EB008432) and by the NIA, NIBIB, NIMH, the National Library of Medicine, and the National Center for Research Resources (AG016570, AG040060, EB01651, MH097268, LM05639, RR019771 to PT). Data collection and sharing for this project was funded by ADNI (NIH Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through contributions from the following: Abbott; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. This research was also supported by NIH grants P30 AG010129 and K01 AG030514 from the National Institute of General Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Head-to-head comparisons of effect sizes for brain biomarkers require many caveats, as therapeutic interventions may affect each biomarker differently. Also, simply reducing the numerical rate of change for different biomarkers many have different functional or clinical consequences for the patient. Some of these issues are discussed in Hua et al., 2013.
Disclosure Statement: The authors have no potential financial or personal conflicts of interest including relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence this work.
References
- Adak S, Illouz K, Gorman W, Tandon R, Zimmerman EA, Guariglia R, Moore MM, Kaye JA. Predicting the rate of cognitive decline in aging and early Alzheimer disease. Neurology. 2004;63(1):108–114. doi: 10.1212/01.wnl.0000132520.69612.ab. [DOI] [PubMed] [Google Scholar]
- Annweiler C, Montero-Odasso M, Hachinski V, Seshadri S, Bartha R, Beauchet O. Vitamin D concentration and lateral cerebral ventricle volume in older adults. Mol Nutr Food Res. 2013;57(2):267–276. doi: 10.1002/mnfr.201200418. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Green AE, Babakchanian S, Hwang KS, Chou YY, Toga AW, Thompson PM. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI), and Alzheimer Disease. Alzheimer Dis Assoc Disord. 2012;26(1):17–27. doi: 10.1097/WAD.0b013e3182163b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- Bernal-Rusiel JL, Greve DN, Reuter M, Fischl B, Sabuncu MR, Initi AsDN. Statistical analysis of longitudinal neuroimage data with Linear Mixed Effects models. Neuroimage. 2013;66:249–260. doi: 10.1016/j.neuroimage.2012.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Parker N, Kurth S, Horn SD. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR Am J Neuroradiol. 1995;16(2):241–251. [PMC free article] [PubMed] [Google Scholar]
- Bourne SK, Conrad A, Konrad PE, Neimat JS, Davis TL. Ventricular width and complicated recovery following deep brain stimulation surgery. Stereotact Funct Neurosurg. 2012;90(3):167–172. doi: 10.1159/000338094. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Klunder AD, Hayashi KM, Protas H, Kepe V, Miller KJ, Huang SC, Barrio JR, Ercoli LM, Siddarth P, Satyamurthy N, Liu J, Toga AW, Bookheimer SY, Small GW, Thompson PM. Plaque and tangle imaging and cognition in normal aging and Alzheimer's disease. Neurobiol Aging. 2010;31(10):1669–1678. doi: 10.1016/j.neurobiolaging.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005a;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005b;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, Lee SE, Lee JY, Aizenstein HJ, Meltzer CC, Liu Y, Toga AW, Becker JT. Cerebral ventricular changes associated with transitions between normal cognitive function, mild cognitive impairment, and dementia. Alzheimer Dis Assoc Disord. 2007a;21(1):14–24. doi: 10.1097/WAD.0b013e318032d2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, Lee SE, Lee JY, Aizenstein HJ, Meltzer CC, Liu Y, Toga AW, Becker JT. Ventricular volume and dementia progression in the Cardiovascular Health Study. Neurobiol Aging. 2007b;28(3):389–397. doi: 10.1016/j.neurobiolaging.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavedo E, Galluzzi S, Pievani M, Boccardi M, Frisoni GB. Norms for imaging markers of brain reserve. J Alzheimers Dis. 2012;31(3):623–633. doi: 10.3233/JAD-2012-111817. [DOI] [PubMed] [Google Scholar]
- Chou YY, Lepore N, Avedissian C, Madsen SK, Parikshak N, Hua X, Shaw LM, Trojanowski JQ, Weiner MW, Toga AW, Thompson PM, Initia ADN. Mapping correlations between ventricular expansion and CSF amyloid and tau biomarkers in 240 subjects with Alzheimer's disease, mild cognitive impairment and elderly controls. Neuroimage. 2009;46(2):394–410. doi: 10.1016/j.neuroimage.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YY, Lepore N, de Zubicaray GI, Cannichael OT, Becker JT, Toga AW, Thompson PM. Automated ventricular mapping alignment reveals genetic effects with multi-atlas fluid image in Alzheimer's disease. Neuroimage. 2008;40(2):615–630. doi: 10.1016/j.neuroimage.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YY, Lepore N, Saharan P, Madsen SK, Hua X, Jack CR, Shaw LM, Trojanowski JQ, Weiner MW, Toga AW, Thompson PM, Initi AsDN. Ventricular maps in 804 ADNI subjects: correlations with CSF biomarkers and clinical decline. Neurobiol Aging. 2010;31(8):1386–1400. doi: 10.1016/j.neurobiolaging.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YY, Lepore N, de Zubicaray GI, Rose SE, Carmichael OT, Becker JT, Toga AW, Thompson PM. Automated 3D mapping and shape analysis of the lateral ventricles via fluid registration of multiple surface-based atlases. 2007 4th IEEE International Symposium on Biomedical Imaging: Macro to Nano. 2007:1288–1291. [Google Scholar]
- Coffey CE, Ratcliff G, Saxton JA, Bryan RN, Fried LP, Lucke JE. Cognitive correlates of human brain aging: A quantitative magnetic resonance imaging investigation. J Neuropsych Clin N. 2001;13(4):471–485. doi: 10.1176/jnp.13.4.471. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Wilkinson WE, Parashos IA, Soady SAR, Sullivan RJ, Patterson LJ, Figiel GS, Webb MC, Spritzer CE, Djang WT. Quantitative Cerebral Anatomy of the Aging Human Brain - a Cross-Sectional Study Using Magnetic-Resonance-Imaging. Neurology. 1992;42(3):527–536. doi: 10.1212/wnl.42.3.527. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis - I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Djamanakova A, Faria AV, Hsu J, Ceritoglu C, Oishi K, Miller MI, Hillis AE, Mori S. Diffeomorphic brain mapping based on T1-weighted images: Improvement of registration accuracy by multichannel mapping. J Magn Reson Imaging. 2013;37(1):76–84. doi: 10.1002/jmri.23790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Isaac V, Sim S, Ng TP, Krishnan KRR, Chee MWL. Associations Between Elevated Homocysteine, Cognitive Impairment, and Reduced White Matter Volume in Healthy Old Adults. Am J Geriat Psychiat. 2013;21(2):164–172. doi: 10.1016/j.jagp.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Ferrarini L, Palm WM, Olofsen H, van Buchem MA, Reiber JH, Admiraal-Behloul F. Shape differences of the brain ventricles in Alzheimer's disease. Neuroimage. 2006;32(3):1060–1069. doi: 10.1016/j.neuroimage.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Ferrarini L, Palm WM, Olofsen H, van der Landen R, van Buchem MA, Reiber JH, Admiraal-Behloul F. Ventricular shape biomarkers for Alzheimer's disease in clinical MR images. Magn Reson Med. 2008;59(2):260–267. doi: 10.1002/mrm.21471. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. P Natl Acad Sci USA. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Amlien IK, Westlye LT, Walhovd KB. Mini-Mental State Examination Is Sensitive to Brain Atrophy in Alzheimer's Disease. Dement Geriatr Cogn. 2009a;28(3):252–258. doi: 10.1159/000241878. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci. 2009b;29(48):15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Sun S, Taylor C, Ward CP, Gamst AC, Petersen RC, Jack CR, Aisen PS, Thal LJ, Study As.D.C. Volumetric MRI vs clinical predictors of Alzheimer disease in mild cognitive impairment. Neurology. 2008;70(3):191–199. doi: 10.1212/01.wnl.0000287091.57376.65. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Geroldi C, Beltramello A, Bianchetti A, Binetti G, Bordiga G, DeCarli C, Laakso MP, Soininen H, Testa C, Zanetti O, Trabucchi M. Radial width of the temporal horn: A sensitive measure in Alzheimer disease. Am J Neuroradiol. 2002;23(1):35–347. [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Lu J, Chen J, Wang Y, Yuan Y, Zhang T, Guo L, Miller LS and the Georgia Centenarian Study. Ventricle Shape Analysis for Centenarians, Elderly Subjects, MCI and AD Patients. MBIA. 2011:84–92. [Google Scholar]
- Gutman BA, Hua X, Rajagopalan P, Chou YY, Wang Y, Yanovsky I, Toga AW, Jack CR, Jr, Weiner MW, Thompson PM. Maximizing power to track Alzheimer's disease and MCI progression by LDA-based weighting of longitudinal ventricular surface features. Neuroimage. 2013;70:386–401. doi: 10.1016/j.neuroimage.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hua X, Hibar DP, Ching CR, Boyle CP, Rajagopalan P, Gutman BA, Leow AD, Toga AW, Jack CR, Jr, Harvey D, Weiner MW, Thompson PM. Unbiased tensor-based morphometry: improved robustness and sample size estimates for Alzheimer's disease clinical trials. Neuroimage. 2013;66:648–661. doi: 10.1016/j.neuroimage.2012.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac V, Sim S, Zheng H, Zagorodnov V, Tai ES, Chee M. Adverse associations between visceral adiposity, brain structure, and cognitive performance in healthy elderly. Front Aging Neurosci. 2011:3. doi: 10.3389/fnagi.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack Weigand SD, Shiung MM, Przybelski SA, O'Brien PC, Gunter JL, Knopman DS, Boeve BF, Smith GE, Petersen RC. Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology. 2008;70(19):1740–1752. doi: 10.1212/01.wnl.0000281688.77598.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, J LW, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Shiung MM, Gunter JL, O'Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62(4):591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65(8):1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22(4):581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kline RP, Pirraglia E, Cheng H, De Santi S, Li Y, Haile M, de Leon MJ, Bekker A. Surgery and brain atrophy in cognitively normal elderly subjects and subjects diagnosed with mild cognitive impairment. Anesthesiology. 2012;116(3):603–612. doi: 10.1097/ALN.0b013e318246ec0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow AD, Yanovsky I, Chiang MC, Lee AD, Klunder AD, Lu A, Becker JT, Davis SW, Toga AW, Thompson PM. Statistical properties of Jacobian maps and the realization of unbiased large-deformation nonlinear image registration. Ieee T Med Imaging. 2007;26(6):822–832. doi: 10.1109/TMI.2007.892646. [DOI] [PubMed] [Google Scholar]
- Liu YW, Paajanen T, Westman E, Zhang Y, Wahlund LO, Simmons A, Tunnard C, Sobow T, Proitsi P, Powell J, Mecocci P, Tsolaki M, Vellas B, Muehlboeck S, Evans A, Spenger C, Lovestone S, Soininen H, Consortium A. APOE epsilon 2 Allele Is Associated with Larger Regional Cortical Thicknesses and Volumes. Dement Geriatr Cogn. 2010;30(3):229–237. doi: 10.1159/000320136. [DOI] [PubMed] [Google Scholar]
- Long XJ, Liao WQ, Jiang CX, Liang D, Qiu BS, Zhang LJ. Healthy Aging: An Automatic Analysis of Global and Regional Morphological Alterations of Human Brain. Acad Radiol. 2012;19(7):785–793. doi: 10.1016/j.acra.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Longstreth WTJ. Brain abnormalities in the elderly: frequency and predictors in the United States (the Cardiovascular Health Study) J Neural Transm-Supp. 1998;(53):9–16. doi: 10.1007/978-3-7091-6467-9_2. [DOI] [PubMed] [Google Scholar]
- Madsen SK, Gutman BA, Joshi SH, Toga AW, Jack CR, Jr, Weiner MW, Thompson PM for the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Mapping dynamic changes in ventricular volume onto the cortical surface in normal aging, MCI, and Alzheimer’s disease; The 16th International Conference on Medical Imaging Computing and Computer Assisted Intervention.2013. [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos T R Soc B. 2001;356(1412):1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, Trojanowski JQ, Toga AW, Beckett L. Ways toward an early diagnosis in Alzheimer's disease: the Alzheimer's Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1(1):55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EA, Holland D, Donohue M, McEvoy LK, Hagler DJ, Dale AM, Brewer JB, Neuroimaging As.D. Six-month atrophy in MTL structures is associated with subsequent memory decline in elderly controls. Neuroimage. 2010;53(4):1310–1317. doi: 10.1016/j.neuroimage.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor SM, Rupsingh R, Borrie M, Smith M, Accomazzi V, Wells JL, Fogarty J, Bartha R, Initi AsDN. Ventricular enlargement as a possible measure of Alzheimers disease progression validated using the Alzheimers disease neuroimaging initiative database. Brain. 2008;131:2443–2454. doi: 10.1093/brain/awn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Guo X, Gustafson D, Borjesson-Hanson A, Sacuiu S, Eckerstrom C, Bigler ED, Skoog I. A population-based study on the influence of brain atrophy on 20-year survival after age 85. Neurology. 2011;76(10):879–886. doi: 10.1212/WNL.0b013e31820f2e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D. Cortical Thickness: Practicalities and Comparisons. University of Wisconsin Statistics 692 project. 2007 [Google Scholar]
- Preul C, Hund-Georgiadis M, Forstmann BU, Lohmann G. Characterization of cortical thickness and ventricular width in normal aging: A morphometric study at 3 Tesla. J Magn Reson Imaging. 2006;24(3):513–519. doi: 10.1002/jmri.20665. [DOI] [PubMed] [Google Scholar]
- Qiu A, Fennema-Notestine C, Dale AM, Miller MI. Regional shape abnormalities in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2009;45(3):656–661. doi: 10.1016/j.neuroimage.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10(5):464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridha BH, Anderson VM, Barnes J, Boyes RG, Price SL, Rossor MN, Whitwell JL, Jenkins L, Black RS, Grundman M, Fox NC. Volumetric MRI and cognitive measures in Alzheimer disease : comparison of markers of progression. J Neurol. 2008;255(4):567–574. doi: 10.1007/s00415-008-0750-9. [DOI] [PubMed] [Google Scholar]
- Sanchez-Benavides G, Gomez-Anson B, Sainz A, Vives Y, Delfino M, Pena-Casanova J. Manual validation of FreeSurfer's automated hippocampal segmentation in normal aging, mild cognitive impairment, and Alzheimer Disease subjects. Psychiat Res-Neuroim. 2010;181(3):219–225. doi: 10.1016/j.pscychresns.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Schott JM, Price SL, Frost C, Whitwell JL, Rossor MN, Fox NC. Measuring atrophy in Alzheimer disease: a serial MRI study over 6 and 12 months. Neurology. 2005;65(1):119–124. doi: 10.1212/01.wnl.0000167542.89697.0f. [DOI] [PubMed] [Google Scholar]
- Serra L, Cercignani M, Lenzi D, Perri R, Fadda L, Caltagirone C, Macaluso E, Bozzali M. Grey and White Matter Changes at Different Stages of Alzheimer's Disease. J Alzheimers Dis. 2010;19(1):147–159. doi: 10.3233/JAD-2010-1223. [DOI] [PubMed] [Google Scholar]
- Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, Sexton G, Kaye JA. Changes in premorbid brain volume predict Alzheimer's disease pathology. Neurology. 2003;61(4):487–492. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Adalsteinsson E, Swan GE, Carmelli D. Differential rates of regional brain change in callosal and ventricular size: a 4-year longitudinal MRI study of elderly men. Cereb Cortex. 2002;12(4):438–445. doi: 10.1093/cercor/12.4.438. [DOI] [PubMed] [Google Scholar]
- Team RDC. R Foundation for Statistical Computing. Vienna, Austria: 2008. R: A language and environment for statistical computing. [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22(4):1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Wahlund LO, Andersson-Lundman G, Basun H, Almkvist O, Bjorksten KS, Saaf J, Wetterberg L. Cognitive functions and brain structures: a quantitative study of CSF volumes on Alzheimer patients and healthy control subjects. Magn Reson Imaging. 1993;11(2):169–174. doi: 10.1016/0730-725x(93)90021-5. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Wang D, Chalk JB, Rose SE, de Zubicaray G, Cowin G, Galloway GJ, Barnes D, Spooner D, Doddrell DM, Semple J. MR image-based measurement of rates of change in volumes of brain structures. Part II: application to a study of Alzheimer's disease and normal aging. Magn Reson Imaging. 2002;20(1):41–48. doi: 10.1016/s0730-725x(02)00472-1. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer's disease. Brain. 2007;130:1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman BT, Harvey DJ, Crawford K, Bernstein MA, Carmichael O, Cole PE, Crane PK, Decarli C, Fox NC, Gunter JL, Hill D, Killiany RJ, Pachai C, Schwarz AJ, Schuff N, Senjem ML, Suhy J, Thompson PM, Weiner M, Jack CR., Jr Standardization of analysis sets for reporting results from ADNI MRI data. Alzheimers Dement. 2012 doi: 10.1016/j.jalz.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qiu C, Lindberg O, Bronge L, Aspelin P, Backman L, Fratiglioni L, Wahlund LO. Acceleration of hippocampal atrophy in a non-demented elderly population: the SNAC-K study. Int Psychogeriatr. 2010;22(1):14–25. doi: 10.1017/S1041610209991396. [DOI] [PubMed] [Google Scholar]