Abstract
Keratodermas comprise a heterogeneous group of highly debilitating and painful disorders characterized by thickening of the skin with marked hyperkeratosis. Some of these diseases are caused by genetic mutation, while other forms are acquired in response to environmental factors. Our understanding of signaling changes that underlie these diseases is limited. In the present study we describe a keratoderma phenotype in mice in response to suprabasal epidermis-specific inhibition of activator protein 1 transcription factor signaling. These mice develop a severe phenotype characterized by hyperplasia, hyperkeratosis, parakeratosis and impaired epidermal barrier function. The skin is scaled, constricting bands encircle the tail and digits, the footpads are thickened and scaled, and loricrin staining is markedly reduced in the cornified layers and increased in the nucleus. Features of this phenotype, including nuclear loricrin localization and pseudoainhum (autoamputation), are characteristic of Vohwinkel Syndrome. We confirm that the phenotype develops in a loricrin null genetic background, indicating that suppressed suprabasal AP1 factor function is sufficient to drive this disease. We also show that the phenotype regresses when suprabasal AP1 factor signaling is restored. Our findings suggest that suppression of AP1 factor signaling in the suprabasal epidermis is a key event in the pathogenesis of keratoderma.
Keywords: TAM67, jun, fos, AP1, keratinocyte differentiation, epidermis, keratoderma, loricrin, Vohwinkel Syndrome
Introduction
Keratodermas comprise a group of disorders characterized by thickening of the epidermis with marked hyperplasia and hyperkeratosis (Christiano, 1997). Keratodermas include both inherited and acquired forms. Inherited forms of this disease are associated with expression of mutant intermediate filaments (keratins), cornified envelope-associated proteins (loricrin, transglutaminase), cohesion proteins (desmoglein 1, plakophilin, desmoplakin), proteins of cell-to-cell communication (connexins), and transmembrane proteins (cathepsin C) (Kimyai-Asadi et al., 2002; Christiano, 1997; Amagai and Stanley, 2012). Most of these mutations are autosomal dominant, although some are recessive (Kimyai-Asadi et al., 2002). In addition, some forms of keratoderma are acquired in response to treatment with drugs, exposure to chemicals, malnutrition, infection or response to cancer (Patel et al., 2007). The underlying biochemical changes in these diseases are not well understood.
Vohwinkel syndrome is a form of keratoderma characterized by honeycomb-like palmoplantar keratoderma, hyperkeratosis, prominent tissue buildup on the dorsal hands and feet, pseudoainhum with digit amputation, and hearing impairment (OMIM 124500) that is caused by a mutation in the connexin 26 gene (Maestrini et al., 1999). In addition, Camisa and Rossana reported a variant of Vohwinkel syndrome characterized by ichthyosiform dermatosis and no hearing impairment (OMIM 604117) (Camisa and Rossana, 1984; Schmuth et al., 2004). This autosomal-dominant disease, also called loricrin keratoderma or keratoderma with ichthyosis, is caused by mutation of the loricrin gene (Korge et al., 1997). The loricrin mutations are frameshift changes due to a single nucleotide insertion in the loricrin coding sequence. The most frequent mutation is 730insG (Korge et al., 1997; Maestrini et al., 1996; O'Driscoll et al., 2002; Matsumoto et al., 2001; Takahashi et al., 1999; Gedicke et al., 2006; Drera et al., 2008), although mutations are also observed at 662insT (Armstrong et al., 1998), 709insC (Ishida-Yamamoto et al., 1997) and c.545-546insG (Song et al., 2008). These mutations result in synthesis of an extended form of loricrin that includes a nuclear localization signal. Instead of being incorporated into the cornified envelope, the mutant loricrin translocates into the nucleus where it produces changes that are not well characterized (Ishida-Yamamoto et al., 1999; Ishida-Yamamoto et al., 2000; Ishida-Yamamoto et al., 1998b).
Mouse model studies support a role for mutant loricrin in the pathogenesis of loricrin keratoderma. Overexpression of wild-type human loricrin in mouse epidermis shows a normal phenotype that is associated with incorporation of human loricrin into the murine cornified envelope (Yoneda and Steinert, 1993). Loricrin knockout mice have also been produced and these mice display only a mild phenotype (Koch et al., 2000; Jarnik et al., 2002). In contrast, transgenic mice expressing mutant loricrin display features of loricrin keratoderma (Suga et al., 2000) and this phenotype is more dramatic when the mutant is expressed in the absence of wild-type loricrin (Suga et al., 2000). The phenotype includes nuclear localization of loricrin, hyperproliferation, hyperkeratosis, parakeratosis and pseudoainhum of the tail (Suga et al., 2000). These findings argue that this is a gain-of-function mutation and that wild-type loricrin is not necessary for the phenotype. However, beyond the accumulation of nuclear loricrin, subsequent events in disease progression are not understood. Recent studies, using HaCaT cell cultures expressing mutant loricrin, suggest that nuclear accumulation of loricrin alters intracellular signaling and that this may contribute to the keratoderma phenotype (Yoneda et al., 2010b; Yoneda et al., 2010a). These findings suggest the accumulation of mutant nuclear loricrin may influence a host of cell processes that lead to enhanced cell proliferation.
In the present study we describe mice which express tetracycline-inducible dominant-negative c-jun (TAM67) in the suprabasal murine epidermis. Dominant-negative c-jun dimerizes with all jun/fos factors to inhibit transcription (Brown et al., 1994). Jun/fos factors are key controllers of keratinocyte proliferation and differentiation (Eckert et al., 2004; Efimova and Eckert, 2000; Efimova et al., 1998; Efimova et al., 2002; Efimova et al., 2003). Remarkably, these mice develop an authentic loricrin keratoderma-like phenotype. Moreover, studies in loricrin null background suggest that loricrin is not required for this response. Based on these findings, we suggest that reduced suprabasal AP1 factor signaling in the suprabasal epidermis is sufficient to cause keratoderma, and that suppressed suprabasal epidermal AP1 factor function may be involved in a range of keratoderma types.
Results
TAM67 in suprabasal epidermis
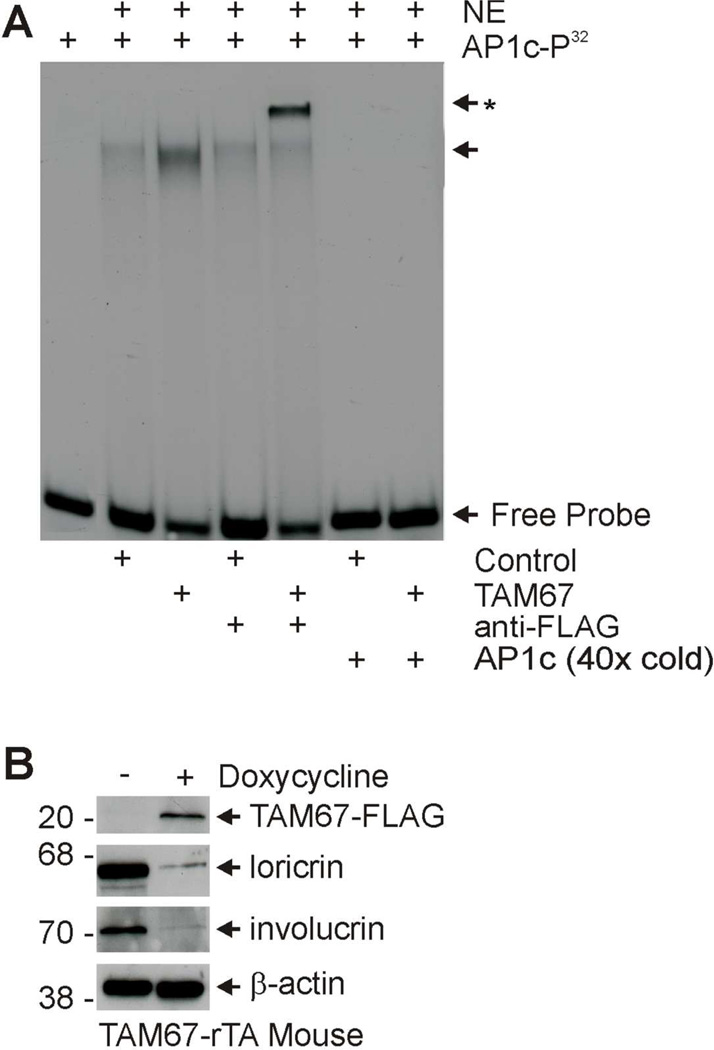
SHK1-TAM67-rTA mice are engineered to encode the TetO-TAM67- FLAG and hINV-rTA gene expression cassettes. The hINV-rTA construct uses the human involucrin promoter to target expression of the reverse tetracycline activator (rTA) to the suprabasal epidermis. Doxycycline binding produces an active form of rTA which binds to TetO to drive suprabasal expression of TAM67-FLAG. TAM67 forms inactive heterodimers with other AP1 factors which interact with AP1 response elements in a normal manner but do not active transcription. This results in reduced expression of AP1 responsive genes (Han et al., 2012). As an initial step in characterization of the role of TAM67-FLAG in epidermis, we confirmed that TAM67-FLAG interacts with AP1 response elements and reduces expression of AP1 target genes. TAM67-rTA mice were treated for three days with or without doxycycline and nuclear epidermal extracts were prepared. Fig. 1A shows an increase in the quantity of shifted AP1c-P32 probe in extract prepared from TAM67-expressing epidermis, and that this binding is reduced by addition of excess radioinert AP1c. In addition, direct TAM67-FLAG binding to AP1c-P32 was confirmed by anti-FLAG supershift (Fig. 1A). Expression of TAM67 in this tissue is expected to reduce expression of AP1 factor-regulated genes. To assess this, we monitored expression of involucrin and loricrin, two genes which require AP1 transcription factors for expression (Jang and Steinert, 2002; Yoneda et al., 1992; Eckert et al., 2004). TAM67-rTA mice were treated with or without 2 mg/ml doxycycline for three days and total epidermal extracts were assayed for involucrin and loricrin. We find that both involucrin and loricrin levels are reduced in TAM67-expressing epidermis (Fig. 1B), thereby providing biochemical evidence for a TAM67 impact on the tissue.
Fig. 1.
TAM67-FLAG interacts with AP1 response element and suppresses AP1-responsive genes. TAM67-rTA mice were treated with (+) or without (−) 2 mg/ml doxycycline in drinking water for 3 days. A Interaction of TAM67 with AP1 site consensus element. Epidermis from control and TAM67-expressing epidermis was collected by high temperature separation (Rorke et al., 2010) and nuclear extracts were prepared and incubated with AP1c-P32 as indicated. FP indicates free probe and NE indicates nuclear extract. The arrow indicates migration of the mobility shifted bands, and the arrow/asterisk indicates migration of the supershifted band (anti-FLAG treated sample). A 40-fold excess of AP1c was added as indicated to demonstrate binding specificity. B Murine epidermis was collected free of the dermis by high temperature separation as previously described (Rorke et al., 2010). Total extract was prepared for immunoblot to detect the indicated proteins. TAM67-FLAG was detected with anti-FLAG. Similar results were observed in each of three experiments.
The phenotype of SKH1-TAM67-rTA mice
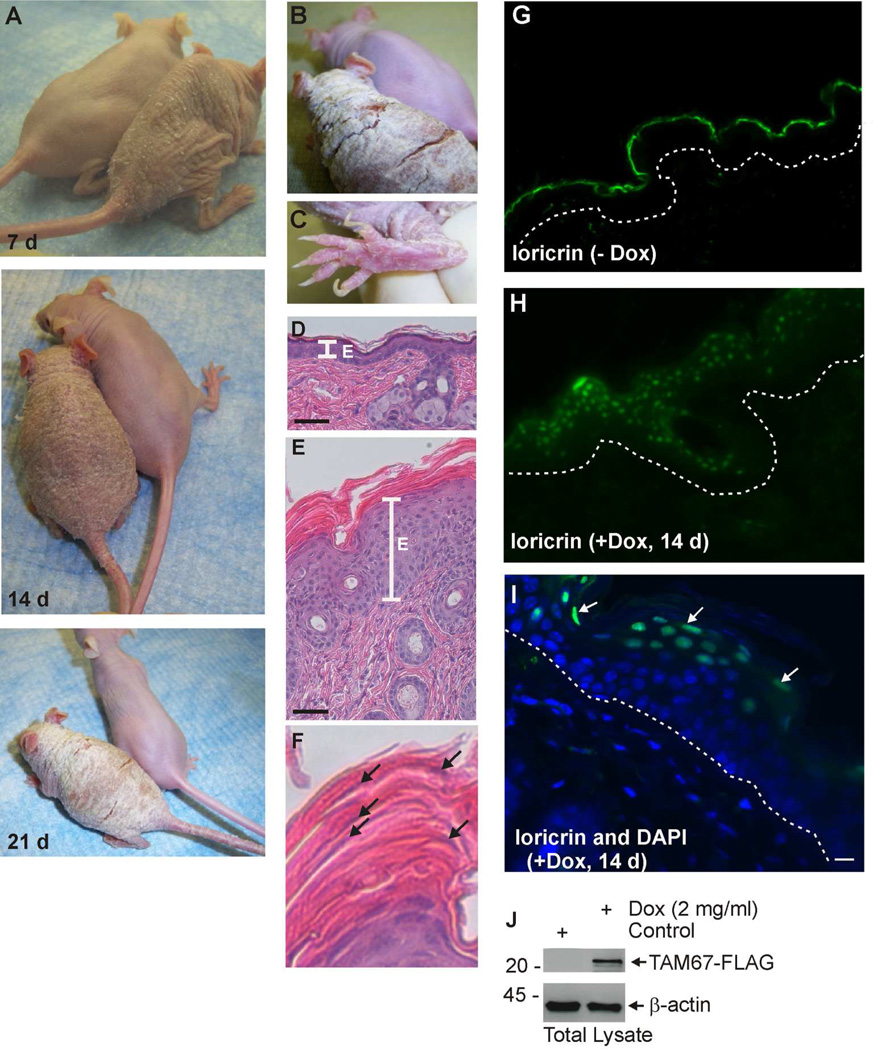
To characterize the phenotype of SKH1-TAM67-rTA mice, littermates were treated with or without 2 mg/ml doxycycline in drinking water for 0 – 21 days. The SKH-1 genetic background was used, as the hairless phenotype permits enhanced visualization of the phenotype. However, it is important to note that the mice are completely immune competent. Fig. 2A shows that TAM67-FLAG expression is associated with development of a honeycomb epidermal phenotype that is evident during the first week and that this phenotype progresses such that the mice develop extensive hyperkeratosis by 21 d. Fig. 2B shows a closer view of the 21 d doxycycline-treated mouse illustrating the extensive hyperkeratosis and fissures that develop across the back of the animal, and Fig. 2C shows the hyperkeratosis that develops on the bottom of the feet and the unusual “club” appearance of the nails. The toes also display a tendency towards pseudoainhum at the junction between the foot and digits. We next examined epidermal histology. Fig. 2D shows the thin epidermis and normal keratinization characteristic of control mice, while Fig. 2E shows the extensive hyperkeratosis observed in the epidermis of doxycycline-treated mice. Fig. 2F shows the extensive parakeratosis (nuclei retention, arrows) in dead cells of the cornified layer.
Fig. 2. Characterization of SKH1-TAM67-rTA mice.
SKH1-TAM67-rTA mice were administered drinking water supplemented with or without 2 mg/ml doxycycline. A Doxycycline-treated and untreated littermates were photographed at 7, 14 and 21 d. B/C Enlarged images showing hyperkeratinization of the dorsal epidermis and foot in mice treated for 21 d with doxycycline. Panel B includes an image of an untreated littermate. D Histological appearance of epidermis from untreated littermate. E Histological appearance of epidermis from 14 d doxycycline-treated littermate. The line labeled “E” marks the extent of the living epidermis. F Histological appearance of the cornified layer from panel E shows incomplete nuclear destruction (parakeratosis) in the cornified layer. The arrows indicate nuclei. G/H Loricrin distribution in untreated and 14 d doxycycline-treated SKH1-TAM67-rTA mice. The dotted lines indicate the dermal/epidermal junction. I Co-staining with DAPI (nuclear) and anti-loricrin confirms loricrin nuclear distribution in epidermis from 14 d doxycycline-treated mouse. J Immunoblot shows accumulation of TAM67-FLAG in 14 d doxycycline-treated mice and the absence of expression in untreated littermate.
Since mutant loricrin redistributes from the cell periphery to the nucleus in human keratoderma, we examined the tissue and subcellular distribution of murine loricrin in the TAM67-expressing mice (Ishida-Yamamoto et al., 1998a; Ishida-Yamamoto, 2003). Fig. 2G shows a thin margin of loricrin staining in the epidermal cornified layer of control mice. Much to our surprise wild-type loricrin redistributes to the nucleus in the suprabasal epidermis of TAM67-FLAG expressing mice (Fig. 2H). Fig. 1I shows sections co-stained with anti-loricrin and DAPI which confirms nuclear loricrin localization in suprabasal cells. Fig. 2J shows that doxycycline treatment results in expression of TAM67-FLAG and confirms the complete lack of expression in the absence of doxycycline. These studies show that SKH-1 mice expressing TAM67 in the suprabasal epidermis develop a phenotype that includes progressive epidermal hyperkeratosis which is associated with parakeratosis, hyperkeratosis of the skin and base of the feet, and nuclear accumulation of loricrin. This phenotype closely resembles features of human keratoderma (Camisa and Rossana, 1984; Schmuth et al., 2004).
Rapid onset of phenotype
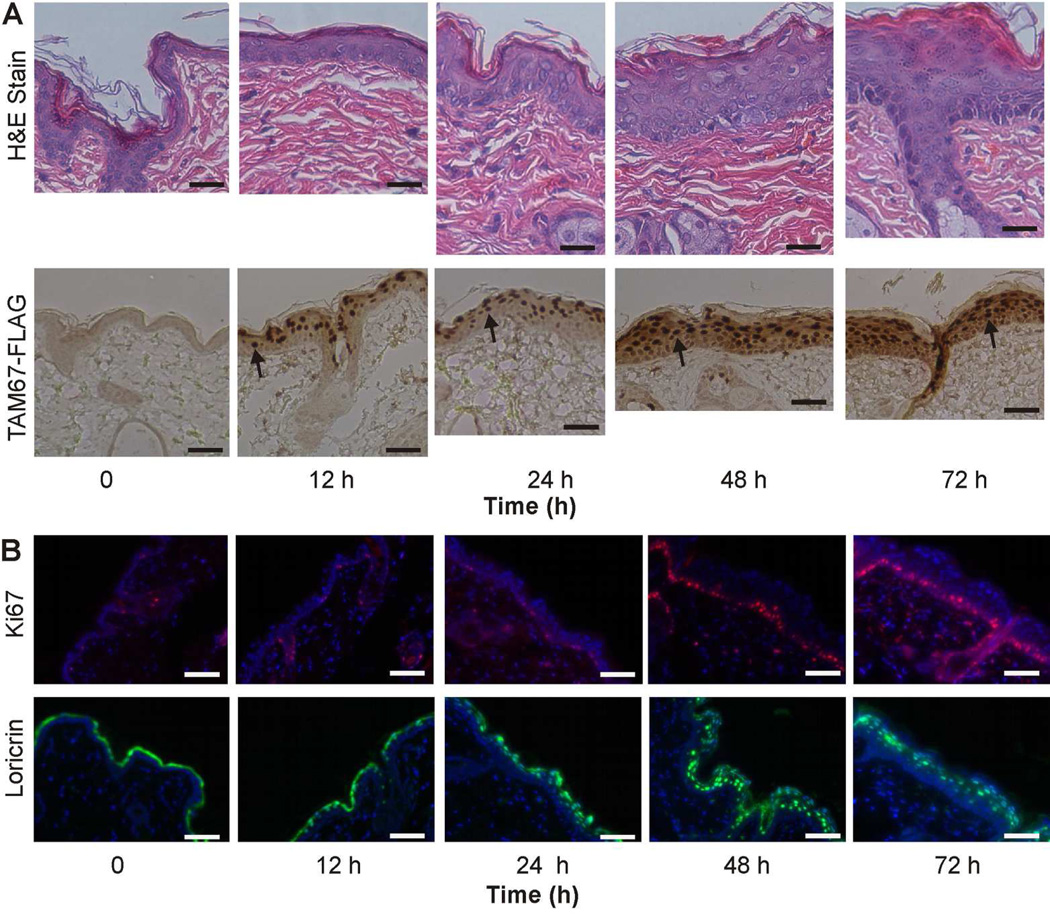
We next studied the time course of phenotype onset in order to understand the sequence of events during disease development. SKH1-TAM67-rTA mice were treated with doxycycline and epidermis was harvested at 0, 12, 24, 48 and 72 h after initiation of treatment. Fig. 3A shows initial evidence of nuclear TAM67-FLAG accumulation in epidermal suprabasal cells at 12 h with continued accumulation at subsequent times (arrows). TAM67-FLAG level, as measured by immunoblot, is maximal by 48 h and is maintained thereafter (not shown). Fig. 3A shows that no TAM67-FLAG is detected in nuclei of basal cells. To determine the time of initiation of epidermal cell hyperproliferation, we monitored Ki67 level. Fig. 3B shows increased Ki67 staining that is evident at 48 and 72 h and is associated with increased epidermal thickness as detected in H&E sections (Fig. 3A). We also monitored loricrin subcellular distribution during this time course (Fig. 3B). At time zero, loricrin is present as a thin band in the cornified layer, but at 24, 48 and 72 h after activation of TAM67-FLAG expression loricrin assumes a punctate intracellular localization (Fig. 3B).
Fig. 3. Time course of impact of TAM67-FLAG on epidermal endpoints.
SKH1-TAM67-rTA mice were administered 2 mg/ml doxycycline in drinking water and at the indicated times tissue was harvested for characterization. A Epidermal morphology (H&E stain) and immunodetection of TAM67-FLAG using a peroxidase-based secondary antibody. The arrows indicate nuclear TAM67-FLAG accumulation in cells of the suprabasal epidermis. B Immunodetection of Ki67 and loricrin. Ki67 is detected in the basal and immediately suprabasal cell layers in TAM67-FLAG expressing mice. Loricrin shifts from staining the margin of the cornified layer 0 and 12 h, to staining of nuclei at 24, 48 and 72 h.
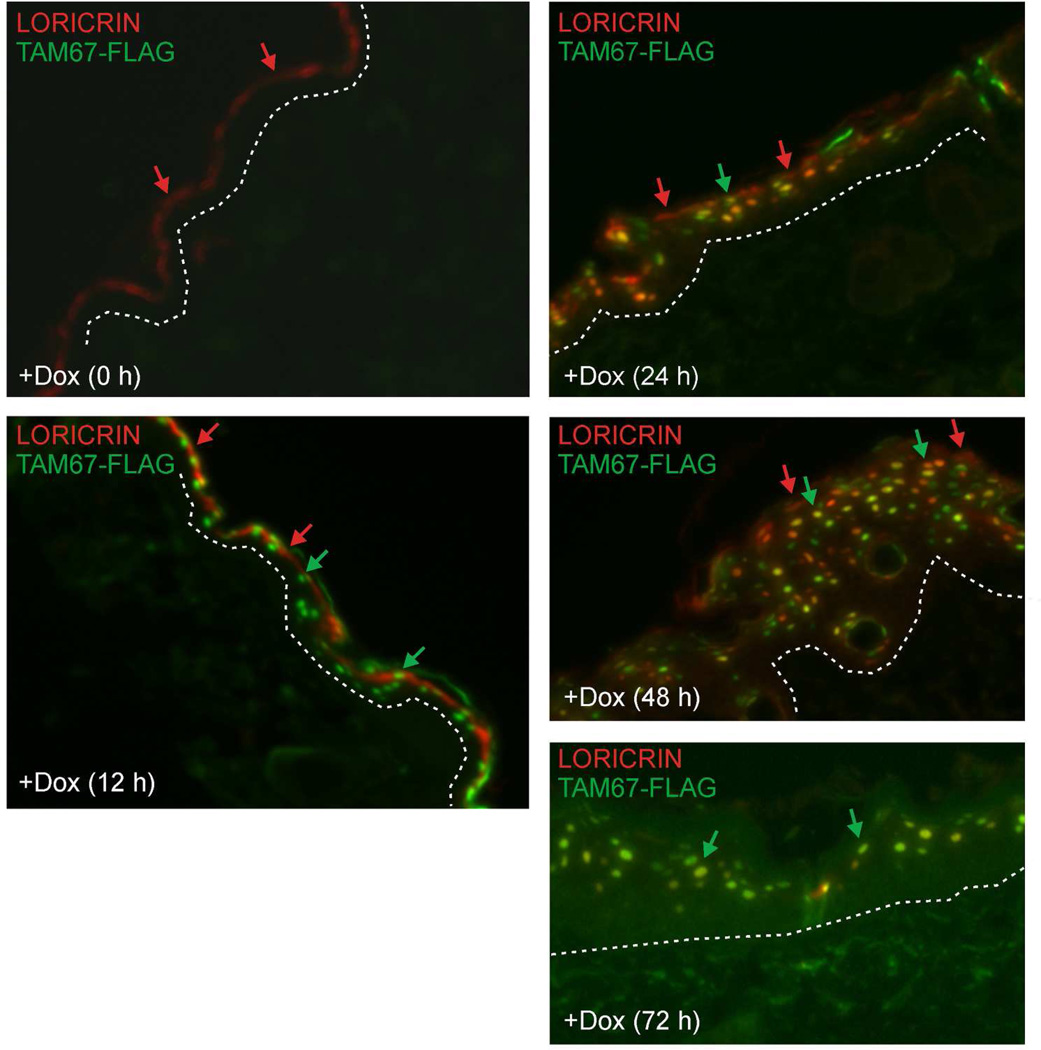
We examined loricrin and TAM67-FLAG localization in detail by co-staining sections with anti-loricrin (red) and anti-TAM67-FLAG (green). At time zero loricrin is detected as part of the cornified envelope (red) and, as expected, no TAM67-FLAG (green) is detected (Fig. 4). At 12 h after initiation of doxycycline treatment, TAM67-FLAG is detected in the nuclei while loricrin remains associated with the cornified envelope. However, by 24 h there is clear evidence for redistribution of loricrin into some nuclei in the suprabasal layer (red). Moreover, this accumulation is maintained at 48 and 72 h (Fig. 4) and 14 d (Fig. 2H and I). These findings indicate that nuclear loricrin accumulation is an early event in phenotype development and that nuclear accumulation is sustained thereafter. It is interesting to note that loricrin is present in nuclei that are strongly and weakly TAM67-FLAG positive (Fig. 4), suggesting that TAM67-FLAG does not directly act to move loricrin to the nucleus. We speculate that loricrin may be moved to the nucleus via carrier protein-dependent transport, but this remains to be determined.
Fig. 4.
Staining of loricrin (red) and TAM67-FLAG (green) showing that TAM67-FLAG appears in the nucleus at 12 h and loricrin appears in the nucleus beginning at 24 h. Red arrows indicate loricrin localization, green arrows indicate TAM67-FLAG localization, and yellow signal indicates co-localization. TAM67-FLAG and loricrin co-localize in many but not all suprabasal nuclei.
Pseudoainhum
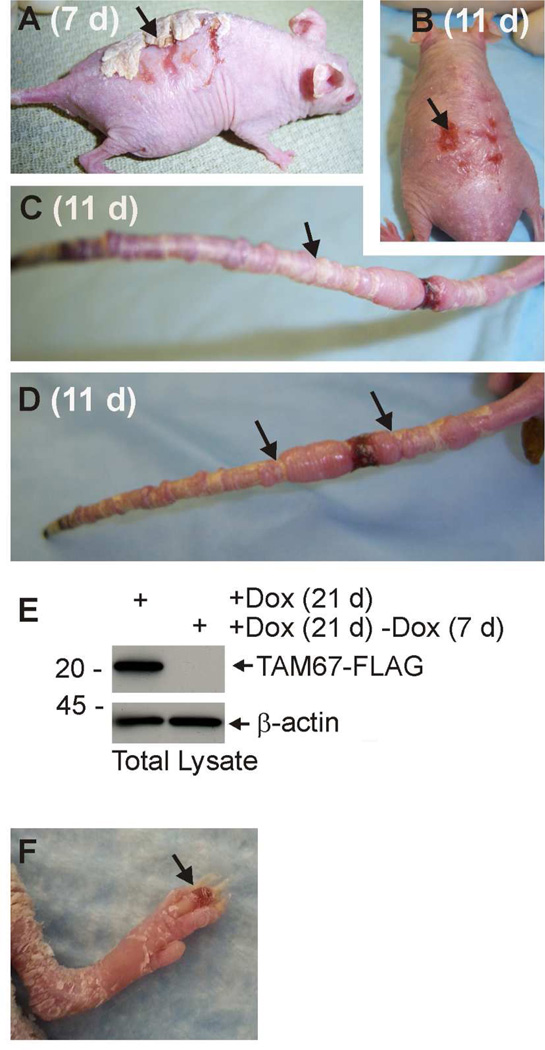
Pseudoainhum (constriction) and autoamputation of the digits is a signature phenotype of loricrin keratoderma patients, and is also observed in mutant loricrin-expressing mice (Ishida-Yamamoto et al., 1997; Suga et al., 2000). As shown in Fig. 5, TAM67 expressing mice develop a severe pseudoainhum response. Constriction bands accumulate on the tail and are particularly evident after removal of doxycycline when the phenotype is permitted to normalize. The mouse in Fig. 5 was treated with doxycycline for 21 d followed by removal of doxycycline. The photographs (Fig. 5A/B) were taken at 7 and 11 d after doxycycline removal. This shows that the accumulated scale is lost with the loss of TAM67 expression, indicating that the phenotype is reversible (Fig. 5A) with some residual scarring that slowly normalizes (Fig. 5B). Fig. 5C and 5D show the tails of two mice at 11 d after removal of doxycycline treatment. The arrows indicate some of the restriction bands on the tail. A significant number of mice lose all or a portion of the tail. Fig. 5E confirms that removal of doxycycline results in loss of TAM67-FLAG expression. Pseudoainhum is also observed on the digits. Fig. 5F is an image showing development of edema in a digit undergoing pseudoainhum. This phenotype mimics the human disease (Seirafi et al., 2011).
Fig. 5. Pseudoainhum (autoamputation) in TAM67-expressing mice.
SKH1-TAM67-rTA mice were treated for 21 d with 2 mg/ml doxycycline and the doxycycline was then removed for 7 or 11 d. A/B Appearance of mice at 7 and 11 d after discontinuation of doxycycline treatment. The accumulated cornified material is progressively shed from the skin surface. The arrows indicate the remaining cornified material and a residual scar. C/D Appearance of the tails of two mice after 21 d doxycycline treatment followed by 11 d without doxycycline. The arrows show representative pseudoainhum bands on each tail. In some mice the tail is lost due to autoamputation. E An immunoblot showing TAM67- FLAG level in 21 d doxycycline treatment mice versus mice treated for 21 d followed by 7 d of discontinued treatment. F Early stage pseudoainhum of digits in mouse treated for 21 d with doxycycline. The arrow indicats pseudoainhum-associated edema.
Role of loricrin
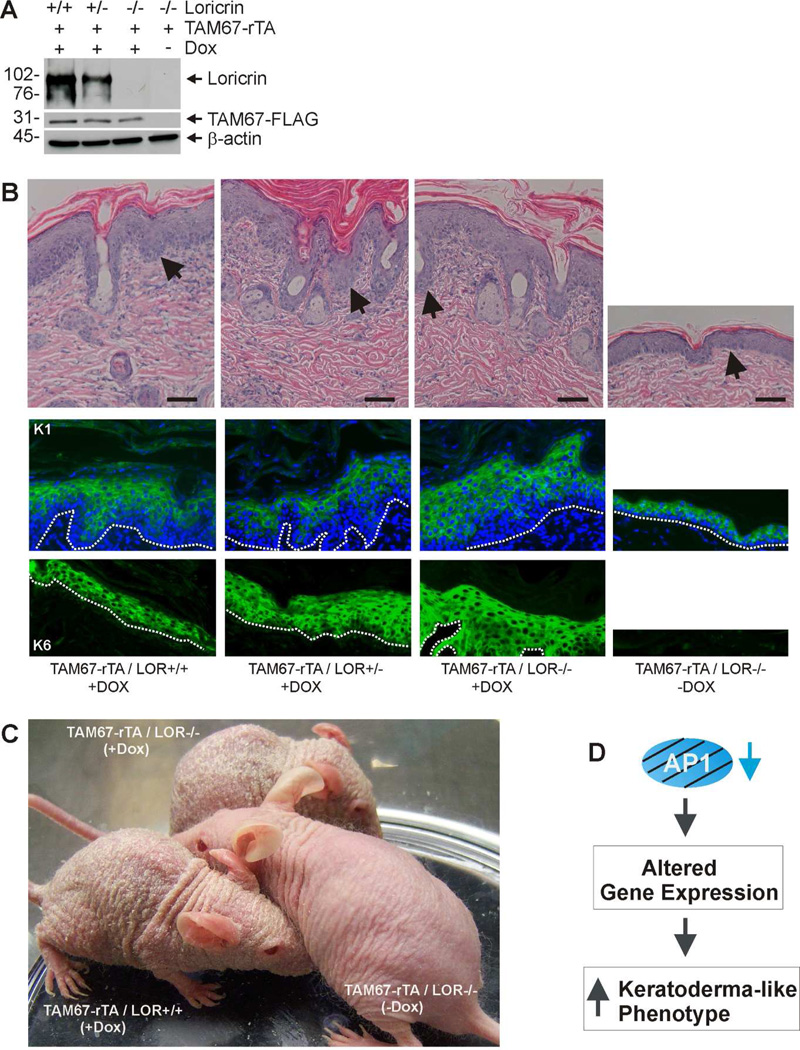
An interesting and unexpected observation is the nuclear accumulation of wild-type loricrin in epidermal keratinocytes in TAM67-expressing mice (Fig. 4C). This begs the question of whether nuclear accumulation of wild-type loricrin is required for keratoderma phenotype development in this model. To assess this, we examined the impact of TAM67 expression in SKH1-TAM67-rTA mice that are homozygous, heterozygous and null for loricrin. Mice were treated for 21 d with doxycycline prior to tissue harvest. Fig. 6A confirms the TAM67 and loricrin expression status of the mice, and shows that doxycycline treatment activates TAM67-FLAG expression at comparable levels in each strain. We next examined the impact of loricrin loss on the TAM67-dependent phenotype. H&E staining shows that TAM67 expression induces an epidermal hyperproliferative phenotype in the presence and absence of loricrin and that this is associated with activation of expression of the hyperproliferation-associated keratin, K6 (Fig. 6B). As a control, we show that suprabasal expression of keratin 1 is maintained (Fig. 6B). However, the most striking observation is that the epidermal scaling keratoderma-like phenotype develops unabated in mice with reduced or absent loricrin (Fig. 6C).
Fig. 6. Suppression of suprabasal AP1 factor signaling is sufficient for phenotype development - loricrin is not required.
A Immunoblot confirmation of mouse genotype. SKH-1 mice of the indicated TAM67-FLAG and loricrin genotypes were treated for 21 d with (+) or without (−) 2 mg/ml doxycycline. At 21 d epidermal extracts were prepared for detection of the indicated proteins. B H&E, K1 and K6 staining of epidermis from 21 d doxycycline-treated mice. Arrows or dotted lines indicate that epidermal/dermal junction. C Appearance of mice of the indicated genotype after 21 d of doxycycline treatment. Absence of loricrin did not prevent phenotype development. D Hypothesis: Inhibition of suprabasal AP1 factor signaling produces a keratoderma-like phenotype that closely resembles human keratoderma by altering gene expression.
Discussion
Keratodermas are a group of diseases characterized by thickening of the epidermis associated with marked hyperplasia and hyperkeratosis (Christiano, 1997). In the course of studies designed to understand AP1 transcription factor function in epidermis, we produced mice in which AP1 transcription factor function is inactivated in the suprabasal epidermis (Rorke et al., 2010). In this model, we induce suprabasal epidermal expression of TAM67, a dominant-negative form of c-jun which inhibits AP1 factor function, using a doxycycline-inducible system. TAM67 dimerizes with and inhibits transcriptional activation potential of all AP1 factors. We show that TAM67-FLAG interacts with AP1 response elements in a competitive manner and that this is associated with reduced expression of representative AP1 factor responsive genes. A remarkable finding is that these mice display a phenotype that includes all of the hallmarks of keratoderma. As AP1 factor inactivation in the basal epidermis produces no such phenotype (Cooper et al., 2003; Young et al., 2002; Young et al., 1999), this implies that epidermal compartment-specific changes in transcription factor signaling can cause keratoderma pathogenesis. Early changes include a progressive increase in skin thickness at 48 to 72 h after TAM67 expression, and a parallel increase in cell proliferation. This is associated with nuclear accumulation of wild-type loricrin and changes in expression of differentiation markers. On a longer time scale, beginning after several days, the mice develop a honeycomb epidermal appearance. This is followed by accumulation of a thick layer of epidermal cornified material, and tail and digit pseudoainhum and autoamputation. The fact that this phenotype so closely mimics human keratoderma strongly suggests that suppression of AP1 factor signaling is an important event in disease development.
Keratoderma includes both inherited and acquired forms. Inherited forms of this disease are associated with mutations in structural proteins (keratins), cornified envelope-associated proteins (loricrin, transglutaminase), cohesion proteins (desmoglein 1, plakophilin, desmoplakin), proteins of cell-to-cell communication (connexins), and transmembrane proteins (cathepsin C) (Kimyai-Asadi et al., 2002; Christiano, 1997; Amagai and Stanley, 2012). Most of these mutations are autosomal dominant, although some are recessive (Kimyai-Asadi et al., 2002). Vohwinkel Syndrome and the Camisa variant of Vohwinkel Syndrome are highly debilitating forms of the disease that display pseudoainhum and autoamputation of the digits and other extremities. Connexin mutations have been identified in Vohwinkel Syndrome (Scott et al., 2012) and targeted expression of mutant connexin in the suprabasal epidermis in mice produces a keratoderma-like phenotype (Schmuth et al., 2004). A mutation in the loricrin gene, which leads to nuclear accumulation of loricrin, is associated with the Carmisa variant of Vohwinkel Syndrome (Suga et al., 2000). In addition, some forms of keratoderma are acquired in response to treatment with drugs, exposure to chemicals, malnutrition, infection or response to cancer (Patel et al., 2007). However, the underlying mechanisms that drive these diseases are not well understood. In particular knowledge of changes in cell signaling is limited.
AP1 factors and keratoderma
AP1 transcription factors (c-jun, junB, junD, c-fos, Fra-1, Fra-2) are important controllers of keratinocyte differentiation. They control differentiation and proliferation, and are frequently perturbed in disease processes (Gerdes et al., 2006; Efimova et al., 2003; Efimova et al., 2004; Crish et al., 1993; Eckert et al., 1997; Eckert et al., 2004; Welter et al., 1995). A role for transcription factors in development of keratoderma has not been studied. The present study is the first showing that direct suppression of AP1 transcription factor function can produce a keratoderma phenotype (Kimyai-Asadi et al., 2002). It is interesting that the phenotype is observed in the absence of mutation in proteins known to be mutated in keratoderma, and that the phenotype is only observed when AP1 factor function is selectively inactivated in the suprabasal epidermis (Cooper et al., 2003; Young et al., 2002; Young et al., 1999). Suprabasal AP1 factor inactivation appears to inhibit/delay normal keratinocyte differentiation and also cause an increase in basal layer proliferation. The latter suggests a potential indirect effect due to incomplete differentiation and may involve paracrine mediators. Given the central role that AP1 factors have in regulating keratinocyte function, it is perhaps not surprising that reducing AP1 factor signaling produces a keratoderma phenotype. It is interesting to speculate that suppressed AP1 factor function may be an underlying response that is common in a range of keratoderma types - both genetically inherited and acquired.
Role of loricrin
An interesting property of the mice produced in the present study is the presence of two signature features commonly associated with the Camisa variant of Vohwinkel Syndrome (loricrin keratoderma). These are nuclear loricrin accumulation and pseudoainhum/autoamputation. Loricrin keratoderma is an interesting form of the disease that is characterized by honeycomb-like palmoplantar keratoderma, hyperkeratosis, tissue buildup on the dorsal hands and feet, nuclear accumulation of mutant loricrin, and pseudoainhum and autoamputation of extremities. Loricrin is a structural protein that is a component of the cornified envelope (Candi et al., 2005; Mehrel et al., 1990). In normal epidermis, it is produced during the terminal stages of differentiation and is then crosslinked as part of the cornified envelope (Hohl et al., 1991; Mehrel et al., 1990). In keratoderma, mutant loricrin accumulates in the nucleus due to a frame shift mutation that adds a nuclear localization signal at the c-terminal end of the protein (Korge et al., 1997; Maestrini et al., 1996; O'Driscoll et al., 2002; Matsumoto et al., 2001; Takahashi et al., 1999; Gedicke et al., 2006; Drera et al., 2008). However, what loricrin does in the nucleus is not known. Although wild-type loricrin is not thought to cause disease, our observation of its accumulation in the nucleus at early times (12 to 24 h) during phenotype development suggests that it could be driving the phenotype. Moreover, some investigators have described nuclear localization of wild-type loricrin (Yoneda et al., 2010a; Yoneda et al., 2010b; Ishida-Yamamoto et al., 1993). We do not know why wild-type loricrin accumulates in the nucleus in the presence of TAM67, but we presume that unknown factors are produced that facilitate this movement. To determine whether wild-type loricrin has a role in disease development, we studied phenotype development in a loricrin-null background. These studies clearly indicate that a complete phenotype develops in the absence of loricrin, indicating that wild-type loricrin has no role. Thus, suprabasal AP1 factor inactivation is sufficient to drive the phenotype.
Pseudoainhum
Pseudoainhum and autoamputation is associated with Vohwinkel Syndrome and the Camisa Vohwinkel Syndrome variant (Maestrini et al., 1999; Camisa and Rossana, 1984; Schmuth et al., 2004; Korge et al., 1997). In our model, suprabasal AP1 factor inactivation produces digit and tail pseudoainhum and autoamputation. The phenotype is particularly evident on the tail. This is a complex aspect of keratoderma that is not well understood (Rashid et al., 2007). The disease is thought to involve a complex series of events that include abnormal vascular ligation, secondary atrophy and fibrosis, and constriction leading to autoamputation (Rashid et al., 2007). It also may involve genetic or systemic components, as the response is often symmetric and bilateral (Rashid et al., 2007). It is particularly interesting that compromised suprabasal AP1 factor function may trigger a series of events that drive this phenomenon.
The fact that the phenotype is inducible and reversible in our model suggests that it will be possible to study pseudoainhum and autoamputation development and regression. Based on these finding, we propose that reduced AP1 transcription factor function in the suprabasal epidermis leads to presently unknown changes in gene expression that drives the keratoderma phenotype (Fig. 6D). We further propose that changes in AP1 factor signaling may be a common event in multiple keratoderma types, and may be involved in the pathogenesis of acquired keratoderma which develops in response to environmental factors.
Materials and Methods
SKH1-TAM67-rTA mice
TAM67 is a dominant-negative form of c-jun which lacks the c-jun amino terminus (Brown et al., 1994). TAM67 forms complexes with all jun and fos transcription factors, but when these complexes interact with DNA response elements they fail to active transcription (Brown et al., 1994). Thus, it can be used to create an AP1 factor function-deficient environment. We cloned TAM67-FLAG into pTRE-Tight to produce pTRE-Tight-TAM67-FLAG. The TetO-TAM67-FLAG-SV40 transcription cassette from this plasmid was microinjected into B6SJL embryos (Rorke et al., 2010) to produce TetO-TAM67-FLAGTG/- (strain 44) mice. A FLAG epitope was included at the carboxyl terminus of TAM67 to facilitate TAM67 detection. These mice were mated with FVB hINV-rTATG/- mice (Jaubert et al., 2004) to produce bi-transgenic B6SJL/FVB TAM67-FLAGTG/- / hINV-rTATG/- mice (TAM67-rTA) (Rorke et al., 2010).
In the present studies, we transferred the TetO-TAM67-FLAG and hINV-rTA genes to the SKH-1 genetic background by breeding for five generations. These mice were then bred to produce SKH1- TAM67-rTA mice. Treatment of these mice with doxycycline results in TAM67-FLAG expression in the suprabasal layers. This is due to involucrin promoter specific targeting of rTA to these layers (Rorke et al., 2010). The mice were genotyped using DNA-dependent PCR and primers that detect each transgene (Rorke et al., 2010). The SKH-1 genetic background was utilized in these studies because the mice are hairless and this facilitates visualization of the epidermis. SKH-1 mice are immune-competent. Loricrin knockout mice (Koch et al., 2000) were bred for five generations into the SKH-1 background. The wild-type and loricrin knockout genotypes were detected using 5’-CACTCCCAGCACAATAAGAA and 5’-TTGCATCGCATTGTCTGAGT, respectively, as the forward primers. The reverse primer was 5’-GCACTGGGAGAGTGGTAAGC for both wild-type and knockout mice. The primers produce bands at approximately 350 (knockout) and 650 (wild-type) nucleotides. The mice were housed in the University of Maryland School of Medicine animal facility in compliance with NIH regulations.
Antibodies and immunological methods
Immunofluorescence was performed using paraffin-embedded formalin-fixed sections as previously described (Crish et al., 1998; Crish et al., 2002; Crish et al., 2006). Loricrin (PRB-145P), K1 and K6 antibodies were from Covance (Emeryville, CA). Ki67 (TEC- 3) antibody was from Dako (Cupertino, CA), and β-actin (A5441) and FLAG (M2) (F4049) specific antibodies were obtained from Sigma (St. Louis, MO). Primary antibody localization was visualized using an appropriate fluorophore-conjugated secondary antibody. For immunoblot, epidermis was separated from dermis, frozen in liquid nitrogen, pulverized and suspended in dye-free Laemmli sample buffer. The suspension was sonicated, particulates were removed by centrifugation, and soluble extract was electrophoresed on a polyacrylamide gel and transferred to nitrocellulose for immunoblot (Crish et al., 1998; Crish et al., 2002; Crish et al., 2006). Unless otherwise indicated, the experiments were repeated three separate times, and sections and extracts were monitored from epidermis of three mice per treatment group.
Gel mobility shift assay
Epidermis was isolated for preparation of nuclear extract using NE-PER Nuclear and Cytoplasmic Extraction Reagent (78833, Pierce Biotechnology, Rockford, IL). TAM67- FLAG binding to double-stranded AP1 consensus (AP1c) oligonucleotide 5′-CGCTTGATGAGTCAGCCGGAA-3′ (E320A, Promega, Madison, WI, AP1 site in bold) was monitored by gel mobility shift assay. Three micrograms of nuclear extract was incubated for 30 min at room temperature in a volume of 20 µl containing 20 mM HEPES, pH 7.5, 10% glycerol, 50 mM KCl, 2 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 1 µg/ml poly(dI:dC), 0.1 mg/ml bovine serum albumin, and 40,000 cpm radioactive double-stranded AP1c-P32 or AP1-5-P32 oligonucleotide. For competition studies, a fixed (40-fold) molar excess of non-radioactive AP1c competitor oligonucleotide was added to the DNA binding reaction. For gel mobility supershift assay, 2 µg of antibody specific for FLAG (F3165, Sigma, St. Louis, MO) was added to the reaction mixture and incubated 1 h at 25 °C. The 32P-labeled probe was then added and the incubation was continued for an additional 30 min at 25 °C. Protein-DNA complexes were resolved in 6% polyacrylamide gels under nondenaturing conditions (Adhikary et al., 2004; Adhikary et al., 2005).
Acknowledgements
This work was supported by NIH R01 AR046494 (R. Eckert) and NIH R01 AR060388 (D. Roop).
Footnotes
Conflict of Interest
The authors have no conflict of interest financial or otherwise.
References
- Adhikary G, Crish J, Lass J, et al. Regulation of involucrin expression in normal human corneal epithelial cells: a role for activator protein one. Invest Ophthalmol Vis Sci. 2004;45:1080–1087. doi: 10.1167/iovs.03-1180. [DOI] [PubMed] [Google Scholar]
- Adhikary G, Crish JF, Gopalakrishnan R, et al. Involucrin expression in the corneal epithelium: an essential role for Sp1 transcription factors. Invest Ophthalmol Vis Sci. 2005;46:3109–3120. doi: 10.1167/iovs.05-0053. [DOI] [PubMed] [Google Scholar]
- Amagai M, Stanley JR. Desmoglein as a target in skin disease and beyond. J Invest Dermatol. 2012;132:776–784. doi: 10.1038/jid.2011.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DK, McKenna KE, Hughes AE. A novel insertional mutation in loricrin in Vohwinkel's Keratoderma. J Invest Dermatol. 1998;111:702–704. doi: 10.1046/j.1523-1747.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Brown PH, Chen TK, Birrer MJ. Mechanism of action of a dominant-negative mutant of c-Jun. Oncogene. 1994;9:791–799. [PubMed] [Google Scholar]
- Camisa C, Rossana C. Variant of keratoderma hereditaria mutilans (Vohwinkel's syndrome). Treatment with orally administered isotretinoin. Arch Dermatol. 1984;120:1323–1328. [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Christiano AM. Frontiers in keratodermas: pushing the envelope. Trends Genet. 1997;13:227–233. doi: 10.1016/S0168-9525(97)01104-9. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, MacGowan J, Ranger-Moore J, et al. Expression of dominant negative c-jun inhibits ultraviolet B-induced squamous cell carcinoma number and size in an SKH-1 hairless mouse model. Mol Cancer Res. 2003;1:848–854. [PubMed] [Google Scholar]
- Crish JF, Bone F, Banks EB, et al. The human involucrin gene contains spatially distinct regulatory elements that regulate expression during early versus late epidermal differentiation. Oncogene. 2002;21:738–747. doi: 10.1038/sj.onc.1205038. [DOI] [PubMed] [Google Scholar]
- Crish JF, Gopalakrishnan R, Bone F, et al. The distal and proximal regulatory regions of the involucrin gene promoter have distinct functions and are required for in vivo involucrin expression. J Invest Dermatol. 2006;126:305–314. doi: 10.1038/sj.jid.5700019. [DOI] [PubMed] [Google Scholar]
- Crish JF, Howard JM, Zaim TM, et al. Tissue-specific and differentiation-appropriate expression of the human involucrin gene in transgenic mice: an abnormal epidermal phenotype. Differentiation. 1993;53:191–200. doi: 10.1111/j.1432-0436.1993.tb00708.x. [DOI] [PubMed] [Google Scholar]
- Crish JF, Zaim TM, Eckert RL. The distal regulatory region of the human involucrin promoter is required for expression in epidermis. J Biol Chem. 1998;273:30460–30465. doi: 10.1074/jbc.273.46.30460. [DOI] [PubMed] [Google Scholar]
- Drera B, Tadini G, Balbo F, et al. De novo occurrence of the 730insG recurrent mutation in an Italian family with the ichthyotic variant of Vohwinkel syndrome, loricrin keratoderma. Clin Genet. 2008;73:85–88. doi: 10.1111/j.1399-0004.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Banks EB, et al. The epidermis: genes on - genes off. J Invest Dermatol. 1997;109:501–509. doi: 10.1111/1523-1747.ep12336477. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Efimova T, et al. Regulation of involucrin gene expression. J Invest Dermatol. 2004;123:13–22. doi: 10.1111/j.0022-202X.2004.22723.x. [DOI] [PubMed] [Google Scholar]
- Efimova T, Broome AM, Eckert RL. A regulatory role for p38 delta MAPK in keratinocyte differentiation. Evidence for p38 delta-ERK1/2 complex formation. J Biol Chem. 2003;278:34277–34285. doi: 10.1074/jbc.M302759200. [DOI] [PubMed] [Google Scholar]
- Efimova T, Broome AM, Eckert RL. Protein kinase Cdelta regulates keratinocyte death and survival by regulating activity and subcellular localization of a p38delta-extracellular signal-regulated kinase 1/2 complex. Mol Cell Biol. 2004;24:8167–8183. doi: 10.1128/MCB.24.18.8167-8183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova T, Deucher A, Kuroki T, et al. Novel protein kinase C isoforms regulate human keratinocyte differentiation by activating a p38 delta mitogen-activated protein kinase cascade that targets CCAAT/enhancer-binding protein alpha. J Biol Chem. 2002;277:31753–31760. doi: 10.1074/jbc.M205098200. [DOI] [PubMed] [Google Scholar]
- Efimova T, Eckert RL. Regulation of human involucrin promoter activity by novel protein kinase C isoforms. J Biol Chem. 2000;275:1601–1607. doi: 10.1074/jbc.275.3.1601. [DOI] [PubMed] [Google Scholar]
- Efimova T, LaCelle P, Welter JF, et al. Regulation of human involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J Biol Chem. 1998;273:24387–24395. doi: 10.1074/jbc.273.38.24387. [DOI] [PubMed] [Google Scholar]
- Gedicke MM, Traupe H, Fischer B, et al. Towards characterization of palmoplantar keratoderma caused by gain-of-function mutation in loricrin: analysis of a family and review of the literature. Br J Dermatol. 2006;154:167–171. doi: 10.1111/j.1365-2133.2005.06995.x. [DOI] [PubMed] [Google Scholar]
- Gerdes MJ, Myakishev M, Frost NA, et al. Activator protein-1 activity regulates epithelial tumor cell identity. Cancer Res. 2006;66:7578–7588. doi: 10.1158/0008-5472.CAN-06-1247. [DOI] [PubMed] [Google Scholar]
- Han B, Rorke EA, Adhikary G, et al. Suppression of AP1 transcription factor function in keratinocyte suppresses differentiation. PLoS One. 2012;7:e36941. doi: 10.1371/journal.pone.0036941. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hohl D, Mehrel T, Lichti U, et al. Characterization of human loricrin. Structure and function of a new class of epidermal cell envelope proteins. J Biol Chem. 1991;266:6626–6636. [PubMed] [Google Scholar]
- Ishida-Yamamoto A. Loricrin keratoderma: a novel disease entity characterized by nuclear accumulation of mutant loricrin. J Dermatol Sci. 2003;31:3–8. doi: 10.1016/s0923-1811(02)00143-3. [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Hohl D, Roop DR, et al. Loricrin immunoreactivity in human skin: localization to specific granules (L-granules) in acrosyringia. Arch Dermatol Res. 1993;285:491–498. doi: 10.1007/BF00376822. [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Kato H, Kiyama H, et al. Mutant loricrin is not crosslinked into the cornified cell envelope but is translocated into the nucleus in loricrin keratoderma. J Invest Dermatol. 2000;115:1088–1094. doi: 10.1046/j.1523-1747.2000.00163.x. [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, McGrath JA, Lam H, et al. The molecular pathology of progressive symmetric erythrokeratoderma: a frameshift mutation in the loricrin gene and perturbations in the cornified cell envelope. Am J Hum Genet. 1997;61:581–589. doi: 10.1086/515518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Takahashi H, Iizuka H. Loricrin and human skin diseases: molecular basis of loricrin keratodermas. Histol Histopathol. 1998a;13:819–826. doi: 10.14670/HH-13.819. [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Takahashi H, Presland RB, et al. Translocation of profilaggrin N-terminal domain into keratinocyte nuclei with fragmented DNA in normal human skin and loricrin keratoderma. Lab Invest. 1998b;78:1245–1253. [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Tanaka H, Nakane H, et al. Programmed cell death in normal epidermis and loricrin keratoderma. Multiple functions of profilaggrin in keratinization. J Investig Dermatol Symp Proc. 1999;4:145–149. doi: 10.1038/sj.jidsp.5640198. [DOI] [PubMed] [Google Scholar]
- Jang SI, Steinert PM. Loricrin expression in cultured human keratinocytes is controlled by a complex interplay between transcription factors of the Sp1, CREB, AP1, and AP2 families. J Biol Chem. 2002;277:42268–42279. doi: 10.1074/jbc.M205593200. [DOI] [PubMed] [Google Scholar]
- Jarnik M, de Viragh PA, Scharer E, et al. Quasi-normal cornified cell envelopes in loricrin knockout mice imply the existence of a loricrin backup system. J Invest Dermatol. 2002;118:102–109. doi: 10.1046/j.0022-202x.2001.01661.x. [DOI] [PubMed] [Google Scholar]
- Jaubert J, Patel S, Cheng J, et al. Tetracycline-regulated transactivators driven by the involucrin promoter to achieve epidermal conditional gene expression. J Invest Dermatol. 2004;123:313–318. doi: 10.1111/j.0022-202X.2004.23203.x. [DOI] [PubMed] [Google Scholar]
- Kimyai-Asadi A, Kotcher LB, Jih MH. The molecular basis of hereditary palmoplantar keratodermas. J Am Acad Dermatol. 2002;47:327–343. doi: 10.1067/mjd.2002.124814. [DOI] [PubMed] [Google Scholar]
- Koch PJ, de Viragh PA, Scharer E, et al. Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J Cell Biol. 2000;151:389–400. doi: 10.1083/jcb.151.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge BP, Ishida-Yamamoto A, Punter C, et al. Loricrin mutation in Vohwinkel's keratoderma is unique to the variant with ichthyosis. J Invest Dermatol. 1997;109:604–610. doi: 10.1111/1523-1747.ep12337534. [DOI] [PubMed] [Google Scholar]
- Maestrini E, Korge BP, Ocana-Sierra J, et al. A missense mutation in connexin26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel's syndrome) in three unrelated families. Hum Mol Genet. 1999;8:1237–1243. doi: 10.1093/hmg/8.7.1237. [DOI] [PubMed] [Google Scholar]
- Maestrini E, Monaco AP, McGrath JA, et al. A molecular defect in loricrin, the major component of the cornified cell envelope, underlies Vohwinkel's syndrome. Nat Genet. 1996;13:70–77. doi: 10.1038/ng0596-70. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Muto M, Seki S, et al. Loricrin keratoderma: a cause of congenital ichthyosiform erythroderma and collodion baby. Br J Dermatol. 2001;145:657–660. doi: 10.1046/j.1365-2133.2001.04412.x. [DOI] [PubMed] [Google Scholar]
- Mehrel T, Hohl D, Rothnagel JA, et al. Identification of a major keratinocyte cell envelope protein, loricrin. J Cell Biochem. 1990;61:1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- O'Driscoll J, Muston GC, McGrath JA, et al. A recurrent mutation in the loricrin gene underlies the ichthyotic variant of Vohwinkel syndrome. Clin Exp Dermatol. 2002;27:243–246. doi: 10.1046/j.1365-2230.2002.01031.x. [DOI] [PubMed] [Google Scholar]
- Patel S, Zirwas M, English JC., III Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1–11. doi: 10.2165/00128071-200708010-00001. [DOI] [PubMed] [Google Scholar]
- Rashid RM, Cowan E, Abbasi SA, et al. Destructive deformation of the digits with auto-amputation: a review of pseudo-ainhum. J Eur Acad Dermatol Venereol. 2007;21:732–737. doi: 10.1111/j.1468-3083.2007.02224.x. [DOI] [PubMed] [Google Scholar]
- Rorke EA, Adhikary G, Jans R, et al. AP1 factor inactivation in the suprabasal epidermis causes increased epidermal hyperproliferation and hyperkeratosis but reduced carcinogen-dependent tumor formation. Oncogene. 2010;29:5873–5882. doi: 10.1038/onc.2010.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuth M, Fluhr JW, Crumrine DC, et al. Structural and functional consequences of loricrin mutations in human loricrin keratoderma (Vohwinkel syndrome with ichthyosis) J Invest Dermatol. 2004;122:909–922. doi: 10.1111/j.0022-202X.2004.22431.x. [DOI] [PubMed] [Google Scholar]
- Scott CA, Tattersall D, O'Toole EA, et al. Connexins in epidermal homeostasis and skin disease. Biochim Biophys Acta. 2012;1818:1952–1961. doi: 10.1016/j.bbamem.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Seirafi H, Khezri S, Morowati S, et al. A new variant of Vohwinkel syndrome: A case report. Dermatol Online J. 2011;17:3. [PubMed] [Google Scholar]
- Song S, Shen C, Song G, et al. A novel c.545-546insG mutation in the loricrin gene correlates with a heterogeneous phenotype of loricrin keratoderma. Br J Dermatol. 2008;159:714–719. doi: 10.1111/j.1365-2133.2008.08657.x. [DOI] [PubMed] [Google Scholar]
- Suga Y, Jarnik M, Attar PS, et al. Transgenic mice expressing a mutant form of loricrin reveal the molecular basis of the skin diseases, Vohwinkel syndrome and progressive symmetric erythrokeratoderma. J Cell Biol. 2000;151:401–412. doi: 10.1083/jcb.151.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Ishida-Yamamoto A, Kishi A, et al. Loricrin gene mutation in a Japanese patient of Vohwinkel's syndrome. J Dermatol Sci. 1999;19:44–47. doi: 10.1016/s0923-1811(98)00049-8. [DOI] [PubMed] [Google Scholar]
- Welter JF, Crish JF, Agarwal C, et al. Fos-related antigen (Fra-1), junB, and junD activate human involucrin promoter transcription by binding to proximal and distal AP1 sites to mediate phorbol ester effects on promoter activity. J Biol Chem. 1995;270:12614–12622. doi: 10.1074/jbc.270.21.12614. [DOI] [PubMed] [Google Scholar]
- Yoneda K, Demitsu T, Manabe M, et al. Expression of wild-type, but not mutant, loricrin causes programmed cell death in HaCaT keratinocytes. J Dermatol. 2010a;37:956–964. doi: 10.1111/j.1346-8138.2010.00932.x. [DOI] [PubMed] [Google Scholar]
- Yoneda K, Demitsu T, Nakai K, et al. Activation of vascular endothelial growth factor receptor 2 in a cellular model of loricrin keratoderma. J Biol Chem. 2010b;285:16184–16194. doi: 10.1074/jbc.M109.056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K, Hohl D, McBride OW, et al. The human loricrin gene. J Biol Chem. 1992;267:18060–18066. [PubMed] [Google Scholar]
- Yoneda K, Steinert PM. Overexpression of human loricrin in transgenic mice produces a normal phenotype. Proc Natl Acad Sci U S A. 1993;90:10754–10758. doi: 10.1073/pnas.90.22.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MR, Farrell L, Lambert P, et al. Protection against human papillomavirus type 16-E7 oncogene-induced tumorigenesis by in vivo expression of dominant-negative c-jun. Mol Carcinog. 2002;34:72–77. doi: 10.1002/mc.10050. [DOI] [PubMed] [Google Scholar]
- Young MR, Li JJ, Rincon M, et al. Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci U S A. 1999;96:9827–9832. doi: 10.1073/pnas.96.17.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]