Abstract
Purpose
Due to suboptimal outcomes in muscle-invasive bladder cancer even with multimodality therapy, determination of potential genetic drivers offers the possibility of improving therapeutic approaches and discovering novel prognostic indicators.
Experimental Design
Using pTN staging, we case-matched 81 patients with resected ≥pT2 bladder cancers for whom perioperative chemotherapy use and disease recurrence status were known. Whole exome sequencing was conducted in 43 cases to identify recurrent somatic mutations and targeted sequencing of 10 genes selected from the initial screening in an additional 38 cases was completed. Mutational profiles along with clinicopathologic information were correlated with recurrence-free survival (RFS) in the patients.
Results
We identified recurrent novel somatic mutations in the gene UNC5C (9.9%), in addition to TP53 (40.7%), KDM6A (21.0%), and TSC1 (12.3%). Patients who were carriers of somatic mutations in DNA repair genes (one or more of ATM, ERCC2, FANCD2, PALB2, BRCA1 or BRCA2) had a higher overall number of somatic mutations (p=0.011). Importantly, after a median follow-up of 40.4 months, carriers of somatic mutations (n=25) in any of these six DNA repair genes had significantly enhanced RFS compared to non-carriers (median 32.4 vs. 14.8 months; hazard ratio of 0.46, 95% CI 0.22 to 0.98; p=0.0435), after adjustment for pathologic pTN staging and independent of adjuvant chemotherapy usage.
Conclusion
Better prognostic outcomes of individuals carrying somatic mutations in DNA repair genes suggest these mutations as favorable prognostic events in muscle-invasive bladder cancer. Additional mechanistic investigation into the previously undiscovered role of UNC5C in bladder cancer is warranted.
Keywords: bladder cancer, exome sequencing, DNA repair mutations, UNC5C
Introduction
Bladder cancer is the sixth most common cancer type in the United States and approximately 72,500 individuals were diagnosed in 2012 (1). Patients with superficial (low-grade papillary) tumors generally have a good prognosis with a 5-year survival rate exceeding 90%, but those with muscle-invasive or locally advanced disease at surgical resection have a significant risk of recurrence with 5-year survival rates of 30-60% (2). Evidence have suggested invasive bladder cancers could have evolved from distinct molecular pathways as compared to papillary-type cancers (3). Systemic chemotherapy options for bladder cancer beyond platinum-based therapy are very limited, and no new drugs have been approved in the United States for metastatic bladder cancer in over 20 years (4). Although a number of newer, molecularly targeting drugs have been developed and approved for multiple cancer types in the last two decades, no such drug has been developed for the treatment of bladder cancer. This is likely due, in part, to the relatively limited molecular understanding of invasive bladder cancers and lack of knowledge about potential genetic drivers of invasive forms of the disease. Hence, it is of priority to discover novel genetic pathways involved in the carcinogenesis of muscle-invasive bladder cancer for the assessment of risk stratification and for development of novel drugs.
To identify such targets in bladder cancer, new technologies in genomics have been applied. High-throughput genotyping of genetic variations has enabled researchers to identify genetic variants that increase the risk of bladder cancer (5). Furthermore, rapid progresses in next generation DNA sequencers have revolutionized the cancer genomics field and a huge amount of information for somatic alterations in various types of human cancer has been accumulated in the last several years. In particular, recent efforts by The Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC) have led to the comprehensive molecular characterization of various solid human cancers. Regarding bladder cancer, a few papers have reported results of somatic mutational analysis and identified genes significantly mutated in this cancer (6-10). However, due to the large scale nature of these genomic projects and the scarcity of information on clinical outcomes associated with the analyzed human samples, there have been few studies that have attempted to comprehensively analyze mutational profiles as paired with clinical outcomes.
In this study, we focused on characterization of somatic mutations in muscle-invasive (stage T2 and above) bladder cancers from 81 patients without any prior neoadjuvant chemotherapy (to avoid the effect of genotoxic agents) to elucidate novel targets and potential molecular signatures associated with clinical outcomes in bladder cancer.
Patients and Methods
Sample collection and processing
We analyzed patient clinical follow-up data from available cases in our institutional biobank that allowed us to characterize cases according to their recurrence outcomes. Case-matching using pTN staging was performed wherever possible to identify approximately equal groups of patients with and without disease recurrence after definitive cystectomy to enable comparison between mutational profiles and recurrence status. Patients who received neoadjuvant chemotherapy before surgical resection of their tumors were intentionally excluded, since cytotoxic and genotoxic effect of chemotherapeutic drugs could bias the genetic make-up of tumor genomes for analysis. A subset of patients received adjuvant chemotherapy if recommended after consultation with a medical oncologist. For the case-matched non-recurrence subjects, notably only patients with a substantial length of clinical follow-up were selected for inclusion (median 40.4 months) to ensure that such cases were truly non-recurrent. A panel of 43 patients with muscle-invasive bladder carcinomas was selected for whole exome sequencing with availability of tumor and corresponding normal control samples (Table 1). An additional set of 38 patients was selected for targeted gene sequencing for a total of 81 patients analyzed (Table 2). Whenever possible, the source of the normal DNA control was DNA extracted from a peripheral venous blood draw. For patients without such a sample available, adjacent normal bladder tissue was used for extraction of normal DNA. A subset of the samples were reviewed by an attending genitourinary oncology pathologist for verification of tumor and normal tissue qualifications, in particular to ensure sufficient tumor nuclei percentage (for tumor samples) and the absence of tumor in the adjacent normal bladder tissue when used as normal controls. The tumor and normal tissue review information has been presented in Supplementary Table 1 and 2 respectively. The overall characteristics of the patient population were also summarized in Supplementary Table 3, illustrating a group of well-matched patients within the 81 cases. Sample collection was conducted under institutional review board approval (University of Chicago IRB #15550-B and 13-0526; Iwate Medical University approved IRB protocol HG H24-20). Pathologic stage at the time of surgical resection and subsequent clinical follow-up information (adjuvant chemotherapy; recurrence data) were recorded. Tissue samples were either frozen in regular OCT media, or formalin-fixed and embedded in paraffin. Genomic DNA (gDNA) was extracted using column purification (Qiagen DNA mini kits) after tissues were frozen in nitrogen and pulverized by the Cryopress (Microtec Co. Ltd., Japan). For samples embedded in paraffin, tissues were either punched or carved out in thin slivers and extracted with the truXTRAC™ FFPE DNA kit (Covaris Inc).
Table 1. Clinical and pathologic characteristics of the 43 patients (Whole Exome Sequencing).
| Study ID | Patient Age (At Surgery) |
Gender | Prior NMIBC |
Pathologic T | Pathologic N | Histologic Type | Adjuvant Chemotherapy |
Recurrence | Location of Recurrence | Time to recurrence (Months) |
Length of follow up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 78 | M | NO | pT3b | pN0 | squamous carcinoma | NO | NO | N/A | N/A | 59.6 |
| A2 | 57 | M | YES | pT2b | pN0 | urothelial carcinoma with squamous differentiation | NO | NO | N/A | N/A | 35.7 |
| A3 | 80 | M | NO | pT4a | pN0 | adenocarcinoma | NO | YES | pelvic mass, lungs | 9.4 | N/A |
| A4 | 62 | M | NO | pT3a | pN0 | urothelial carcinoma | NO | YES | liver, bones | 16.4 | N/A |
| A5 | 55 | F | NO | pT2b | pN0 | urothelial carcinoma with squamous differentiation | NO | NO | N/A | N/A | 25.6 |
| A6 | 72 | F | Unknown | pT4a | pN0 | adenocarcinoma | NO | YES | lungs, stomach | 1.4 | N/A |
| A7 | 79 | M | NO | pT3a | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 47.3 |
| A8 | 70 | M | NO | pT3b | pN0 | urothelial carcinoma | NO | YES | pelvic mass | 5.3 | N/A |
| A9 | 54 | F | Yes | pT2b | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 42.5 |
| A10 | 73 | M | Yes | pT3a | pN0 | urothelial carcinoma | NO | YES | retroperitoneal nodes, lungs | 7.1 | N/A |
| A11 | 55 | M | NO | pT2a | pN0 | urothelial carcinoma | NO | YES | pelvic mass/lungs/liver/bones | 14.4 | N/A |
| A12 | 67 | M | Unknown | pT2b | pN0 | urothelial carcinoma with squamous differentiation | NO | NO | N/A | N/A | 32.4 |
| A13 | 67 | F | NO | pT3a | pN0 | urothelial carcinoma | NO | YES | liver, bones | 2.5 | N/A |
| A14 | 68 | F | YES | pT3a | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 40.4 |
| A15 | 74 | M | NO | pT4a | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 43.4 |
| A16# | 72 | M | YES | pT3a | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 23.2 |
| A17 | 59 | M | NO | pT3b | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 37.1 |
| A18 | 59 | F | YES | pT3a | pN0 | urothelial carcinoma with squamous differentiation | NO | NO | N/A | N/A | 33.8 |
| A19 | 50 | M | NO | pT3b | pN0 | urothelial carcinoma | YES | YES | pelvic mass | 5.4 | N/A |
| A20 | 64 | M | YES | pT3a | pN1 | urothelial carcinoma | YES | NO | N/A | N/A | 53.9 |
| A21 | 43 | F | YES | pT2b | pN0 | urothelial carcinoma with squamous differentiation | NO | NO | N/A | N/A | 13.9 |
| A22 | 65 | M | YES | pT3b | pN1 | urothelial carcinoma | YES | YES | pelvic mass/lungs/liver/bones | 6.9 | N/A |
| A23 | 69 | M | NO | pT3b | pN0 | urothelial carcinoma | NO | YES | lungs | 5.8 | N/A |
| A24 | 74 | F | YES | pT3a | pN0 | urothelial carcinoma | NO | YES | lungs | 7.9 | N/A |
| A25 | 59 | M | NO | pT2b | pN0 | urothelial carcinoma with squamous differentiation | NO | NO | N/A | N/A | 21.8 |
| A26 | 55 | M | NO | pT3a | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 14.8 |
| A27 | 59 | M | NO | pT4a | pN2 | urothelial carcinoma | NO | YES | retroperitoneal nodes/lungs/liver/bones | 1.5 | N/A |
| A28 | 57 | M | NO | pT3a | pN1 | urothelial carcinoma | YES | YES | pelvic mass, sigmoid colon | 6.9 | N/A |
| A29 | 84 | M | NO | pT3a | pN0 | urothelial carcinoma with squamous differentiation | NO | NO | N/A | N/A | 14.9 |
| A30 | 45 | M | NO | pT4 | pN2 | urothelial carcinoma | YES | YES | retroperitoneal and mesenteric nodes, small bowel | 11.0 | N/A |
| A31# | 71 | M | NO | pT2a | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 12.7 |
| A32 | 77 | M | NO | pT2a | pN0 | urothelial carcinoma | NO | YES | pelvic mass, liver | 43.3 | N/A |
| A33 | 68 | M | NO | pT3a | pN1 | urothelial carcinoma | YES | YES | unknown sites | 3.9 | N/A |
| A34 | 56 | M | NO | pT3a | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 12.2 |
| A35 | 71 | F | NO | pT2b | pN2 | urothelial carcinoma | YES | NO | N/A | N/A | 39.7 |
| A36 | 59 | M | NO | pT4 | pN0 | squamous carcinoma | YES | YES | retroperitoneal nodes | 1.1 | N/A |
| A37 | 73 | M | NO | pT4 | pN0 | urothelial carcinoma | YES | NO | N/A | N/A | 45.3 |
| A38 | 79 | M | NO | pT3a | pN2 | urothelial carcinoma | YES | YES | mediastinal nodes, pelvic mass | 26.3 | N/A |
| A39 | 62 | M | NO | pT3a | pN2 | urothelial carcinoma | YES | YES | retroperitoneal nodes, liver, bones | 31.6 | N/A |
| A40 | 52 | M | NO | pT3b | pN1 | urothelial carcinoma with squamous differentiation | YES | NO | N/A | N/A | 46.2 |
| A41 | 68 | F | NO | pT3b | pN2 | urothelial carcinoma | YES | NO | N/A | N/A | 37.5 |
| A42 | 82 | M | NO | pT2 | pN0 | urothelial carcinoma with neuroendocrine features | NO | NO | N/A | N/A | 21.9 |
| A43 | 79 | M | Unknown | pT2a | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 6.6 |
| Median age= 67 Range (43-84) |
%M= 76.7 %F= 23.3 |
% prior NMIBC= 23.2% |
% pT2=27.9 % pT3=55.8 %pT4=16.3 |
% pN(+)= 25.6 % pN(-)= 74.4 |
Median time to recurrence= 6.9 |
See footnote* |
Median length of follow-up for non-recurrent cases = 34.7 months. Length of follow-up is defined as the difference between the date of surgery and the last clinic visit. Length of follow-up is calculated only for patients without recurrence to demonstrate the length of the intervals for these patients without evidence of disease recurrence (NED). Patients with recurrent disease met the primary study endpoint at the time of recurrence, and therefore length of follow-up in these patients is not applicable. NMIBC= non-muscle invasive bladder cancer.
excluded from mutational profile and clinical outcomes correlation since, although patient had no evidence of documented disease recurrence, patient died shortly after last follow-up of unknown causes and it could not be excluded that death was related to disease recurrence.
Table 2. Clinical and pathologic characteristics of the 38 patients (Targeted Sequencing Cohort).
| Study ID | Patient Age (At surgery) |
Gender | Prior NMIBC |
Pathologic T | Pathologic N | Histologic Type | Adjuvant Chemotherapy |
Recurrence | Site of Recurrence | Time to recurrence (Months) |
Length of follow up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A44 | 77 | M | YES | pT3a | pN2 | urothelial carcinoma | YES | YES | retroperitoneal/mesenteric nodes | 17.5 | N/A |
| A45 | 71 | M | YES | pT3a | pN2 | urothelial carcinoma | NO | YES | lungs | 1.9 | N/A |
| A46 | 56 | M | NO | pT3b | pN0 | urothelial carcinoma w/ squamous differentiation | YES | YES | lungs | 6.4 | N/A |
| A47 | 58 | M | NO | pT2 | pN0 | squamous carcinoma | NO | YES | urethra | 13.0 | N/A |
| A48 | 74 | F | YES | pT2b | pN0 | urothelial carcinoma | NO | YES | external iliac node | 21.1 | N/A |
| A49 | 76 | M | NO | pT3a | pN0 | urothelial carcinoma | NO | YES | lungs/pelvic mass | 2.8 | N/A |
| A50 | 51 | F | NO | pT3b | pN0 | urothelial carcinoma | YES | YES | periaortic/mesenteric nodes | 12.7 | N/A |
| A51 | 59 | M | YES | pT2b | pN2 | urothelial carcinoma | YES | YES | retroperitoneal nodes, lungs, bones | 10.7 | N/A |
| A52 | 60 | F | NO | pT3b | pN2 | urothelial carcinoma | YES | YES | mediastinal and retroperitoneal nodes/lungs | 10.8 | N/A |
| A53 | 70 | M | YES | pT4 | pN0 | urothelial carcinoma w/ squamous differentiation | YES | YES | sigmoid colon | 13.0 | N/A |
| A54 | 62 | M | NO | pT2 | pN0 | urothelial carcinoma | NO | YES | lungs, bones | 4.2 | N/A |
| A55 | 87 | M | NO | pT2b | pN0 | urothelial carcinoma | NO | YES | Iliac nodes | 5.0 | N/A |
| A56 | 81 | F | YES | pT3a | pN0 | urothelial carcinoma | NO | YES | liver, bones | 26.0 | N/A |
| A57 | 58 | M | YES | pT2 | pN0 | urothelial carcinoma | NO | YES | liver | 9.5 | N/A |
| A58 | 89 | F | YES | pT2 | pN0 | urothelial carcinoma w/ squamous differentiation | NO | YES | unknown | 13.0 | N/A |
| A59 | 54 | F | NO | pT3b | pN1 | urothelial carcinoma | YES | NO | N/A | N/A | 48.2 |
| A60 | 39 | M | YES | pT3a | pN0 | urothelial carcinoma | YES | NO | N/A | N/A | 57.2 |
| A61 | 40 | M | NO | pT2a | pN0 | squamous carcinoma | NO | NO | N/A | N/A | 58.1 |
| A62 | 68 | F | NO | pT2a | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 59.4 |
| A63 | 65 | F | NO | pT3a | pN0 | urothelial carcinoma w/ sarcomatoid differentiation | YES | NO | N/A | N/A | 51.1 |
| A64 | 53 | F | NO | pT2a | pN2 | urothelial carcinoma | YES | NO | N/A | N/A | 57.5 |
| A65 | 58 | M | NO | pT3b | pN2 | urothelial carcinoma | YES | NO | N/A | N/A | 62.5 |
| A66 | 64 | M | Unknown | pT4a | pN0 | urothelial carcinoma | YES | NO | N/A | N/A | 75.3 |
| A67 | 64 | M | NO | pT2a | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 59.3 |
| A68 | 57 | M | NO | pT2a | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 62.5 |
| A69 | 60 | M | Unknown | pT2b | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 60.6 |
| A70 | 73 | F | YES | pT3 | pN0 | urothelial carcinoma | NO | YES | pelvic mass | 3.8 | N/A |
| A71 | 67 | M | YES | pT3a | pN0 | urothelial carcinoma | NO | YES | pelvic mass | 20.1 | N/A |
| A72 | 58 | M | YES | pT3a | pN0 | urothelial carcinoma | NO | YES | pelvic mass, lungs | 6.1 | N/A |
| A73 | 80 | M | NO | pT3b | pN0 | urothelial carcinoma | NO | YES | lungs | 1.9 | N/A |
| A74 | 74 | F | YES | pT3b | pN0 | urothelial carcinoma | YES | YES | lungs, brain | 6.5 | N/A |
| A75 | 60 | F | NO | pT3b | pN0 | urothelial carcinoma | NO | YES | pelvic mass | 6.6 | N/A |
| A76 | 56 | F | YES | pT3 | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 27.4 |
| A77 | 44 | M | YES | pT3a | pN0 | urothelial carcinoma w/ squamous differentiation | NO | NO | N/A | N/A | 34.0 |
| A78 | 50 | F | Unknown | pT3a | pN0 | urothelial carcinoma w/ squamous differentiation | NO | NO | N/A | N/A | 26.3 |
| A79 | 76 | F | YES | pT3b | pN0 | urothelial carcinoma w/ myxoid stromal change | NO | NO | N/A | N/A | 32.4 |
| A80 | 67 | M | YES | pT3a | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 48.0 |
| A81 | 77 | M | YES | pT3 | pN0 | urothelial carcinoma | NO | NO | N/A | N/A | 46.0 |
| Median Age= 63 Range (39-89) |
%M= 57.9 %F=39.5 |
% prior NMIBC= 47% |
%pT2=34.2 %pT3=60.5 %pT4=5.3 |
%pN(+)=18.4 %pN(−)=81.6 |
Median Time to Recurrence=9.5 |
See footnote* |
Median length of follow-up for non-recurrent cases is 57.2 months. Length of follow up is defined as the difference between the date of surgery and the last clinic visit. Length of follow-up is calculated only for patients without recurrence to demonstrate the length of the intervals for these patients without evidence of disease recurrence (NED). Patients with recurrent disease met the primary study endpoint at the time of recurrence, and therefore length of follow-up without recurrence in these patients is not applicable. NMIBC= non-muscle invasive bladder cancer.
Whole exome sequencing
DNA libraries for whole-exome sequencing were constructed using the Ion Plus fragment library kit (Life Technologies, Carlsbad, CA, USA). Enrichment of the exonic regions was carried out by a probe hybridization approach using the Ion Targetseq Exome work flow (Life Technologies, Carlsbad, CA, USA) according to the supplier’s protocol. The final exome libraries were quantitated on the Agilent Tapestation system (Agilent Technologies, Santa Clara, CA, USA) before proceeding to the template preparation step. Optimal amount of final exome library (~7pM) was used in the template reaction on the OneTouch2 instrument (Life Technologies, Carlsbad, CA, USA) to achieve monoclonal amplification on the Ion Sphere Particles (ISPs) according to the standard protocol (11). The sample was loaded onto Ion Proton P1 v2 chip and sequenced on the Ion Proton instrument (Life Technologies, Carlsbad, CA, USA)
Targeted gene sequencing
A customized primer set for a selected panel of 10 genes was designed on the Ion Ampliseq Designer (Version 3.4.3) to maximize the exon coverage (99.7%) of target genes. Amplicon and multiplex library construction were conducted using the Ion Ampliseq Library 2.0 kit (Life Technologies, Carlsbad, CA, USA) and IonXpress barcode kit 1 and 2. The individual final libraries were quantitated on the Agilent Tapestation system (Agilent Technologies, Santa Clara, CA, USA) before making a mixture of approximately equal amounts and proceeding to the template preparation step. An optimal amount of final library (~7pM) was used in the template reaction on the OneTouch2 instrument (Life Technologies, Carlsbad, CA, USA) to achieve monoclonal amplification on the Ion Sphere Particles (ISPs) according to the standard protocol (11). The sample was loaded onto Ion Proton P1 v2 chip and sequenced on the Ion Proton instrument (Life Technologies, Carlsbad, CA, USA) as before.
Data processing and bioinformatics analysis
Sequencing reads were base-called and aligned to the human reference genome hg19 using TMAP on the Ion Torrent analysis server (Life Technologies, Carlsbad, CA, USA). Before somatic variant calling was carried out, we conducted filtering of the full BAM files generated by the sequencing reaction. Using the Picard (12) tools, we excluded sequence reads (1) with mapping quality value (MQV) less than 30, (2) less than 50bp in length, (3) ≥5% in overall base mismatches, and (4) those that were considered to be PCR duplicates (only for whole exome sequencing data, but not for targeted sequencing data). After applying these filtering criteria, somatic variants in each bladder carcinoma were detected using EbCall (13) and VarScan2 (14) respectively for exome data sets and targeted sequencing data sets. To minimize platform specific errors in our exome data set, EbCall is able to reduce the number of false positive calls by analyzing the platform-specific mutations found in 10 other reference normal DNAs. The variant list was further streamlined by applying p-value thresholds (EbCall p value [p<0.01] AND Fisher p value [p<0.05]) to minimize false positive calls. The EbCall parameters for single nucleotide variants (SNV) were as follows: minimum depth=10x, minimum variant support depth=4x, Minimum base quality=15, somatic P value threshold=0.05. The indel parameters were as follows: minimum depth=20x, minimum variant support depth=4x, Minimum base quality=15, somatic P value threshold=0.001, and mutation supported by reads from both strands. As SNVs causing an amino-acid substitution or a non-sense mutation are more likely to be functionally relevant for the pathogenesis of cancers, we focused our efforts on these non-silent variants. Additionally these variants should be somatic in nature, in effect only occurring in the tumor tissues but not in the normal germline DNA. To accomplish that, we shortlisted non-silent somatic SNVs that are present at a frequency ≥ 10% in the tumor samples, and < 5% in the normal control DNA (standard threshold for filtering of somatic variants) (14), and somatic indels present at a frequency ≥ 20% in the tumor samples, and < 3% in the normal control DNA. We determined the Sanger sequencing validation rate by calculating the number of variants that could be validated as true as a percentage of all variants that were selected for validation. The significance of the mutated genes was calculated by applying the MuSic (15) and MutSigCV (16) algorithm, and establishing a cut-off P value threshold of 0.01 that translates into a −log10(P value) of 2. We analyzed the effects of mutations in DNA repair genes on recurrence-free survival by the Kaplan-Meier method and conducted a multivariate analysis with the Cox proportional hazards regression model to compensate for the effects of pathological pTN staging on our observations using the software R (version 3.0.2) (17). Two of the 43 cases were excluded from the clinical correlation analysis as the patients died shortly after follow up without direct evidence of disease recurrence (indicated as # in Table 1).
Results
We analyzed a total of 81 surgically resected muscle-invasive bladder carcinomas from patients who did not receive neoadjuvant chemotherapy (to avoid the potential bias of selection of a subset of tumor cells caused by chemotherapy). Detailed clinical information for the cases is provided in Tables 1 and 2. For whole exome sequencing of the first 43 cases on the Ion Proton, we obtained overall total sequencing output with an average read length of approximately 150 bases and an average sequencing depth of 187.5× per base. We applied our customized filtering pipeline followed by the detection of somatic variants in the data sets using EbCall (13). Applying two independent mutation significance calculation algorithms, Genome MuSic (15) and MutSigCV (16), we computed the significance levels of frequently mutated genes, accounting for the gene size and background mutation rate (Supplementary Table 4 and 5). The MutSigCV algorithm additionally considers the replication timing and transcriptional activity of each gene.
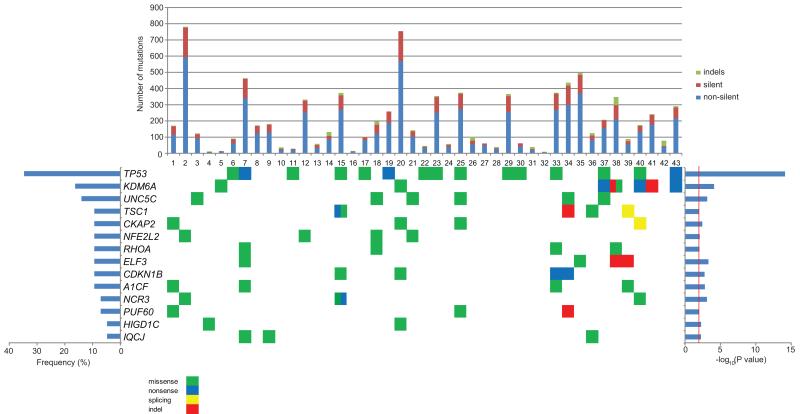
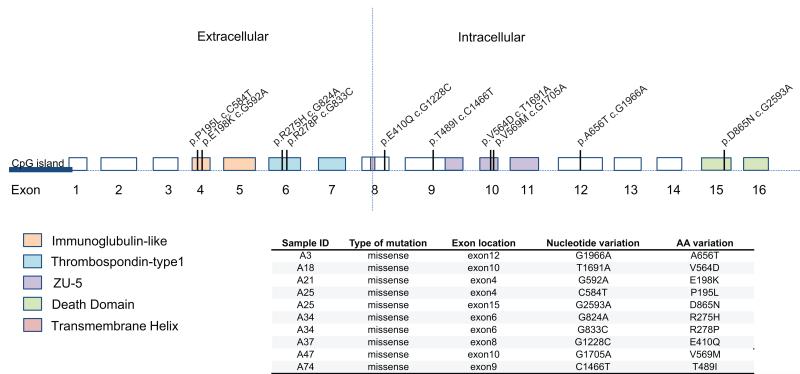
We concentrated exclusively on non-silent somatic mutations that were likely to be functionally relevant. Figure 1 shows the most significantly mutated genes defined by both the MuSic and MutSigCV algorithms in our panel of 43 cases, with their mutation frequencies derived from whole exome sequencing. We selected genes with a threshold cut off of –log10(p value) ≥ 2 for inclusion in Figure 1. Among 139 somatic variants selected for validation (from frequently-mutated genes), 128 (92.0%) were verified by the Sanger sequencing method. The number and type (indels, silent and non-silent mutations) of somatic mutations for each case are also summarized in the top panel of Figure 1. From our exome sequencing analysis, the top 3 most frequent significantly mutated genes were TP53 (34.8%), KDM6A (16.3%), and UNC5C (14.0%). UNC5C was not previously reported to be significantly mutated in human cancers, although somatic mutations were documented in a small number of tumors in the TCGA datasets (18). We carried out more detailed analysis of the type of mutations observed (Figure 2). All called mutations in UNC5C found by exome sequencing were confirmed by follow-up Sanger sequencing. All of the non-silent mutations in UNC5C discovered in our study (verified by the Sanger method) caused amino-acid substitutions and were scattered within multiple exons as shown in Figure 2. Two cases (case no. 25 and 34) harbored two mutations each. In addition to the novel finding of UNC5C in our bladder cancer cases, we had also observed somatic mutations in other genes that had been recently reported in previous publications (6, 8, 10), as listed in Supplementary table 6, providing more confirmatory evidence for their frequency.
Figure 1.
The significantly mutated genes derived from both Genome MuSic and MutSigCV algorithms were represented in this figure. In the top panel, a graphical representation of the number and type of somatic mutations observed in the significantly mutated genes of each sample was presented. The frequencies (% of analyzed samples) of mutations in each gene are depicted in the bar graph on the left and −log10(p-value) is shown on the right panel.
Figure 2.
Schematic representation of the UNC5C gene indicating exon-intron regions and the nucleotide and amino acid location of the somatic mutations observed.
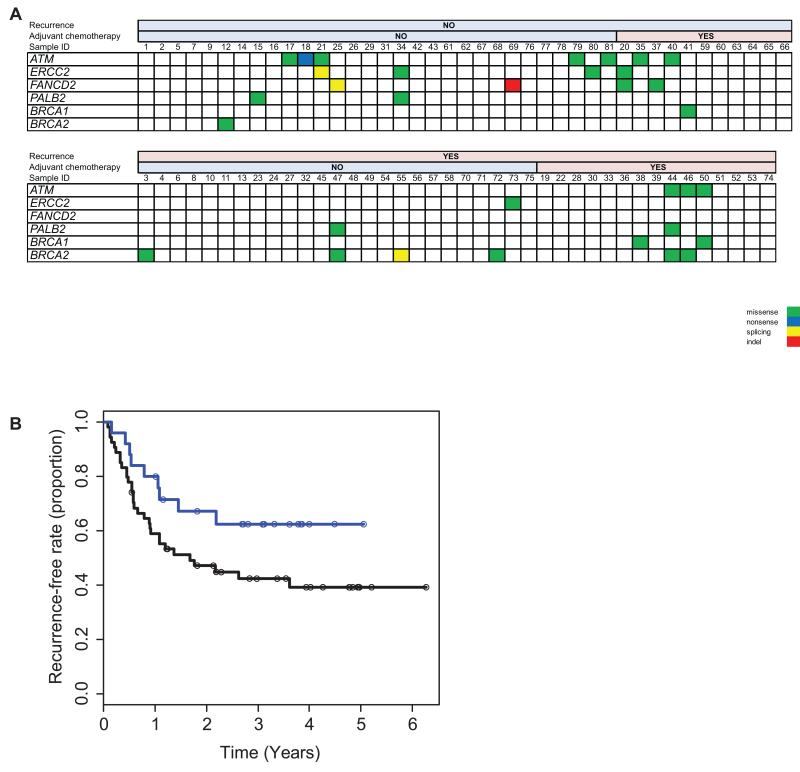
To incorporate the analysis of clinical pathological data together with the associated mutational profiles, we analyzed for recurrently mutated genes that may belong to a similar biological or functional pathway. Interestingly, we detected that somatic mutations in six DNA repair genes (whose mutations were found in at least two cases), ATM (5 mutations in 5 cases),ERCC2 (3 mutations in 3 cases), FANCD2 (3 mutations in 3 cases), PALB2 (5 mutations in 2 cases), BRCA1 (2 mutations in 2 cases), and BRCA2 (2 mutations in 2 cases), had a likelihood to occur more frequently in non-recurrent bladder cancer cases. We also found that tumors with mutations in at least one of these DNA repair genes had significantly higher overall numbers of somatic mutations (307.4 mutations/case) as compared to those without a mutation in any of them (155.4 mutations/case) (two tailed Student’s T-test, p=0.011). Since aberrations in the repair pathway are known to influence clinical outcomes (19), we were interested to further examine if mutations in these genes can be relevant for the pathogenesis or clinical outcome of bladder cancer.
Hence, we further analyzed these six repair genes and the most frequently mutated genes detected by exome sequencing (TP53, KDM6A, UNC5C, TSC1) in an additional 38 cases of muscle-invasive bladder cancers with similar clinical characteristics by targeted-gene panel sequencing. The targeted sequencing achieved higher overall coverage than whole exome sequencing and we summarize the total number of somatic mutations observed in the 10 genes in the 81 cases in Table 3.
Table 3. Summary of observed somatic mutation frequencies in 81 muscle-invasive bladder cancers.
| Gene | No. of mutated cases | Frequency % (n=81) |
|---|---|---|
| TP53 | 33 | 40.7 |
| KDM6A | 17 | 21.0 |
| TSC1 | 10 | 12.3 |
| ATM | 10 | 12.3 |
| UNC5C | 8 | 9.9 |
| BRCA2 | 7 | 8.6 |
| ERCC2 | 5 | 6.2 |
| FANCD2 | 4 | 4.9 |
| PALB2 | 4 | 4.9 |
| BRCA1 | 3 | 3.7 |
We also stratified 79 patients with the clinicopathological information to examine the relationship between the mutational profile of the DNA repair genes and recurrence free survival (RFS) (#16 and 31 as indicated in Table 1 were excluded from mutational profile and clinical outcomes correlation since, although patient had no evidence of documented disease recurrence, patient died shortly after last follow-up of unknown causes and it could not be excluded that death was related to disease recurrence). We found that carriers of mutations in DNA repair genes (either of ATM, ERCC2, FANCD2, PALB2, BRCA1 or BRCA2) were more frequently represented in the non-recurrent disease group (Figure 3A). Furthermore, carriers of somatic mutations in DNA repair genes have improved RFS in a Kaplan-Meier analysis curve (Figure 3B). Although recurrent and non-recurrent cases were well matched in our samples set (see Supplementary Table 3), we carried out a multivariate Cox proportional hazards regression to adjust for pT and pN stages to account for the known prognostic factors of the pT stage and nodal status in our analysis. After multivariate regression, we found that mutations in DNA repair genes remained significantly associated with longer recurrence free survival (p= 0.0435, hazard ratio of 0.46, 95% CI 0.22 to 0.98). In other words, for patients who were carriers of somatic mutations in DNA repair genes, they had a 50% reduced relative risk of disease recurrence as compared to patients who were non-carriers. Carriers of mutations in these DNA repair genes (n=25) had a median disease free survival period of 32.4 months (9 patients recurred within this group, 16 have not in the observation period), whereas non-carriers (n=54) had a median disease free survival of only 14.8 months (23 patients had non-recurrent disease, 31 patients had recurrent disease).
Figure 3.
A. Frequencies of mutations in each DNA repair gene detected in our panel of bladder carcinoma patients in relationship to status of recurrence and adjuvant chemotherapy. B. Kaplan-Meier analysis of the effect of mutations in DNA repair genes on recurrence-free survival. A blue line denotes carriers of mutations in DNA repair genes (n=25). A black line denotes non-carriers of mutations in DNA repair genes (n=54). The open circle markers on survival curves are censored cases.
As somatic mutations that are deleterious in nature to protein function are more likely to play an important role for a disease phenotype, it is of interest to investigate if the observed somatic mutations in DNA repair genes are likely to affect their biological function. Therefore using four commonly utilized predictors of missense mutations on protein function (20), namely SIFT(21) (Sorting Intolerant From Tolerant) program, Polyphen-2(22), LRT(23) (likelihood ratio test), and Mutation taster (24), we demonstrate the predictions for all the observed somatic mutations in DNA repair genes in Supplementary Table 7. For example, we observed that the majority of missense somatic mutations in the ATM gene were predicted to be deleterious by all 4 predictor algorithms.
Discussion
Our analysis identified at relatively high frequency a novel significantly mutated gene UNC5C (9.9%) that was previously unreported in earlier studies (6, 7, 10). UNC5C is a member of the netrin-1 receptor family that functions as a dependence receptor (25), and two well-known cancer related proteins, RET and Patched, have also been classified as dependence receptors (26, 27). When the ligand netrin-1 is present, these dependence receptors generate survival signals to cells. On the other hand, when the ligand is absent, the receptors send pro-apoptotic signals to trigger cell death (25). Significant somatic mutations of UNC5C have not been previously reported in bladder cancer, although a search of the TCGA bladder cancer dataset shows that a small number of samples were found to harbor UNC5C mutations (10). Previous reports have suggested tumor suppressive effects of the UNC5C gene product due to its down regulation in colorectal malignancies through promoter methylation (28, 29). Also, certain germline variants of UNC5C were reported in individuals predisposed to familial colorectal carcinoma (30), and the inactivation of Unc5h3 in mice, the human UNC5C ortholog, was shown to enhance progression of intestinal tumors (31). The down-regulated expression of netrin-1 receptors like DCC and UNC5C through specific genetic alterations in tumors is likely to reduce pro-apoptotic signals and enhance survival of tumor cells (32). More recently, the function of the UNC5 family of receptors has been implicated in the regulation of cell death processes in bladder cancers (33, 34). More specifically, bladder cancer specimens were found to have low UNC5A and UNC5D expression, and further depletion of the endogenous levels of UNC5A and UNC5D gene expression reduced the amount of cell death induced by chemotherapeutic agents (33, 34). Considering the aforementioned evidence, we hypothesize that the UNC5C gene could also harbor tumor suppressive effects, and its somatic mutations in bladder cancer may inactivate protein function and increase cancer cell survival, or cause a constitutive activation of the receptor pathway (sending a survival signal to the cells and reducing cellular apoptosis) that can result in over-proliferation of cancer cells and accelerate progression of muscle-invasive bladder carcinoma. Moreover, as UNC5C encodes for a cellular signaling receptor, this pathway could suggest its potential as a valuable molecular target. To determine whether UNC5C somatic mutations may be associated with specific histological characteristics of muscle invasive bladder cancers, we examined the histological types as presented in Table 1 and we found that four of eight cases with UNC5C somatic mutations were classified to have some squamous-cell component; one was squamous carcinoma of the bladder, and three were urothelial carcinomas with squamous differentiation. One case was an adenocarcinoma. The remaining three cases were transitional urothelial carcinomas. Although the number of cases is too small to make definitive conclusions, the frequent observation of UNC5C somatic mutations in our patient population might be associated with the squamous-cell phenotype. This may explain why UNC5C mutations were frequently observed in our patient population, and not in other studies if the previous study populations lacked bladder cancer samples with significant squamous differentiation. However we are aware of the limitations to the significance of our findings regarding UNC5C, as we did not observe a significant correlation with clinical outcome. Also with a relatively limited numbers of tumors, there is a need for additional direct functional evidence using carcinogenesis models.
In our exome sequencing study, we also found novel significant somatic mutations at the frequency of 9.3% in CDKN1B, RHOA, CKAP2 and A1CF that were previously unreported in bladder cancer, though CDKN1B and RHOA are involved in cell cycle control and cell motility, which may contribute to invasive and metastatic processes. CDKN1B was reported to be somatically mutated in small intestinal neuroendocrine tumors and associated with hereditary prostate cancer risk (35, 36). CKAP2 encodes a microtubule-associated protein that regulates cellular mitotic exit during division and its up regulation has been detected in gastric cancer (37). A1CF encodes for APOBEC1 complementation factor (ACF), a RNA-binding protein involved in the RNA editing processes that impacts cellular survival (38). It will be of interest to investigate the significance of these genes in bladder cancer by additional functional studies.
As compared to recently published reports (6-8, 10) that delineated several significantly mutated genes in bladder cancer, our present study found several genes, with the significantly high frequency of somatic mutations, such as TP53 (40.7%), KDM6A (21.0%), TSC1 (12.3%), NFE2L2 (9.3%) and ELF3 (9.3%) in agreement with the previous studies (6, 7, 10). Additionally, in the TCGA study (10), the authors found frequent mutations in the genes TP53 (49%), KDM6A (24%), RB1 (13%), NFE2L2 (8%), ELF3 (8%), whose mutation frequency were very similar to our findings (Figure 1 and Supplementary Table 6). Conversely, a number of other significantly mutated genes reported previously were found to be non-significant in our analysis (Supplementary Table 6); for example, STAG2 mutations were frequently observed in previous reports (6, 8, 10), but we found no somatic mutation among 43 tumors in our exome study although it has been acknowledged in the previous publication that STAG2 mutations were less frequent in invasive bladder cancers (8). Mutations in the ARID1A gene, which was reported as significantly mutated in multiple cancer types (18), were found in 4 cases, but this gene was also judged to be non-significant in our analysis. It is of note that even if the list of recurrently mutated genes significantly overlapped among the different studies, the gene lists were not completely identical with one another. It is not unexpected that there are differences in the significant mutational profiles established by different groups, and the differences between our findings and the previously reported data could also be partly explained by the analytical algorithms utilized. In our analysis we used EbCall for somatic mutation calling, and both Genome MuSic and MutSigCV to estimate significance. Genes that were designated as significantly mutated by these two different algorithms can be more confidently accorded higher importance and may be more likely to exert functional significance. In addition, clinical and pathological differences between the sequenced populations could have contributed to this disparity as well, especially since we selected a population with only muscle-invasive disease (as opposed to non-invasive disease) without any prior chemotherapy. The exclusion of bladder tumors treated with neoadjuvant chemotherapy in our studies has allowed us to establish an accurate mutational profile without certain biases exerted by genotoxic drugs on the molecular make-up of the tumors. Most importantly, it has also been shown that the application of specific sequencing platforms like the Ion Torrent may capture variants that are missed on other platforms (39), although some commonly reported genes could be missed. Overall the use of alternative platforms will enable us to build a more comprehensive molecular landscape of human cancer, instead of using information from only one sequencing technology. Finally the strength of our study lies in the comprehensive dataset of clinical outcomes that were carefully curated and correlated with mutational profiles.
As a result, we found that carriers of somatic mutations in the six DNA repair genes (either of ATM, ERCC2, FANCD2, PALB2, BRCA1 or BRCA2) harbored an overall higher larger number of somatic mutations; we also observed that this group of patients had an improved recurrence-free survival. This could be counterintuitive, as we would have expected aberrations in the DNA repair pathway might worsen the outcome of cancer patients by the progressive accumulation of genetic mutations that failed to be repaired. However, it is important to consider that amino acid substitutions caused by missense mutations can generate novel cancer-specific antigens that could become targets of our immune system, particularly of cytotoxic T lymphocytes (40). Hence, it could be hypothesized that mutations in DNA repair genes and subsequent cancer-specific antigen generation may have enhanced immune activity and resulted in better clinical outcomes including the observed longer recurrence-free survival. Although our data showed an interesting correlation between somatic mutations in DNA repair genes and recurrence free survival, there is a need to further validate the findings in an even larger cohort (and ideally prospectively) in future studies prior to clinical use. The potential clinical utility of this information is that, if prospectively validated, it could provide important additional prognostic information about patients undergoing radical cystectomy (beyond pathologic stage) to better inform clinical decisions about recurrence risk and use of adjuvant therapy.
In conclusion, we have identified novel frequent somatic mutations of UNC5C in muscle-invasive bladder cancers. We also revealed a significant association between mutations in DNA repair genes and improved clinical outcomes of bladder cancer patients that, if validated, could be applied as part of potential prognostic algorithms during clinical decision-making when treating patients with this difficult disease.
Supplementary Material
Translational Relevance.
Muscle-invasive bladder cancers frequently recur and often have a poor prognosis despite combined surgical and chemotherapy approaches. Therefore the discovery of novel genetic drivers for this disease is critical. We performed next-generation sequencing of a total of 81 muscle-invasive bladder cancers and identified previously unreported somatic mutations of UNC5C (9.9%) that warrants further investigation as a molecular target. Next we examined the relationship of somatic mutational profile with recurrence-free survival and found that the presence of somatic mutations in one or more of six DNA repair genes (ATM, ERCC2, FANCD2, PALB2, BRCA1 and BRCA2) was significantly associated with enhanced recurrence-free survival after adjustment for pathologic TN staging. We propose that the better prognostic outcomes of individuals carrying somatic mutations in these DNA repair genes suggest their utility as favorable prognostic events in muscle-invasive bladder cancer, a novel and potentially significant clinical finding.
Acknowledgements
We would like to express our gratitude to Drs. Rui Yamaguchi, Seiya Imoto, and Satoru Miyano (Human Genome Center, Institute of Medical Science, University of Tokyo) for their bioinformatics expertise and support. We would also like to thank Dr. Hirofumi Arakawa for very useful input on this manuscript.
Acknowledgement of research support for this study: Support for the clinical collection and annotation of samples was provided by a Cancer Research Foundation Young Investigator Award (PHO) and a sub-award from NIH N02 CO07009-65 (GDS). PHO is supported by NIH K23 GM 100288-01A1. This study is also supported partly by The University of Chicago Comprehensive Cancer Center Support Grant (CCSG: P30 CA014599).
Footnotes
COI statement: The authors have no conflict of interest to disclose
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–25. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 3.Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol. 2006;24:5552–64. doi: 10.1200/JCO.2006.08.2073. [DOI] [PubMed] [Google Scholar]
- 4.Guy L, Mahammedi H, Bastide C, Bruyere F, Karsenty G, Bay JO. Medical treatment of bladder cell carcinoma. Progres en urologie: journal de l’Association francaise d’urologie et de la Societe francaise d’urologie. 2013;23:1238–45. doi: 10.1016/j.purol.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Bambury RM, Rosenberg JE. Actionable mutations in muscle-invasive bladder cancer Curr Opin Urol. 2013;23:472–8. doi: 10.1097/MOU.0b013e328363a3cd. [DOI] [PubMed] [Google Scholar]
- 6.Guo G, Sun X, Chen C, Wu S, Huang P, Li Z, et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nature genetics. 2013 doi: 10.1038/ng.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nature genetics. 2011;43:875–8. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon DA, Kim JS, Bondaruk J, Shariat SF, Wang ZF, Elkahloun AG, et al. Frequent truncating mutations of STAG2 in bladder cancer. Nature genetics. 2013 doi: 10.1038/ng.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyer G, Hanrahan AJ, Milowsky MI, Al-Ahmadie H, Scott SN, Janakiraman M, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research N Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothberg JM, Hinz W, Rearick TM, Schultz J, Mileski W, Davey M, et al. An integrated semiconductor device enabling non-optical genome sequencing. Nature. 2011;475:348–52. doi: 10.1038/nature10242. [DOI] [PubMed] [Google Scholar]
- 12.Alec Wysoker KT, Mike McCowan, Nils Homer, Tim Fennell. cited; A set of tools (in Java) for working with next generation sequencing data in the BAM. Available from: http://picard.sourceforge.net/
- 13.Shiraishi Y, Sato Y, Chiba K, Okuno Y, Nagata Y, Yoshida K, et al. An empirical Bayesian framework for somatic mutation detection from cancer genome sequencing data. Nucleic acids research. 2013;41:e89. doi: 10.1093/nar/gkt126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome research. 2012;22:568–76. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dees ND, Zhang Q, Kandoth C, Wendl MC, Schierding W, Koboldt DC, et al. MuSiC: identifying mutational significance in cancer genomes. Genome research. 2012;22:1589–98. doi: 10.1101/gr.134635.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Team RC. R: A Language and Environment for Statistical Computing. 2014 cited; Available from: http://www.R-project.org.
- 18.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–9. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouwman P, Jonkers J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer. 2012;12:587–98. doi: 10.1038/nrc3342. [DOI] [PubMed] [Google Scholar]
- 20.Chun S, Fay JC. Identification of deleterious mutations within three human genomes. Genome research. 2009;19:1553–61. doi: 10.1101/gr.092619.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic acids research. 2003;31:3812–4. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nature methods. 2010;7:248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doniger SW, Kim HS, Swain D, Corcuera D, Williams M, Yang SP, et al. A catalog of neutral and deleterious polymorphism in yeast. PLoS genetics. 2008;4:e1000183. doi: 10.1371/journal.pgen.1000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nature methods. 2010;7:575–6. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 25.Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer. 2004;4:978–87. doi: 10.1038/nrc1504. [DOI] [PubMed] [Google Scholar]
- 26.Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, et al. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J. 2000;19:4056–63. doi: 10.1093/emboj/19.15.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301:843–6. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 28.Shin SK, Nagasaka T, Jung BH, Matsubara N, Kim WH, Carethers JM, et al. Epigenetic and genetic alterations in Netrin-1 receptors UNC5C and DCC in human colon cancer. Gastroenterology. 2007;133:1849–57. doi: 10.1053/j.gastro.2007.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hibi K, Mizukami H, Shirahata A, Goto T, Sakata M, Sanada Y. Aberrant methylation of the netrin-1 receptor genes UNC5C and DCC detected in advanced colorectal cancer. World journal of surgery. 2009;33:1053–7. doi: 10.1007/s00268-008-9909-x. [DOI] [PubMed] [Google Scholar]
- 30.Coissieux MM, Tomsic J, Castets M, Hampel H, Tuupanen S, Andrieu N, et al. Variants in the netrin-1 receptor UNC5C prevent apoptosis and increase risk of familial colorectal cancer. Gastroenterology. 2011;141:2039–46. doi: 10.1053/j.gastro.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernet A, Mazelin L, Coissieux MM, Gadot N, Ackerman SL, Scoazec JY, et al. Inactivation of the UNC5C Netrin-1 receptor is associated with tumor progression in colorectal malignancies. Gastroenterology. 2007;133:1840–8. doi: 10.1053/j.gastro.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazelin L, Bernet A, Bonod-Bidaud C, Pays L, Arnaud S, Gespach C, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–4. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Y, Yu M, Chen Y, Wang Y, Wang J, Yang C, et al. DNA damage-inducible gene, UNC5A, functions as a tumor-suppressor in bladder cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:6887–91. doi: 10.1007/s13277-014-1930-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Y, Yu M, Chen Y, Wang Y, Wang J, Yang C, et al. Down-Regulation of UNC5D in Bladder Cancer: UNC5D as a Possible Mediator of Cisplatin Induced Apoptosis in Bladder Cancer Cells. The Journal of urology. 2014;192:575–82. doi: 10.1016/j.juro.2014.01.108. [DOI] [PubMed] [Google Scholar]
- 35.Francis JM, Kiezun A, Ramos AH, Serra S, Pedamallu CS, Qian ZR, et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nature genetics. 2013;45:1483–6. doi: 10.1038/ng.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang BL, Zheng SL, Isaacs SD, Wiley KE, Turner A, Li G, et al. A polymorphism in the CDKN1B gene is associated with increased risk of hereditary prostate cancer. Cancer research. 2004;64:1997–9. doi: 10.1158/0008-5472.can-03-2340. [DOI] [PubMed] [Google Scholar]
- 37.Bae CD, Sung YS, Jeon SM, Suh Y, Yang HK, Kim YI, et al. Up-regulation of cytoskeletal-associated protein 2 in primary human gastric adenocarcinomas. Journal of cancer research and clinical oncology. 2003;129:621–30. doi: 10.1007/s00432-003-0484-0. [DOI] [PubMed] [Google Scholar]
- 38.Mehta A, Kinter MT, Sherman NE, Driscoll DM. Molecular cloning of apobec-1 complementation factor, a novel RNA-binding protein involved in the editing of apolipoprotein B mRNA. Molecular and cellular biology. 2000;20:1846–54. doi: 10.1128/mcb.20.5.1846-1854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boland JF, Chung CC, Roberson D, Mitchell J, Zhang X, Im KM, et al. The new sequencer on the block: comparison of Life Technology’s Proton sequencer to an Illumina HiSeq for whole-exome sequencing. Human genetics. 2013;132:1153–63. doi: 10.1007/s00439-013-1321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nature medicine. 2013;19:747–52. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.